Impact of Oil Addition on Physicochemical Properties and In Vitro Digestibility of Extruded Pineapple Stem Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pineapple Stem Starch Isolation
2.3. Determination of Amylose Content
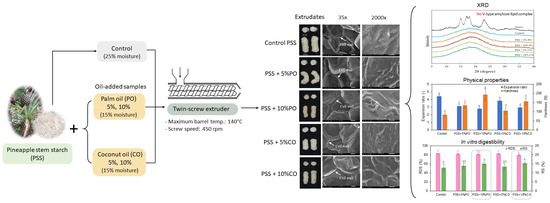
2.4. Sample Preparation
2.5. Extrusion Process
2.6. Physical Properties of Extrudates
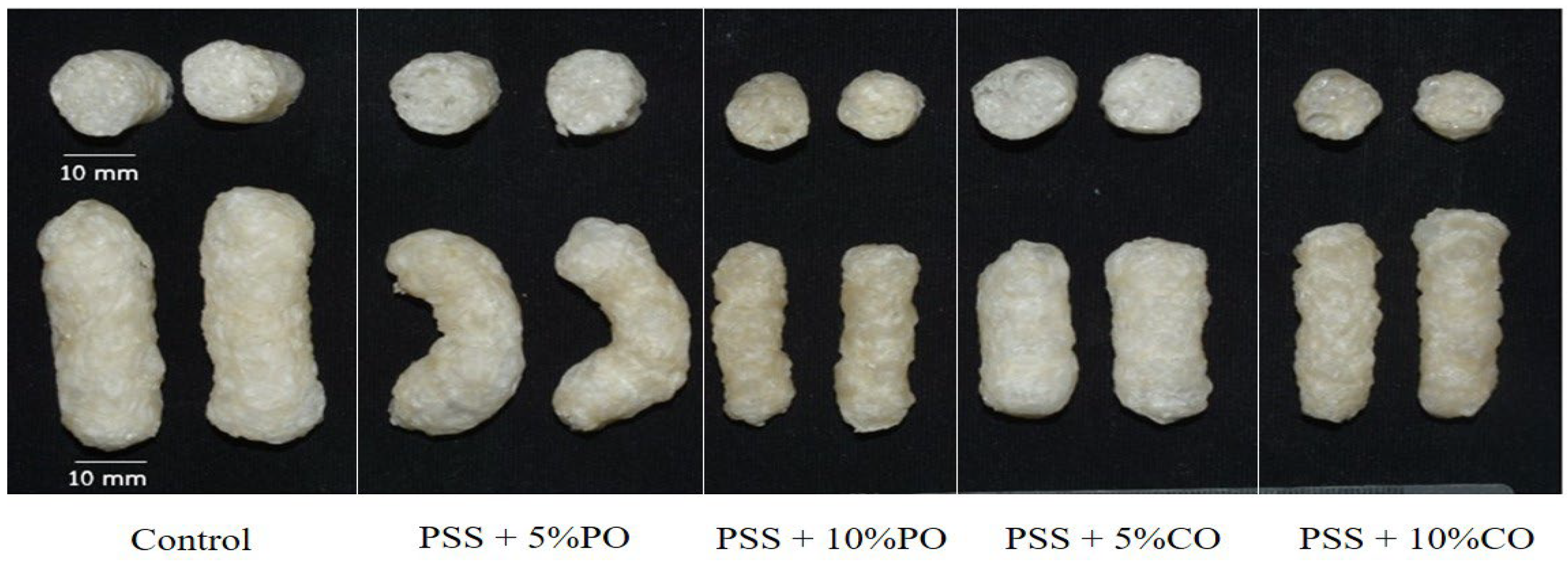
2.6.1. Photograph
2.6.2. Expansion Ratio
2.6.3. Bulk Density
2.6.4. Hardness
2.6.5. Water Absorption Index and Water Solubility Index
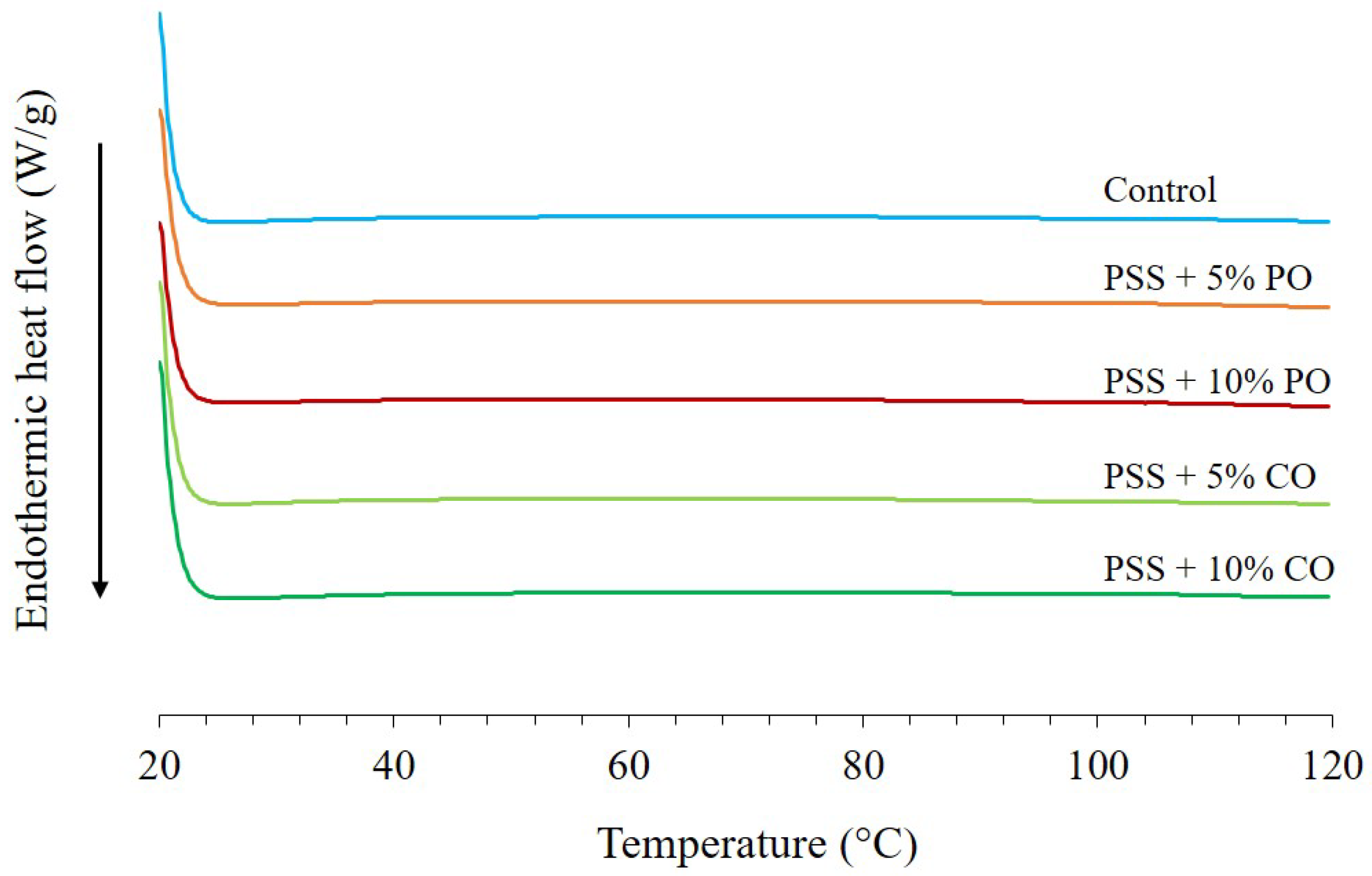
2.6.6. Thermal Properties
2.7. Structural Properties of Extrudates
2.7.1. Scanning Electron Microscopy
2.7.2. X-ray Diffraction
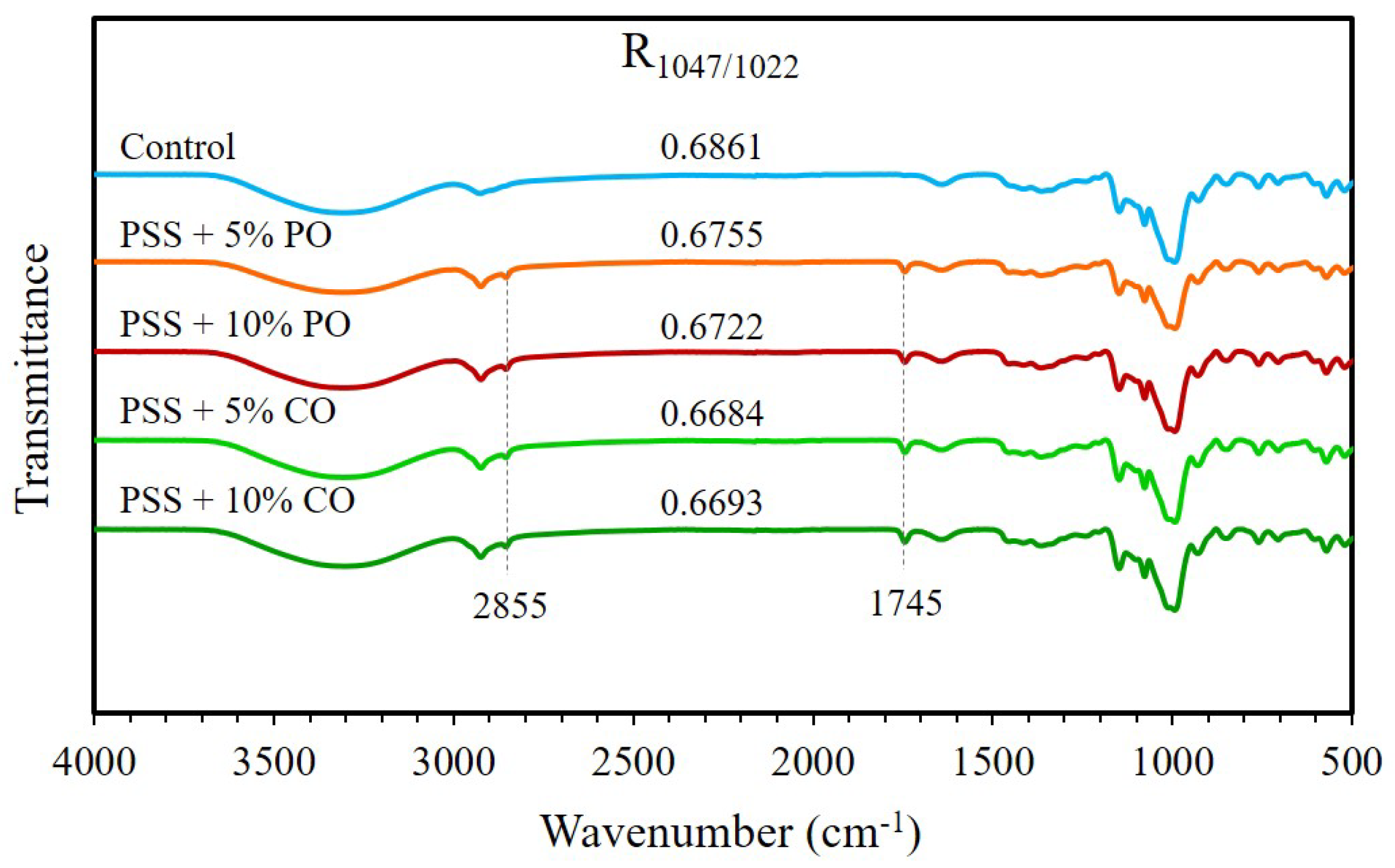
2.7.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Chemical Properties
2.8.1. Moisture Content and Water Activity (aw)
2.8.2. Total Phenolic Content
2.9. In Vitro Starch Digestibility
2.10. Statistical Analysis
3. Results
3.1. Amylose Content of Pineapple Stem Starch
3.2. Physical Properties of Extrudates
3.2.1. Visual Appearance
3.2.2. Expansion Ratio
3.2.3. Bulk Density
3.2.4. Hardness
3.2.5. Water Absorption Index and Water Solubility Index
3.3. Structural Properties of Extrudates
3.3.1. Scanning Electron Microscopy
3.3.2. X-ray Diffraction
3.3.3. Thermal Properties
3.3.4. FTIR Spectra
3.4. Chemical Properties of Extrudate
3.4.1. Moisture Content and Water Activity
3.4.2. Total Phenolic Content
3.5. In Vitro Starch Digestibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, A.; Hyde, K.D.; Jayawardena, R.S. First report of Colletotrichum fructicola causing fruit rot and leaf-tip dieback on pineapple in Northern Thailand. Plants 2023, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crops Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Kengkhetkit, N.; Amornsakchai, T. Utilisation of pineapple leaf waste for plastic reinforcement: 1. A novel extraction method for short pineapple leaf fiber. Ind. Crops Prod. 2012, 40, 55–61. [Google Scholar] [CrossRef]
- Manohar, J.; Gayathri, R.; Vishnupriya, V. Tenderisation of meat using bromelain from pineapple extract. Int. J. Pharm. Sci. Rev. Res. 2016, 39, 81–85. [Google Scholar]
- Sriprablom, J.; Suphantharika, M.; Smith, S.M.; Amornsakchai, T.; Pinyo, J.; Wongsagonsup, R. Physicochemical, rheological, in-vitro digestibility, and emulsifying properties of starch extracted from pineapple stem agricultural waste. Foods 2023, 12, 2028. [Google Scholar] [CrossRef]
