A Facile Surface Modification Scheme for Medical-Grade Titanium and Polypropylene Using a Novel Mussel-Inspired Biomimetic Polymer with Cationic Quaternary Ammonium Functionalities for Antibacterial Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
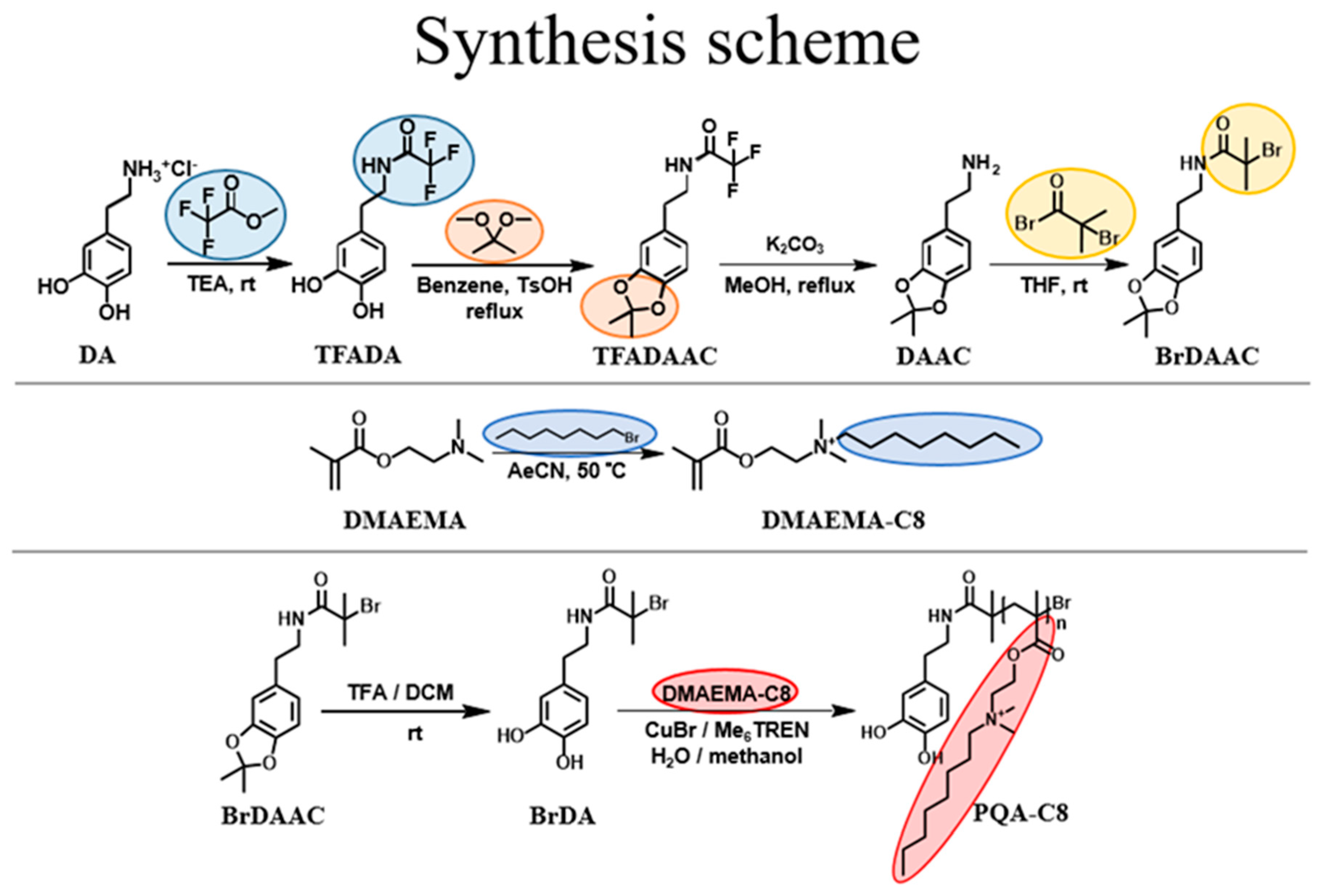
2.2. Synthesis of PQA-C8
2.2.1. Synthesis of TFADA
2.2.2. Synthesis of TFADAAC
2.2.3. Synthesis of DAAC
2.2.4. Synthesis of BrDAAC
2.2.5. Synthesis of BrDA
2.2.6. Synthesis of DMAEMA-C8
2.2.7. Synthesis of PQA-C8
2.3. Surface Modification of Different Substrates
2.4. Characterization
2.5. Antibacterial Test
2.6. Cytotoxicity Assay
2.7. Copper Ion Release Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. PQA-C8 Synthesis
3.2. Surface Characterization
3.2.1. Surface Morphology
Surface Morphology of the Modified Titanium Substrates
Surface Morphology of the Modified Polypropylene Substrates
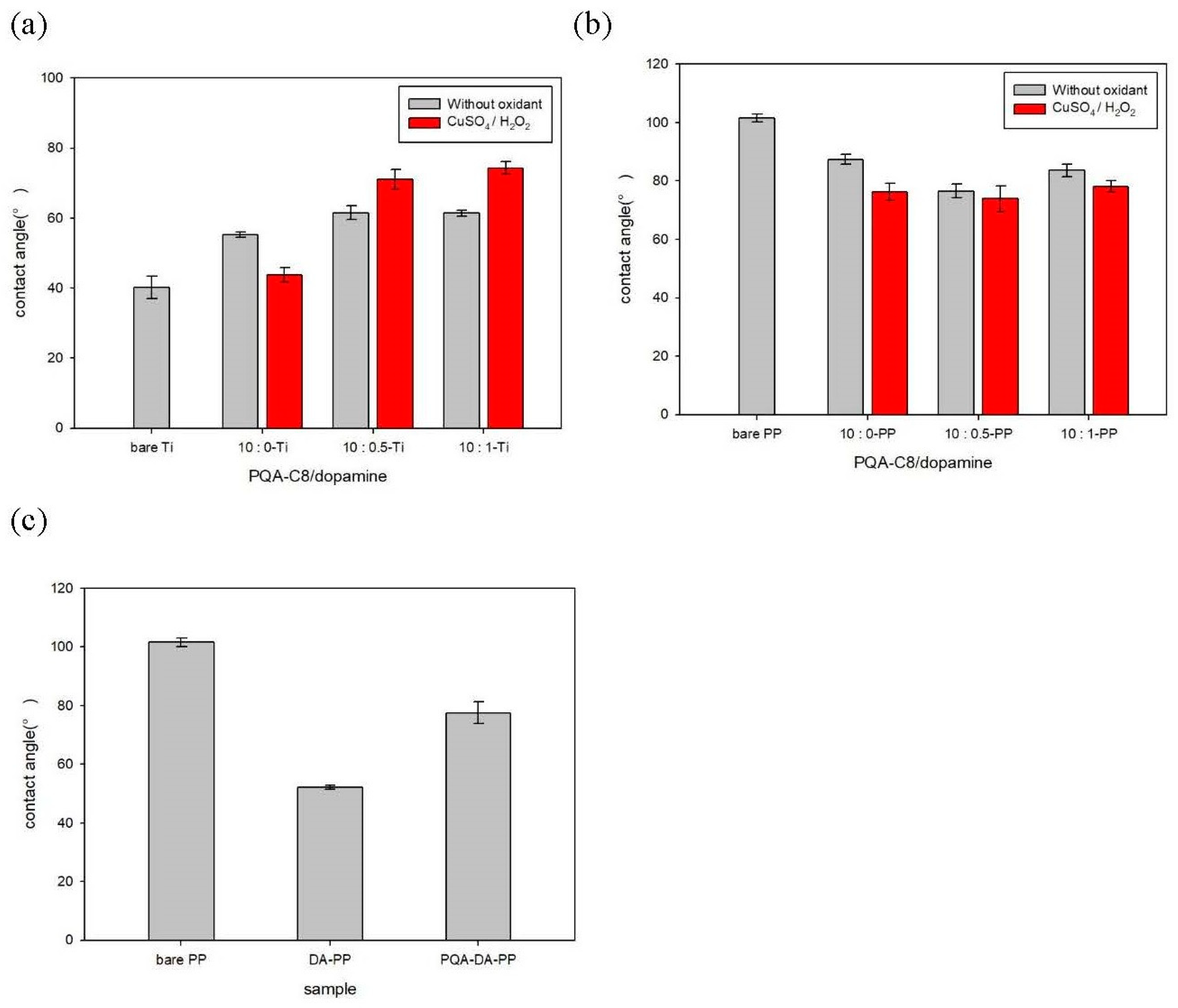
3.2.2. Surface Hydrophilicity
Surface Hydrophilicity of the Modified Titanium Substrates
Surface Hydrophilicity of the Modified Polypropylene Substrates
3.2.3. XPS Analysis
Surface Chemical Characteristics of the Modified Titanium Substrates
Surface Chemical Characteristics of the Modified Polypropylene Substrates
3.3. Antibacterial Assay
3.4. Cytotoxicity Assay
3.5. Copper Ion Release Test
3.6. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Page, K.; Wilson, M.; Parkin, I.P. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J. Mater. Chem. 2009, 19, 3819–3831. [Google Scholar] [CrossRef]
- Lin, P.A.; Cheng, C.H.; Hsieh, K.T.; Lin, J.C. Effect of alkyl chain length and fluorine content on the surface characteristics and antibacterial activity of surfaces grafted with brushes containing quaternized ammonium and fluoro-containing monomers. Colloids Surf. B Biointerfaces 2021, 202, 111674. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef]
- Li, W.L.; Thian, E.S.; Wang, M.; Wang, Z.Y.; Ren, L. Surface Design for Antibacterial Materials: From Fundamentals to Advanced Strategies. Adv. Sci. 2021, 8, 2100368. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, H.C.; Yu, H.; Yan, S.J.; Luan, S.F. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater. Sci. 2020, 8, 4095–4108. [Google Scholar] [CrossRef]
