Exploring the Interplay of Antimicrobial Properties and Cellular Response in Physically Crosslinked Hyaluronic Acid/ε-Polylysine Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HA/ε-PL Hydrogels
2.3. Sterilization of HA/ε-PL Hydrogels
2.4. Lyophilization of HA/ε-PL Hydrogels
2.5. Physicochemical Characterisation
2.5.1. Swelling Behaviour
2.5.2. Gel Fraction
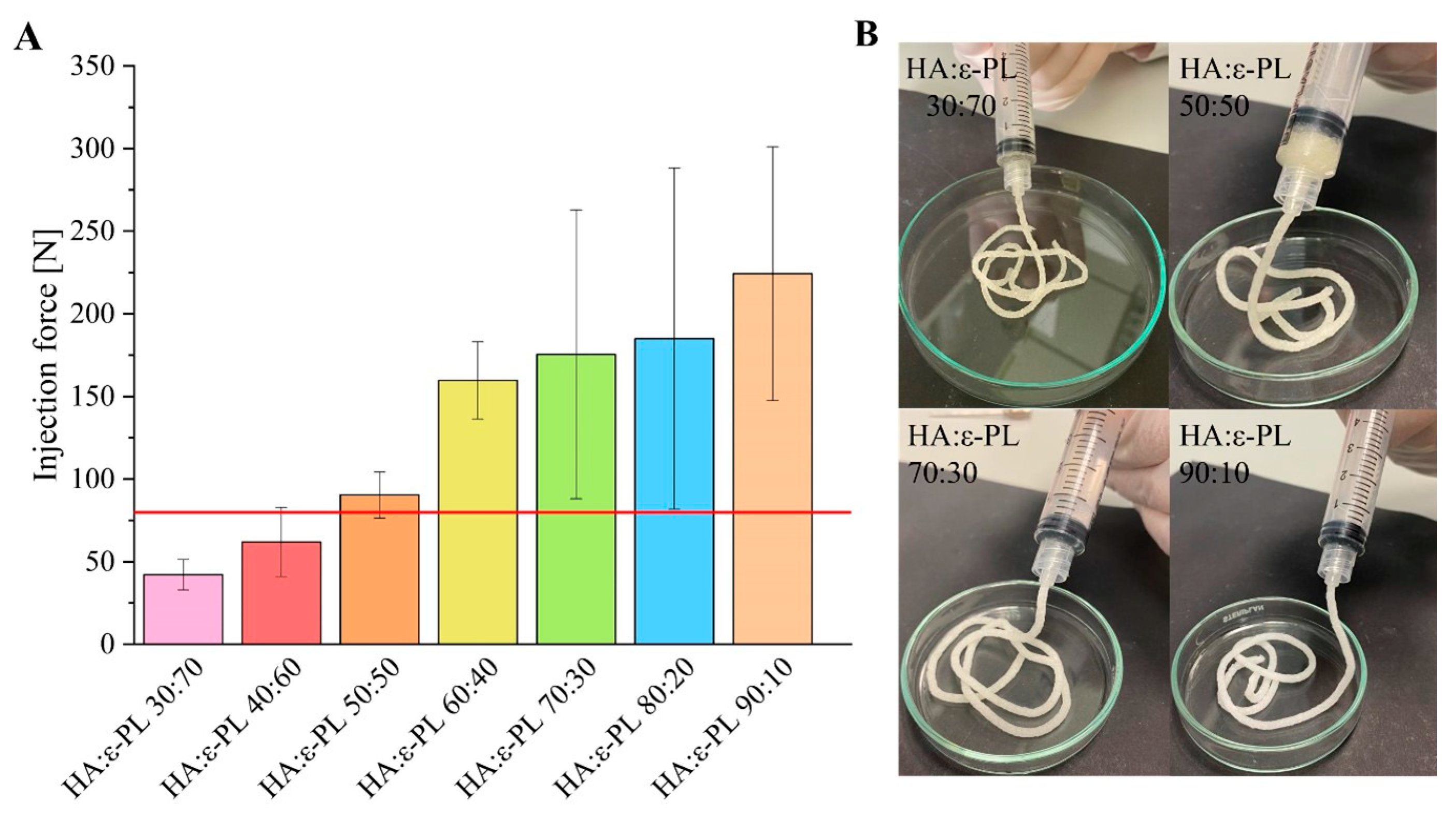
2.5.3. Injection Force
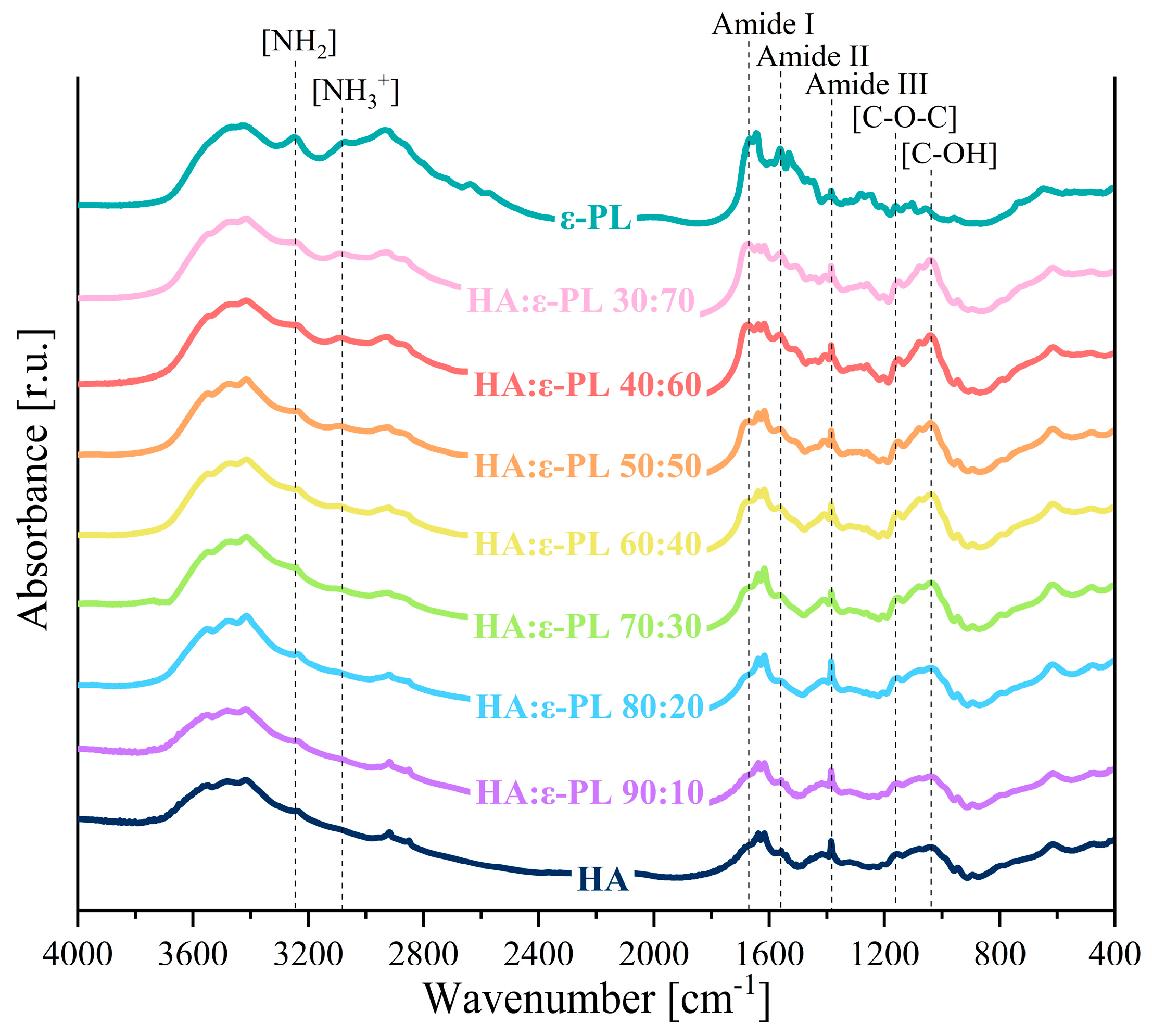
2.5.4. Molecular Structure
2.5.5. Morphology
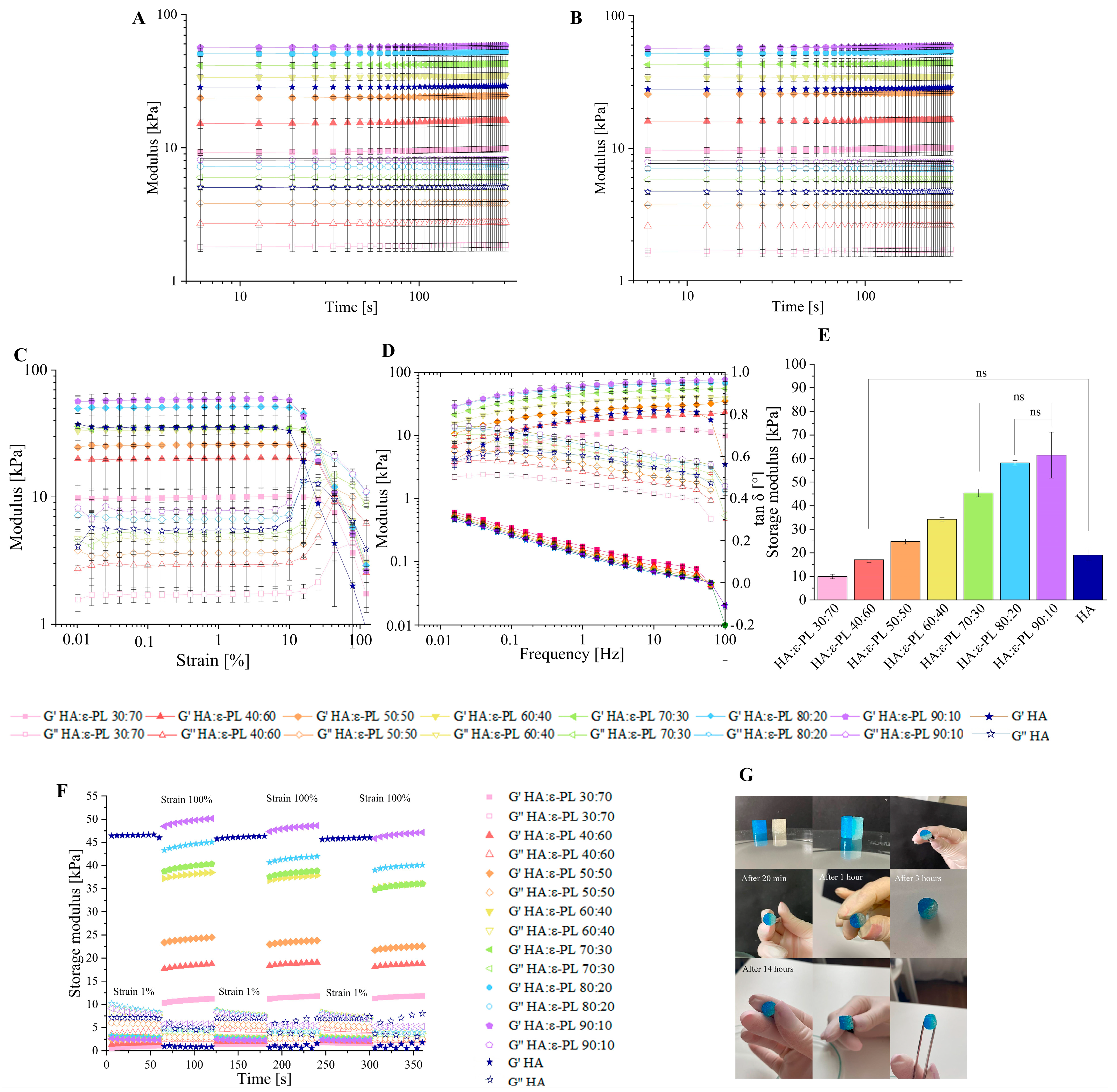
2.5.6. Oscillatory Rheology
2.6. In Vitro Antimicrobial Characterisation
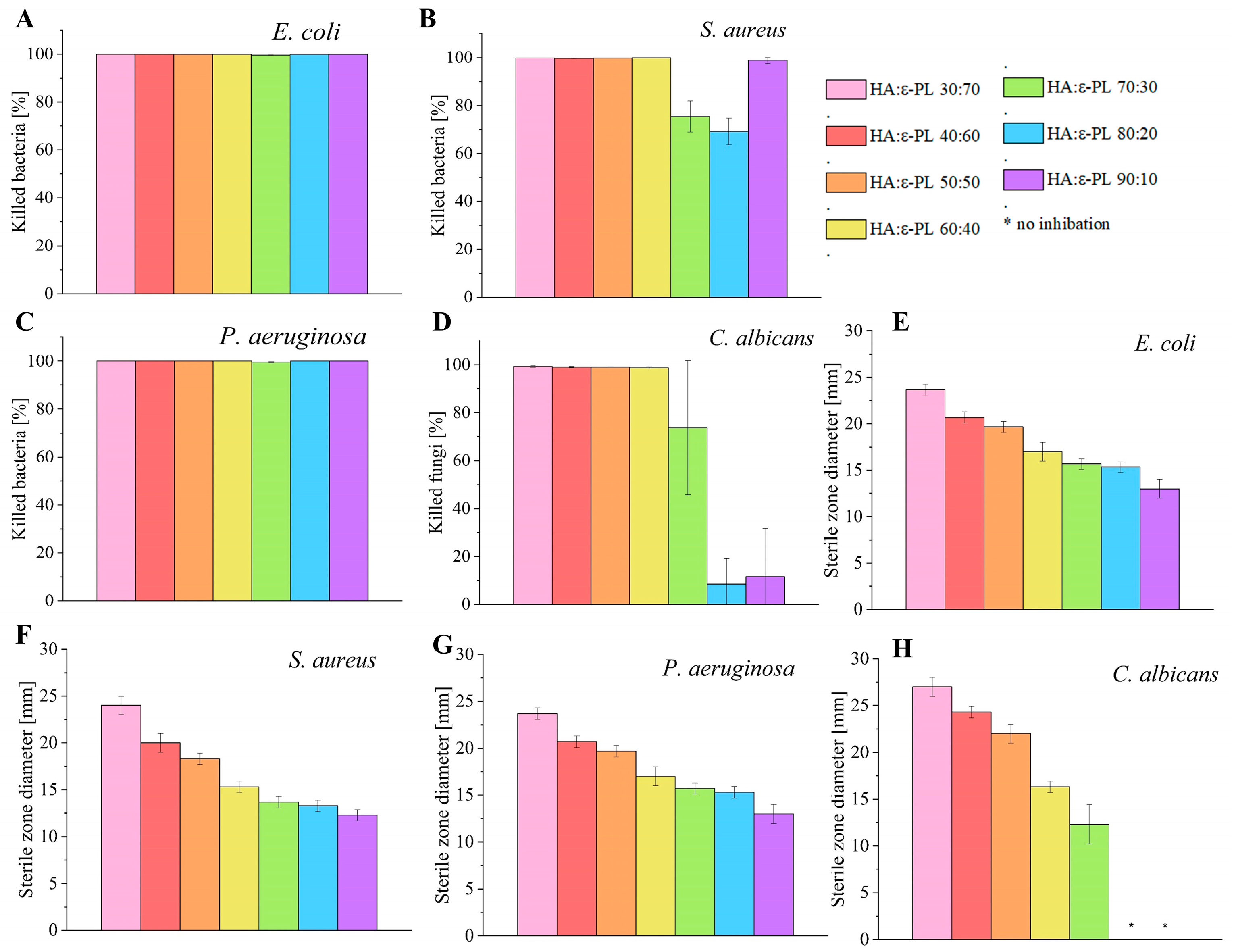
2.6.1. Agar Well Diffusion Method
2.6.2. Dynamic Contact Test
2.7. In Vitro Cytotoxicity Assay
2.7.1. Extract Test
2.7.2. Direct Contact Assay
2.7.3. Cell Viability Staining
2.8. Hemocompatibility of Hydrogels
2.9. Statistical Analysis
3. Results and Discussion
3.1. Influence of HA/ε-PL Ratio on Hydrogel Swelling Behaviour and Gel Fraction
3.2. Influence of the HA/ε-PL Ratio on Hydrogel Crosslinking Ability, Mechanical and Rheological Properties
3.3. Injection Force Measurements
3.4. Chemical Characterization
3.5. Influence of HA/ε-PL Ratio on the Hydrogel Morphology
3.6. Antimicrobial Properties of HA/ε-PL Hydrogels
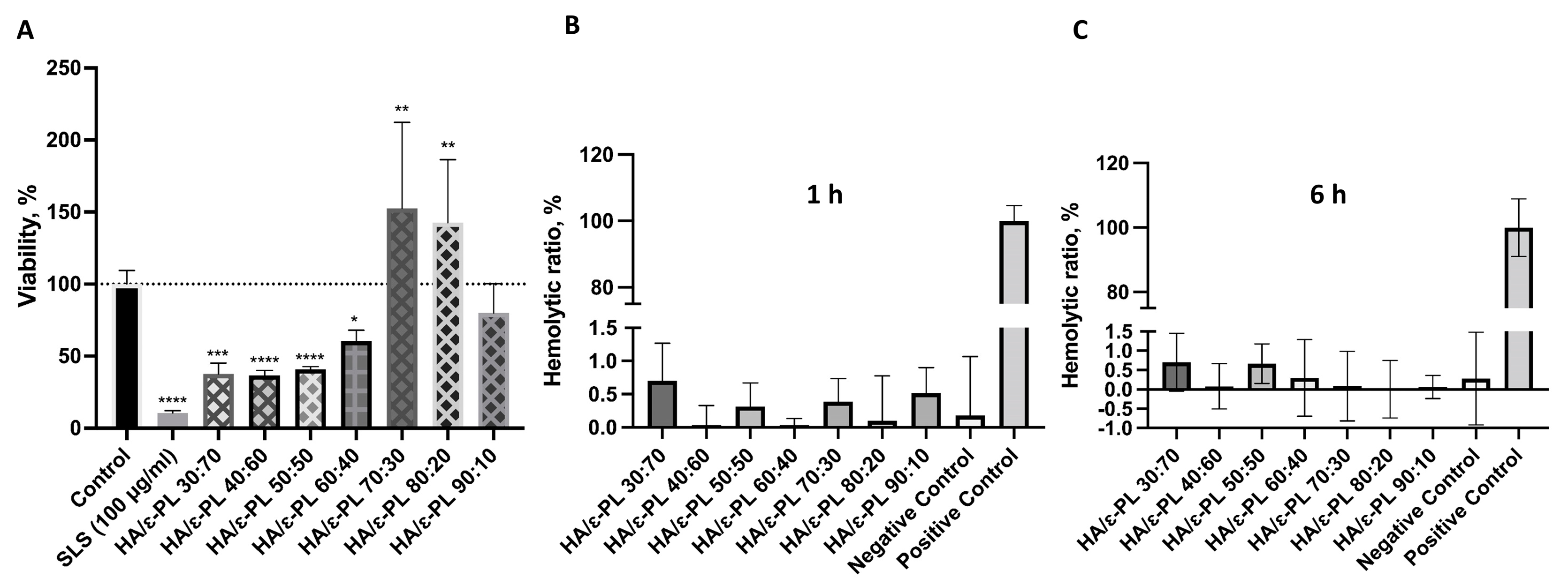
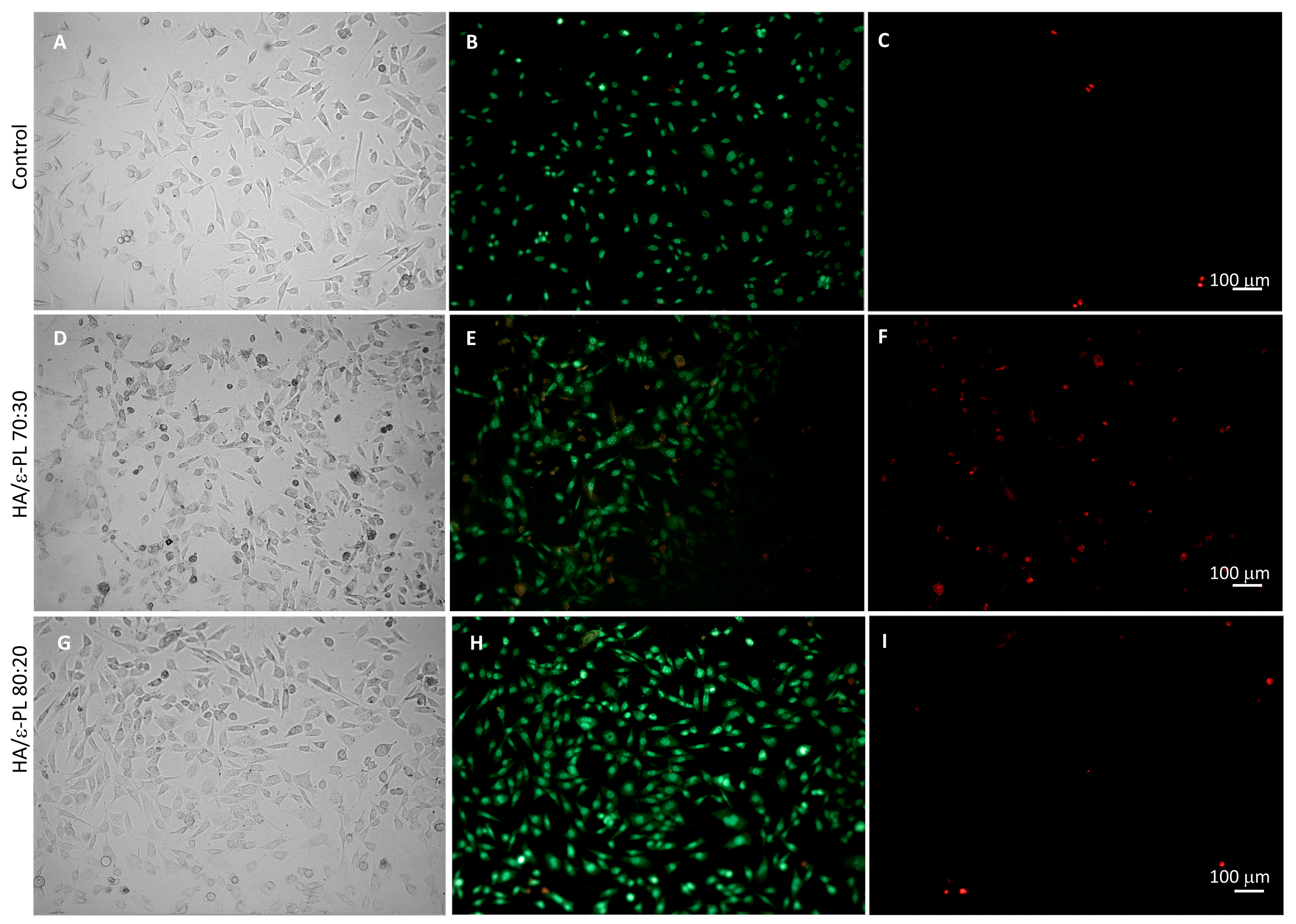
3.7. In Vitro Cell Biocompatibility of HA/ε-PL Hydrogels
3.7.1. Biocompatibility and Hemocompatibility of Hydrogels
3.7.2. Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; He, Q.; Zhou, F.; Zhang, J.; Liang, R.; Yao, X.; Bunpetch, V.; Li, J.; Zhang, S.; Ouyang, H. Current Intelligent Injectable Hydrogels for In Situ Articular Cartilage Regeneration. Polym. Rev. 2020, 60, 203–225. [Google Scholar] [CrossRef]