- Tangsrianugul, N.; Hongsanyatham, S.; Kapcum, C.; Sangayuth, N.; Boonsanong, N.; Somprasong, N.; Smith, S.M.; Amornsakchai, T.; Pinyo, J.; Wongsagonsup, R. Physicochemical and sensory properties of corn grits and pineapple stem starch-based extruded snacks enriched with oyster mushroom powder. Int. J. Food Sci. Technol. 2023, 58, 1528–1540. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Hanna, M.A. Extrusion processing conditions for amylose-lipid complexing. Cereal Chem. 1994, 71, 587–593. [Google Scholar]
- Fellows, P.J. Extrusion cooking. In Food Processing Technology, 4th ed.; Fellows, P.J., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 753–780. [Google Scholar]
- Riaz, M.N. Extruded snacks. In Handbook of Food Science Technology and Engineering; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 281–288. [Google Scholar]
- De Pilli, T.; Jouppila, K.; Ikonen, J.; Kansikas, J.; Derossi, A.; Severini, C. Study on formation of starch-lipid complexes during extrusion-cooking of almond flour. J. Food Eng. 2008, 87, 495–504. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, S.; Singh, M.; Singh, N.; Shevkani, K.; Singh, B. Effect of banana flour, screw speed and temperature on extrusion behavior of corn extrudates. J. Food Sci. Technol. 2015, 52, 4276–4285. [Google Scholar] [CrossRef]
- Thachil, M.T.; Chouksey, M.K.; Gudipati, V. Amylose-lipid complex formation during extrusion cooking: Effect of added lipid type and amylose level on corn-based puffed snacks. Int. J. Food Sci. Technol. 2014, 49, 309–316. [Google Scholar] [CrossRef]
- Shukri, R.; Alavi, S.; Dogan, H.; Shi, Y.-C. Properties of extruded cross-linked waxy maize starches and their effects on extruded oat flour. Carbohydr. Polym. 2021, 253, 117259. [Google Scholar] [CrossRef]
- Su, C.-W.; Kong, M.-S. Effects of soybean oil, cellulose, and SiO2 addition on the lubrication and product properties of rice extrusion. J. Food Eng. 2007, 78, 723–729. [Google Scholar] [CrossRef]
- De Pilli, T.; Derossi, A.; Talja, R.A.; Jouppila, K.; Severini, C. Study of starch-lipid complexes in model system and real food produced using extrusion-cooking technology. Innov. Food Sci. Emerg. Technol. 2011, 12, 610–616. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Hanna, M.A. Amylose-lipid complex formation during single-screw extrusion of various corn starches. Cereal Chem. 1994, 71, 582–587. [Google Scholar]