- Krishnan, S.; Ward, R.J.; Hexemer, A.; Sohn, K.E.; Lee, K.L.; Angert, E.R.; Fischer, D.A.; Kramer, E.J.; Ober, C.K. Surfaces of fluorinated pyridinium block copolymers with enhanced antibacterial activity. Langmuir 2006, 22, 11255–11266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Ma, Y.; Han, Q. Surface-oriented fluorinated pyridinium silicone with enhanced antibacterial activity on cotton via supercritical impregnation. Cellulose 2018, 25, 1499–1511. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. VI. Antibacterial activity of fibers surface-treated with phosphonium salts containing trimethoxysilane groups. J. Appl. Polym. Sci. 1994, 52, 641–647. [Google Scholar] [CrossRef]
- Lenoir, S.; Pagnoulle, C.; Galleni, M.; Compère, P.; Jérôme, R.; Detrembleur, C. Polyolefin matrixes with permanent antibacterial activity: Preparation, antibacterial activity, and action mode of the active species. Biomacromolecules 2006, 7, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Liu, Y.; Du, J.; Li, R.; Ren, X.; Huang, T.-S. Biocidal activity of n-halamine methylenebisacrylamide grafted cotton. J. Eng. Fibers Fabr. 2015, 10, 147–154. [Google Scholar] [CrossRef]
- Lim, X. Do we know enough about the safety of quat disinfectants? Chem. Eng. News 2020, 98, 28. [Google Scholar]
- Hanawa, T. Titanium-Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Gulati, K.; Ding, C.Y.; Guo, T.Q.; Guo, H.Z.; Yu, H.J.; Liu, Y. Craniofacial therapy: Advanced local therapies from nano-engineered titanium implants to treat craniofacial conditions. Int. J. Oral Sci. 2023, 15, 15. [Google Scholar] [CrossRef]
- Hossain, M.T.; Shahid, M.A.; Mahmud, N.; Habib, A.; Rana, M.M.; Khan, S.A.; Hossain, M.D. Research and application of polypropylene: A review. Discov. Nano 2024, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Mirel, S.; Pusta, A.; Moldovan, M.; Moldovan, S. Antimicrobial Meshes for Hernia Repair: Current Progress and Perspectives. J. Clin. Med. 2022, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, E.; Downey, C.; Doxford-Hook, E.; Bryant, M.G.; Culmer, P. The use of polymeric meshes for pelvic organ prolapse: Current concepts, challenges, and future perspectives. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2020, 108, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Manzano, L.A.; Vázquez-Villegas, P.; Prado-Cervantes, L.V.; Franco-Gómez, K.X.; Carbajal-Ocaña, S.; Sotelo-Cortés, D.L.; Atehortúa-Benítez, V.; Delgado-Rodríguez, M.; Membrillo-Hernández, J. Advances in Material Modification with Smart Functional Polymers for Combating Biofilms in Biomedical Applications. Polymers 2023, 15, 3021. [Google Scholar] [CrossRef] [PubMed]