- Bueno, E.M.; Glowacki, J. Cell-free and cell-based approaches for bone regeneration. Nat. Rev. Rheumatol. 2009, 5, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, K. Current requirements for polymeric biomaterials in otolaryngology. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2009, 8, Doc11. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Physical properties of crosslinked hyaluronic acid hydrogels. J. Mater. Sci. Mater. Med. 2008, 1911, 3335–33343. [Google Scholar] [CrossRef]
- Jin, Y.; Koh, R.H.; Kim, S.-H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng. C 2020, 115, 111096. [Google Scholar] [CrossRef]
- Wang, L.; Dong, S.; Liu, Y.; Ma, Y.; Zhang, J.; Yang, Z.; Jiang, W.; Yuan, Y. Fabrication of Injectable, Porous Hyaluronic Acid Hydrogel Based on an In-Situ Bubble-Forming Hydrogel Entrapment Process. Polymers 2020, 12, 1138. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, D.; Ma, S.; Zhang, J.; Gao, F.; Guan, F.; Yao, M. Dual-enzymatically crosslinked and injectable hyaluronic acid hydrogels for potential application in tissue engineering. RSC Adv. 2020, 10, 2870–2876. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, J.; Liu, Y.; Xiao, M.; Li, Y.; Yang, J.; Yang, J.; Liu, W. Injectable Hyaluronic Acid Hydrogel Loaded with Functionalized Human Mesenchymal Stem Cell Aggregates for Repairing Infarcted Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 6926–6937. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef]
- Alinejad, Y.; Adoungotchodo, A.; Grant, M.P.; Epure, L.M.; Antoniou, J.; Mwale, F.; Lerouge, S. Injectable Chitosan Hydrogels with Enhanced Mechanical Properties for Nucleus Pulposus Regeneration. Tissue Eng. Part A 2019, 25, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable Chitosan-Based Thermosensitive Hydrogel/Nanoparticle-Loaded System for Local Delivery of Vancomycin in the Treatment of Osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef]