- Hasjim, J.; Ai, Y.; Jane, J. Novel Applications of Amylose-Lipid Complex as Resistant Starch Type 5. In Resistance Starch: Sources, Applications and Health Benefits; Shi, Y.-C., Maningat, C.C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 79–94. [Google Scholar]
- Pinyo, J.; Wongsagonsup, R.; Panthong, N.; Kantiwong, P.; Huang, Q.; Tangsrianugul, N.; Suphantharika, M. Effects of different edible oils on in vitro starch digestibility and physical properties of rice starch and rice flour. Int. J. Food Sci. Technol. 2024, 59, 170–180. [Google Scholar] [CrossRef]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical composition and health benefits of coconut oil: An overview. J. Sci. Food Agric. 2021, 101, 2182–2193. [Google Scholar] [CrossRef]
- Liau, K.M.; Lee, Y.Y.; Chen, C.K.; Rasool, A.H.G. An open-label pilot study to assess the efficacy and safety of virgin coconut oil in reducing visceral adiposity. Pharmacology 2011, 2011, 949686. [Google Scholar] [CrossRef]
- Szulczewska-Remi, A.; Nogala-Kałucka, M.; Nowak, K.W. Study on the influence of palm oil on blood and liver biochemical parameters, beta-carotene and tocochromanols content as well as antioxidant activity in rats. J. Food Biochem. 2019, 43, e12707. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and nutritional properties of palm oil and palmitic acid: Effects on health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Silalahi, J. Nutritional values and health protective properties of coconut oil. Indones. J. Pharm. Clin. Res. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Marcus, J.B. Lipids Basics: Fats and Oils in Foods and Health; Academic Press: Cambridge, MA, USA, 2013; pp. 231–277. [Google Scholar]
- Cervantes-Ramírez, J.E.; Cabrera-Ramirez, A.H.; Morales-Sánchez, E.; Rodriguez-García, M.E.; Reyes-Vega, M.L.; Ramírez-Jiménez, A.K.; Contreras-Jiménez, B.L.; Gaytán-Martínez, M. Amylose-lipid complex formation from extruded maize starch mixed with fatty acids. Carbohydr. Polym. 2020, 246, 116555. [Google Scholar] [CrossRef]
- Rinju, R.; Harikumaran-Thampi, B.-S. Characteristics of starch extracted from the stem of pineapple plant (Ananas comosus)—An agro waste from pineapple farms. Braz. Arch. Biol. Technol. 2021, 64, e21190276. [Google Scholar] [CrossRef]