- Drobota, M.; Ursache, S.; Aflori, M. Surface Functionalities of Polymers for Biomaterial Applications. Polymers 2022, 14, 2307. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.F.; Si, Z.Y.; Luo, Y.; Feng, P.P.; Wu, X.J.; Hou, W.J.; Zhu, Y.B.; Chan-Park, M.B.; Xu, L.; Huang, D.M. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Hu, Q.; Wang, X.Y.; Zhang, W. A review on recent trends of the antibacterial nonwovens air filter materials: Classification, fabrication, and application. Sep. Purif. Technol. 2024, 330, 125404. [Google Scholar] [CrossRef]
- Zhang, R.C.; Han, B.; Liu, X.M. Functional Surface Coatings on Orthodontic Appliances: Reviews of Friction Reduction, Antibacterial Properties, and Corrosion Resistance. Int. J. Mol. Sci. 2023, 24, 6919. [Google Scholar] [CrossRef]
- Wu, N.; Gao, H.Y.; Wang, X.; Pei, X.B. Surface Modification of Titanium Implants by Metal Ions and Nanoparticles for Biomedical Application. ACS Biomater. Sci. Eng. 2023, 9, 2970–2990. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, W.; Yu, M.; Wang, Z.; Yang, Z.; Xing, X.; Chen, X.; Niu, L.; Yu, F.; Xiao, Y.; et al. Mussel-inspired polymer with catechol and cationic Lys functionalities for dentin wet bonding. Mater. Today Bio 2023, 18, 100506. [Google Scholar] [CrossRef]
- Lee, S.B.; Gonzalez-Cabezas, C.; Kim, K.M.; Kim, K.N.; Kuroda, K. Catechol-Functionalized Synthetic Polymer as a Dental Adhesive to Contaminated Dentin Surface for a Composite Restoration. Biomacromolecules 2015, 16, 2265–2275. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.H.; Zhou, Y.S.; Zhou, F.; Liu, W.M.; Wang, Z.K. Mussel-inspired hydrogels: From design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef] [PubMed]
- Texido, R.; Cabanach, P.; Kaplan, R.; Garcia-Bonillo, C.; Perez, D.; Zhang, S.; Borros, S.; Pena-Francesch, A. Bacteriophobic Zwitterionic/Dopamine Coatings for Medical Elastomers. Adv. Mater. Interfaces 2022, 9, 2201152. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Della Vecchia, N.F.; Avolio, R.; Alfè, M.; Errico, M.E.; Napolitano, A.; d’Ischia, M. Building-Block Diversity in Polydopamine Underpins a Multifunctional Eumelanin-Type Platform Tunable Through a Quinone Control Point. Adv. Funct. Mater. 2013, 23, 1331–1340. [Google Scholar] [CrossRef]