- Ghosh, M.; Halperin-Sternfeld, M.; Grinberg, I.; Adler-Abramovich, L. Injectable Alginate-Peptide Composite Hydrogel as a Scaffold for Bone Tissue Regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, K.; Ma, S.; Liu, T.; Yao, M.; Li, J.; Wang, X.; Guan, F. Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment. e-Polymers 2019, 19, 87–91. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Sha, D.; Liu, Y.; Xu, J.; Shi, K.; Yu, C.; Ji, X. Injectable and antibacterial ε-poly(l-lysine)-modified poly(vinyl alcohol)/chitosan/AgNPs hydrogels as wound healing dressings. Polymer 2021, 212, 123155. [Google Scholar] [CrossRef]
- Sun, A.; He, X.; Li, L.; Li, T.; Liu, Q.; Zhou, X.; Ji, X.; Li, W.; Qian, Z. An injectable photopolymerized hydrogel with antimicrobial and biocompatible properties for infected skin regeneration. NPG Asia Mater. 2020, 12, 25. [Google Scholar] [CrossRef]
- Gong, C.; Lu, C.; Li, B.; Shan, M.; Wu, G. Injectable dopamine-modified poly(α,β-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system. J. Biomed. Mater. Res. Part A 2017, 105, 1000–1008. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Yan, Y.; He, C.; Li, X. Fabrication of injectable hydrogels from silk fibroin and angiogenic peptides for vascular growth and tissue regeneration. Chem. Eng. J. 2021, 418, 129308. [Google Scholar] [CrossRef]
- Yuan, T.; Li, Z.; Zhang, Y.; Shen, K.; Zhang, X.; Xie, R.; Liu, F.; Fan, W. Injectable Ultrasonication-Induced Silk Fibroin Hydrogel for Cartilage Repair and Regeneration. Tissue Eng. Part A 2021, 27, 1213–1224. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Ren, Y.; Wang, P.; Pu, Y.; Yang, R.; Wang, X.; Tan, X.; Ye, Z.; Maurizot, V.; et al. Mussel-Inspired Dual-Cross-linking Hyaluronic Acid/ϵ-Polylysine Hydrogel with Self-Healing and Antibacterial Properties for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 27876–27888. [Google Scholar] [CrossRef]