- Latt, S.S.; Patomchaiviwat, V.; Sriamornsak, P.; Piriyaprasarth, S. Modification of pineapple starch from stem and rhizome using multiple desired response optimization and its characterization. Pharm. Sci. Asia 2019, 46, 206–217. [Google Scholar] [CrossRef]
- Bumrungnok, K.; Threepopnatkul, P.; Amornsakchai, T.; Chia, C.H.; Wongsagonsup, R.; Smith, S.M. Toward a circular bioeconomy: Exploring pineapple stem starch film as protective coating for fruits and vegetables. Polymers 2023, 15, 2493. [Google Scholar] [CrossRef]
- Thongphang, C.; Namphonsane, A.; Thanawan, S.; Chia, C.H.; Wongsagonsup, R.; Smith, S.M.; Amornsakchai, T. Toward a circular bioeconomy: Development of pineapple stem starch composite as a plastic-sheet substitute for single-use applications. Polymers 2023, 15, 2388. [Google Scholar] [CrossRef]
- Pardhi, S.D.; Singh, B.; Nayik, G.A.; Dar, B.N. Evaluation of functional properties of extruded snacks developed from brown rice grits by using response surface methodology. J. Saudi Soc. Agric. Sci. 2019, 18, 7–16. [Google Scholar] [CrossRef]
- Tepsongkroh, B.; Jangchud, K.; Jangchud, A.; Charunuch, C.; Prinyawiwatkul, W. Healthy brown rice-based extrudates containing straw mushrooms: Effect of feed moisture and mushroom powder contents. J. Food Process. Preserv. 2019, 43, e14089. [Google Scholar] [CrossRef]
- Kapcum, C.; Pasada, K.; Kantiwong, P.; Sroysang, B.; Phiwtawee, J.; Suphantharika, M.; Belur, P.D.; Agoo, E.M.G.; Janairo, J.I.B.; Wongsagonsup, R. Effects of different cooking methods on chemical compositions, in vitro starch digestibility and antioxidant activity of taro (Colocasia esculenta) corms. Int. J. Food Sci. Technol. 2022, 57, 5144–5154. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Kallu, S.; Kowalski, R.J.; Ganjyal, G.M. Impacts of cellulose fiber particle size and starch type on expansion during extrusion processing. J. Food Sci. 2017, 82, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, E.; Veena, R.; Kumar, N.M. Polyphenol Oxidase, Beyond Enzyme Browning. In Microbial Bioprospecting for Sustainable Development, 1st ed.; Singh, J., Sharma, D., Kumar, G., Sharma, M.R., Eds.; Springer Nature Singapore Pte. Ltd.: Singapore, 2018; pp. 203–222. [Google Scholar]