- Feinberg, H.; Hanks, T.W. Polydopamine: A bioinspired adhesive and surface modification platform. Polym. Int. 2022, 71, 578–582. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine—Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Jin, X.; Yuan, J.; Shen, J. Zwitterionic polymer brushes via dopamine-initiated ATRP from PET sheets for improving hemocompatible and antifouling properties. Colloids Surf. B Biointerfaces 2016, 145, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wang, W.C.; Xu, F.J.; Zhang, L.Q.; Yang, W.T. Preparation of pH-sensitive membranes via dopamine-initiated atom transfer radical polymerization. J. Membr. Sci. 2011, 367, 7–13. [Google Scholar] [CrossRef]
- Ma, W.; Yang, P.; Li, J.; Li, S.; Li, P.; Zhao, Y.; Huang, N. Immobilization of poly(MPC) brushes onto titanium surface by combining dopamine self-polymerization and ATRP: Preparation, characterization and evaluation of hemocompatibility in vitro. Appl. Surf. Sci. 2015, 349, 445–451. [Google Scholar] [CrossRef]
- Yan, H.H.; Li, L.L.; Wang, Z.L.; Wang, Y.; Guo, M.; Shi, X.C.; Yeh, J.M.; Zhang, P.B. Mussel-Inspired Conducting Copolymer with Aniline Tetramer as Intelligent Biological Adhesive for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, C.; Zhang, Z.T.; Roland, J.D.; Lee, B.P. Antimicrobial property of halogenated catechols. Chem. Eng. J. 2021, 403, 126340. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wan, X.; Xiao, K.; Lin, W.; Li, J.; Li, Z.; Luo, F.; Tan, H.; Li, J.; Fu, Q. Anti-biofilm surfaces from mixed dopamine-modified polymer brushes: Synergistic role of cationic and zwitterionic chains to resist staphyloccocus aureus. Biomater. Sci. 2019, 7, 5369–5382. [Google Scholar] [CrossRef] [PubMed]
- Golabchi, A.; Wu, B.; Cao, B.; Bettinger, C.J.; Cui, X.T. Zwitterionic polymer/polydopamine coating reduce acute inflammatory tissue responses to neural implants. Biomaterials 2019, 225, 119519. [Google Scholar] [CrossRef]
- Deng, L.; Li, S.; Qin, Y.; Zhang, L.; Chen, H.; Chang, Z.; Hu, Y. Fabrication of antifouling thin-film composite nanofiltration membrane via surface grafting of polyethyleneimine followed by zwitterionic modification. J. Membr. Sci. 2021, 619, 118564. [Google Scholar] [CrossRef]
- Cheng, K.; Zhang, N.; Yang, N.; Hou, S.; Ma, J.; Zhang, L.; Sun, Y.; Jiang, B. Rapid and robust modification of PVDF ultrafiltration membranes with enhanced permselectivity, antifouling and antibacterial performance. Sep. Purif. Technol. 2021, 262, 118316. [Google Scholar] [CrossRef]
- Ma, M.-Q.; Zhang, C.; Chen, T.-T.; Yang, J.; Wang, J.-J.; Ji, J.; Xu, Z.-K. Bioinspired Polydopamine/Polyzwitterion Coatings for Underwater Anti-Oil and -Freezing Surfaces. Langmuir 2019, 35, 1895–1901. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.-N.; Du, Y.; Ma, M.-Q.; Xu, Z.-K. CuSO4/H2O2-Triggered Polydopamine/Poly(sulfobetaine methacrylate) Coatings for Antifouling Membrane Surfaces. Langmuir 2017, 33, 1210–1216. [Google Scholar] [CrossRef]