- Han, C.; Zhang, H.; Wu, Y.; He, X.; Chen, X. Dual-crosslinked hyaluronan hydrogels with rapid gelation and high injectability for stem cell protection. Sci. Rep. 2020, 10, 14997. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-healing hyaluronic acid hydrogels based on dynamic Schiff base linkages as biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef] [PubMed]
- Ščeglovs, A.; Salma-Ancane, K. Novel Hydrogels and Composite Hydrogels Based on ԑ-Polylysine, Hyaluronic Acid and Hydroxyapatite. Key Eng. Mater. 2020, 850, 242–248. [Google Scholar] [CrossRef]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Capillo, M.C.; Grimaldi, G.; Alonci, G.; Rauso, R.; Guida, S.; Mocchi, R. Comparative Physicochemical Analysis among 1,4-Butanediol Diglycidyl Ether Cross-Linked Hyaluronic Acid Dermal Fillers. Gels 2021, 7, 139. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.; Morais, T.P.; Zaini, P.A.; Campos, C.S.; Almeida-Souza, H.O.; Dandekar, A.M.; Nascimento, R.; Goulart, L.R. Antimicrobial activity of Epsilon-Poly-l-lysine against phytopathogenic bacteria. Sci. Rep. 2020, 10, 11324. [Google Scholar] [CrossRef]
- Shukla, S.C.; Singh, A.; Pandey, A.K.; Mishra, A. Review on production and medical applications of ɛ-polylysine. Biochem. Eng. J. 2012, 65, 70–81. [Google Scholar] [CrossRef]
- Yoshida, T.; Nagasawa, T. Epsilon-Poly-l-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26. [Google Scholar] [CrossRef]
- Tan, Z.; Shi, Y.; Xing, B.; Hou, Y.; Cui, J.; Jia, S. The antimicrobial effects and mechanism of ε-poly-lysine against Staphylococcus aureus. Bioresour. Bioprocess. 2019, 6, 11. [Google Scholar] [CrossRef]
- Alkekhia, D.; Shukla, A. Influence of poly-l-lysine molecular weight on antibacterial efficacy in polymer multilayer films. J. Biomed. Mater. Res. Part A 2019, 107, 1324–1339. [Google Scholar] [CrossRef]
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent Advances in Epsilon-Poly-L-Lysine and L-Lysine-Based Dendrimer Synthesis, Modification, and Biomedical Applications. Front. Chem. 2021, 9, 659304. [Google Scholar] [CrossRef]