- Salata, C.C.; Leonel, M.; Trombini, F.R.M.; Mischan, M.M. Extrusion of blends of cassava leaves and cassava flour: Physical characteristics of extrudates. Food Sci. Technol. 2014, 34, 501–506. [Google Scholar] [CrossRef]
- Camire, M.E.; Camire, A.; Krumhar, K. Chemical and nutritional changes in foods during extrusion. Crit. Rev. Food Sci. Nutr. 1990, 29, 35–51. [Google Scholar] [CrossRef]
- Cai, C.; Tian, Y.; Sun, C.; Jin, Z. Resistant structure of extruded starch; Effect of fatty acids with different chain lengths and degree of unsaturation. Food Chem. 2022, 374, 131510. [Google Scholar] [CrossRef]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Yves, O.R.; Christian, F.B.; Akum, O.B.; Theodore, T.; Bienvenu, K. Physical and mechanical properties of pineapple fibers (leaves, stems and roots) from Awae Cameroon for the improvement of composite materials. J. Fiber Sci. Technol. 2020, 76, 378–386. [Google Scholar] [CrossRef]
- Chao, C.; Yu, J.; Wang, S.; Copeland, L.; Wang, S. Mechanism underlying the formation of complexes between maize starch and lipids. J. Agric. Food Chem. 2018, 66, 272–278. [Google Scholar] [CrossRef]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch-lipid and starch-lipid-protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, P.; Chen, X.D. Mechanistic insights into the influence of flavonoids from dandelion on physicochemical properties and in vitro digestibility of cooked potato starch. Food Hydrocoll. 2022, 130, 107714. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starches. IOP Conf. Ser. Earth Environ. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Ratnaningsih, N.; Suparmo; Harmayani, E.; Marsono, Y. Physicochemical properties, in vitro starch digestibility, and estimated glycemic index of resistant starch from cowpea (Vigna unguiculata) starch by autoclaving-cooling cycles. Int. J. Biol. Macromol. 2020, 142, 191–200. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, Z.; Chen, L.; Qiu, Z.; Li, T. Effect of starch-catechin interaction on regulation of starch digestibility during hot-extrusion 3D printing: Structural analysis and simulation study. Food Chem. 2022, 393, 133394. [Google Scholar] [CrossRef]
- Guo, T.; Hou, H.; Liu, Y.; Chen, L.; Zheng, B. In vitro digestibility and structural control of rice starch-unsaturated fatty acid complexes by high-pressure homogenization. Carbohydr. Polym. 2021, 256, 117607. [Google Scholar] [CrossRef]