- Li, S.; Chen, N.; Li, Y.; Li, X.; Zhan, Q.; Ban, J.; Zhao, J.; Hou, X.; Yuan, X. Metal-crosslinked ɛ-poly-L-lysine tissue adhesives with high adhesive performance: Inspiration from mussel adhesive environment. Int. J. Biol. Macromol. 2020, 153, 1251–1261. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, Z.; Hu, J.; Liu, Y. Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing applications. Mater. Sci. Eng. C 2021, 128, 112319. [Google Scholar] [CrossRef]
- Mayandi, V.; Wen Choong, A.C.; Dhand, C.; Lim, F.P.; Aung, T.T.; Sriram, H.; Dwivedi, N.; Periayah, M.H.; Sridhar, S.; Fazil, M.H.; et al. Multifunctional Antimicrobial Nanofiber Dressings Containing ε-Polylysine for the Eradication of Bacterial Bioburden and Promotion of Wound Healing in Critically Colonized Wounds. ACS Appl. Mater. Interfaces 2020, 12, 15989–16005. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Tracuma, E.; Loca, D. Hyaluronic Acid/Polylysine Composites for Local Drug Delivery: A Review. Key Eng. Mater. 2020, 850, 213–218. [Google Scholar] [CrossRef]
- Tian, W.M.; Hou, S.P.; Ma, J.; Zhang, C.L.; Xu, Q.Y.; Lee, I.S.; Li, H.D.; Spector, M.; Cui, F.Z. Hyaluronic Acid–Poly-D-Lysine-Based Three-Dimensional Hydrogel for Traumatic Brain Injury. Tissue Eng. 2005, 11, 513–525. [Google Scholar] [CrossRef]
- Amato, G.; Grimaudo, M.A.; Alvarez-Lorenzo, C.; Concheiro, A.; Carbone, C.; Bonaccorso, A.; Puglisi, G.; Musumeci, T. Hyaluronan/Poly-L-lysine/Berberine Nanogels for Impaired Wound Healing. Pharmaceutics 2020, 13, 34. [Google Scholar] [CrossRef]
- Guo, J.; Wei, C.; Wang, X.; Hou, Y.; Guo, W. An in situ mechanical adjustable double crosslinking hyaluronic acid/poly-lysine hydrogel matrix: Fabrication, characterization and cell morphology. Int. J. Biol. Macromol. 2021, 180, 234–241. [Google Scholar] [CrossRef]