- Raza, H.; Ameer, K.; Ren, X.; Liang, Q.; Chen, X.; Chen, H.; Ma, H. Physicochemical properties and digestion mechanism of starch-linoleic acid complex induced by multi-frequency power ultrasound. Food Chem. 2021, 364, 130392. [Google Scholar] [CrossRef]
- Kendler, C.; Dunchardt, A.; Karbstein, H.P.; Emin, M.A. Effect of oil content and oil addition point on the extrusion processing of wheat gluten-based meat analogues. Foods 2021, 10, 697. [Google Scholar] [CrossRef]
- He, T.; Wang, K.; Zhao, L.; Chen, Y.; Zhou, W.; Liu, F.; Hu, Z. Interaction with longan seed polyphenols affects the structure and digestion properties of maize starch. Carbohydr. Polym. 2021, 256, 117537. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Rong, L.; Chen, Y.; Yu, Q.; Shen, M.; Xie, J. Purple red rice bran anthocyanins reduce the digestibility of rice starch by forming V-type inclusion complexes. Food Res. Int. 2023, 166, 112578. [Google Scholar] [CrossRef]
- Amoako, D.B.; Awika, J.M. Polymeric tannins significantly alter properties and in vitro digestibility of partially gelatinized intact starch granule. Food Chem. 2016, 208, 10–17. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C. Persimmon tannin decreased the glycemic response through decreasing the digestibility of starch and inhibiting α-amylase, α-glucosidase and intestinal glucose uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef]
- Kumar, A.; Sahoo, U.; Baisakha, B.; Okpani, O.A.; Ngangkham, U.; Parameswaran, C.; Basak, N.; Kumar, G.; Sharma, S.G. Resistant starch could be decisive in determining the glycemic index of rice cultivars. J. Cereal Sci. 2018, 79, 348–353. [Google Scholar] [CrossRef]


| Sample | Expansion Ratio (-) | Bulk Density (g/cm3) | Hardness (N) | WAI (-) | WSI (-) | Moisture Content (%) | Water Activity (-) |
|---|---|---|---|---|---|---|---|
| Control | 4.41 ± 0.13 a | 0.11 ± 0.01 d | 84.04 ± 8.67 e | 0.50 ± 0.08 c | 0.91 ± 0.01 a | 6.77 ± 0.06 a | 0.48 ± 0.01 a |
| PSS + 5% PO | 3.14 ± 0.21 c | 0.16 ± 0.01 c | 134.50 ± 14.46 c | 0.60 ± 0.04 b | 0.85 ± 0.02 b | 6.47 ± 0.76 a | 0.45 ± 0.01 c |
| PSS + 10% PO | 2.79 ± 0.09 e | 0.27 ± 0.02 a | 192.47 ± 30.55 a | 0.84 ± 0.01 a | 0.73 ± 0.01 c | 7.62 ± 0.57 a | 0.47 ± 0.01 ab |