- Simonson, A.W.; Lawanprasert, A.; Goralski, T.D.P.; Keiler, K.C.; Medina, S.H. Bioresponsive peptide-polysaccharide nanogels—A versatile delivery system to augment the utility of bioactive cargo. Nanomedicine Nanotechnology. Biol. Med. 2019, 17, 391–400. [Google Scholar] [CrossRef]
- Salma-Ancane, K.; Sceglovs, A.; Tracuma, E.; Wychowaniec, J.K.; Aunina, K.; Ramata-Stunda, A.; Nikolajeva, V.; Loca, D. Effect of crosslinking strategy on the biological, antibacterial and physicochemical performance of hyaluronic acid and ɛ-polylysine based hydrogels. Int. J. Biol. Macromol. 2022, 208, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Vadala, G.; Russo, F.; Musumeci, M.; D’Este, M.; Cattani, C.; Catanzaro, G.; Tirindelli, M.C.; Lazzari, L.; Alini, M.; Giordano, R.; et al. Clinically relevant hydrogel-based on hyaluronic acid and platelet rich plasma as a carrier for mesenchymal stem cells: Rheological and biological characterization. J. Orthop. Res. 2017, 35, 2109–2116. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, H.; Xu, C.; Yu, D.; Xu, H.; Hu, Y. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether. RSC Adv. 2020, 10, 7206–7213. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Op’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kulichikhin, V.G.; Malkin, A.Y. The rheological characterisation of typical injection implants based on hyaluronic acid for contour correction. Rheol. Acta 2016, 55, 223–233. [Google Scholar] [CrossRef]
- Hájovská, P.; Chytil, M.; Kalina, M. Rheological study of albumin and hyaluronan-albumin hydrogels: Effect of concentration, ionic strength, pH and molecular weight. Int. J. Biol. Macromol. 2020, 161, 738–745. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.; Xiang, X.; Diao, Y.; Li, B.; Shi, L.; Ran, R. Self-healable, tough and highly stretchable hydrophobic association/ionic dual physically cross-linked hydrogels. RSC Adv. 2017, 7, 12063–12073. [Google Scholar] [CrossRef]
- Devi VK, A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Capillo, M.C.; Sommatis, S.; Maccario, C.; Alonci, G.; Rauso, R.; Galadari, H.; Guida, S.; Mocchi, R. Rheological Investigation as Tool to Assess Physicochemical Stability of a Hyaluronic Acid Dermal Filler Cross-Linked with Polyethylene Glycol Diglycidyl Ether and Containing Calcium Hydroxyapatite, Glycine and L-Proline. Gels 2021, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Vo, A.; Doumit, M.; Rockwell, G. The Biomechanics and Optimization of the Needle-Syringe System for Injecting Triamcinolone Acetonide into Keloids. J. Med. Eng. 2016, 2016, 5162394. [Google Scholar] [CrossRef]
- Rył, A.; Owczarz, P. Injectability of Thermosensitive, Low-Concentrated Chitosan Colloids as Flow Phenomenon through the Capillary under High Shear Rate Conditions. Polymers 2020, 12, 2260. [Google Scholar] [CrossRef]
- Cilurzo, F.; Selmin, F.; Minghetti, P.; Adami, M.; Bertoni, E.; Lauria, S.; Montanari, L. Injectability Evaluation: An Open Issue. AAPS PharmSciTech 2011, 12, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Meng, Y.; Shi, Y.; Li, Y.; Chen, J.; Sheng, F. Properties of epsilon-polylysine·HCl/high-methoxyl pectin polyelectrolyte complexes and their commercial application. J. Food Process. Preserv. 2020, 44, e14320. [Google Scholar] [CrossRef]
- Lopes, T.D.; Riegel-Vidotti, I.C.; Grein, A.; Tischer, C.A.; de Faria-Tischer, P.C.S. Bacterial cellulose and hyaluronic acid hybrid membranes: Production and characterization. Int. J. Biol. Macromol. 2014, 67, 401–440. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, M.; Locs, J.; Loca, D. Hyaluronan Hydrogel/Calcium Phosphates Composites for Medical Application. Key Eng. Mater. 2016, 721, 219–223. [Google Scholar] [CrossRef]