| PSS + 5% CO | 3.84 ± 0.09 b | 0.13 ± 0.01 d | 105.07 ± 16.60 d | 0.60 ± 0.03 b | 0.85 ± 0.02 b | 6.70 ± 0.82 a | 0.42 ± 0.01 d |
| PSS + 10% CO | 2.92 ± 0.10 d | 0.25 ± 0.01 b | 155.64 ± 16.36 b | 0.86 ± 0.04 a | 0.71 ± 0.01 c | 7.29 ± 0.37 a | 0.46 ± 0.00 b |
| Expansion Ratio (-) | Bulk Density (g/cm3) | Hardness (N) | WAI (-) | WSI (-) | |
|---|---|---|---|---|---|
| Expansion ratio (-) | 1 | ||||
| Bulk density (g/cm3) | −0.856 ** | 1 | |||
| Hardness (N) | −0.880 ** | 0.922 ** | 1 | ||
| WAI (-) | −0.782 ** | 0.923 ** | 0.901 ** | 1 | |
| WSI (-) | 0.790 ** | −0.927 ** | −0.908 ** | −0.980 ** | 1 |
| Sample | TPC (mg GAE/100 g) | Screw Speed (rpm) | Torque (N·m) | Die Pressure (bar) |
|---|---|---|---|---|
| Native | 53.93 ± 0.78 a | - | - | - |
| Control | 50.02 ± 0.79 b | 450 | 40.05 | 22 |
| PSS + 5% PO | 35.69 ± 0.45 d | 450 | 47.90 | 56 |
| PSS + 10% PO | 41.43 ± 1.11 c | 450 | 42.40 | 28 |
| SS + 5% CO | 32.44 ± 0.55 e | 450 | 43.19 | 60 |
| PSS + 10% CO | 39.86 ± 1.10 c | 450 | 41.62 | 36 |
| Sample | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| Control | 82.97 ± 0.23 a | 4.10 ± 1.76 a | 12.93 ± 1.85 b |
| PSS + 5% PO | 82.28 ± 1.13 a | 3.79 ± 1.94 a | 13.93 ± 1.32 ab |
| PSS + 10% PO | 81.24 ± 1.49 ab | 3.67 ± 1.51 a | 15.09 ± 0.77 a |
| PSS + 5% CO | 82.79 ± 0.80 a | 3.74 ± 1.47 a | 13.47 ± 1.63 ab |
| PSS + 10% CO | 79.79 ± 1.73 b | 4.89 ± 1.50 a | 15.32 ± 0.56 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisitthichai, J.; Wannaphruek, P.; Sriprablom, J.; Suphantharika, M.; Smith, S.M.; Amornsakchai, T.; Wongsagonsup, R. Impact of Oil Addition on Physicochemical Properties and In Vitro Digestibility of Extruded Pineapple Stem Starch. Polymers 2024, 16, 210. https://doi.org/10.3390/polym16020210
Nisitthichai J, Wannaphruek P, Sriprablom J, Suphantharika M, Smith SM, Amornsakchai T, Wongsagonsup R. Impact of Oil Addition on Physicochemical Properties and In Vitro Digestibility of Extruded Pineapple Stem Starch. Polymers. 2024; 16(2):210. https://doi.org/10.3390/polym16020210
Chicago/Turabian StyleNisitthichai, Juthamath, Phimraphat Wannaphruek, Jiratthitikan Sriprablom, Manop Suphantharika, Siwaporn Meejoo Smith, Taweechai Amornsakchai, and Rungtiwa Wongsagonsup. 2024. "Impact of Oil Addition on Physicochemical Properties and In Vitro Digestibility of Extruded Pineapple Stem Starch" Polymers 16, no. 2: 210. https://doi.org/10.3390/polym16020210