- Carneiro, J.; Döll-Boscardin, P.M.; Fiorin, B.C.; Nadal, J.M.; Farago, P.V.; de Paula, J.P. Development and characterization of hyaluronic acid-lysine nanoparticles with potential as innovative dermal filling. Braz. J. Pharm. Sci. 2016, 52, 645–651. [Google Scholar] [CrossRef]
- Rozenberg, M.; Shoham, G. FTIR spectra of solid poly-l-lysine in the stretching NH mode range. Biophys. Chem. 2007, 125, 166–171. [Google Scholar] [CrossRef] [PubMed]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Jonnalagadda, J.B.; Rivero, I.V.; Dertien, J.S. In vitro chondrocyte behavior on porous biodegradable poly (e-caprolactone)/polyglycolic acid scaffolds for articular chondrocyte adhesion and proliferation. J. Biomater. Sci. Polym. Ed. 2015, 26, 401–419. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]


| HA/ε-PL Ratio | Porosity ± STDEV, % |
|---|---|
| 30:70 | 71.08 ± 0.65 |
| 40:60 | 69.38 ± 0.79 |
| 50:50 | 70.35 ± 0.18 |
| 60:40 | 73.53 ± 0.54 |
| 70:30 | 82.49 ± 0.58 |
| 80:20 | 76.69 ± 1.5 |
| 90:10 | 82.28 ± 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aunina, K.; Ramata-Stunda, A.; Kovrlija, I.; Tracuma, E.; Merijs-Meri, R.; Nikolajeva, V.; Loca, D. Exploring the Interplay of Antimicrobial Properties and Cellular Response in Physically Crosslinked Hyaluronic Acid/ε-Polylysine Hydrogels. Polymers 2023, 15, 1915. https://doi.org/10.3390/polym15081915
Aunina K, Ramata-Stunda A, Kovrlija I, Tracuma E, Merijs-Meri R, Nikolajeva V, Loca D. Exploring the Interplay of Antimicrobial Properties and Cellular Response in Physically Crosslinked Hyaluronic Acid/ε-Polylysine Hydrogels. Polymers. 2023; 15(8):1915. https://doi.org/10.3390/polym15081915
Chicago/Turabian StyleAunina, Kristine, Anna Ramata-Stunda, Ilijana Kovrlija, Eliza Tracuma, Remo Merijs-Meri, Vizma Nikolajeva, and Dagnija Loca. 2023. "Exploring the Interplay of Antimicrobial Properties and Cellular Response in Physically Crosslinked Hyaluronic Acid/ε-Polylysine Hydrogels" Polymers 15, no. 8: 1915. https://doi.org/10.3390/polym15081915