- He, Y.; Xu, L.; Feng, X.; Zhao, Y.; Chen, L. Dopamine-induced nonionic polymer coatings for significantly enhancing separation and antifouling properties of polymer membranes: Codeposition versus sequential deposition. J. Membr. Sci. 2017, 539, 421–431. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jiang, W.; Lei, L.L.; Wang, Y.; Xu, R.N.; Qin, L.; Wei, Q.B. Mussel-Inspired Multicomponent Codeposition Strategy toward Antibacterial and Lubricating Multifunctional Coatings on Bioimplants. Langmuir 2022, 38, 7157–7167. [Google Scholar] [CrossRef]
- Zhang, Q.; Nurumbetov, G.; Simula, A.; Zhu, C.; Li, M.; Wilson, P.; Kempe, K.; Yang, B.; Tao, L.; Haddleton, D.M. Synthesis of well-defined catechol polymers for surface functionalization of magnetic nanoparticles. Polym. Chem. 2016, 7, 7002–7010. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Q.; Wang, D.; Wang, L.; Lin, F.; Wilson, P.; Haddleton, D.M. Bioinspired coating of TiO2 nanoparticles with antimicrobial polymers by Cu(0)-LRP: Grafting to vs. grafting from. Polym. Chem. 2017, 8, 6570–6580. [Google Scholar] [CrossRef]
- Miao, Z.; Li, D.; Zheng, Z.; Zhang, Q. Synthesis of chitosan-mimicking cationic glycopolymers by Cu(0)-LRP for efficient capture and killing of bacteria. Polym. Chem. 2019, 10, 4059–4066. [Google Scholar] [CrossRef]
- Zhang, Q.; Wilson, P.; Li, Z.; McHale, R.; Godfrey, J.; Anastasaki, A.; Waldron, C.; Haddleton, D.M. Aqueous Copper-Mediated Living Polymerization: Exploiting Rapid Disproportionation of CuBr with Me6TREN. J. Am. Chem. Soc. 2013, 135, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Yang, L.; Yang, P.; Jiang, S.H.; Liu, X.H.; Li, Y.W. Polydopamine free radical scavengers. Biomater. Sci. 2020, 8, 4940–4950. [Google Scholar] [CrossRef]
- Morel, J.; Mcneilly, O.; Grundy, S.; Brown, T.; Gunawan, C.; Amal, R.; Scott, J.A. Nanoscale Titanium Surface Engineering via Low-Temperature Hydrothermal Etching for Enhanced Antimicrobial Properties. ACS Appl. Mater. Inter. 2023, 15, 46247–46260. [Google Scholar] [CrossRef]
- Methling, R.; Dückmann, O.; Simon, F.; Wolf-Brandstetter, C.; Kuckling, D. Antimicrobial Brushes on Titanium via “Grafting to” Using Phosphonic Acid/Pyridinium Containing Block Copolymers. Macromol. Mater. Eng. 2023, 308, 2200665. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Q.H.; Chen, J.H.; Mei, T.; Wang, W.W.; Li, M.F.; Wang, D. N-Halamine-Based Polypropylene Melt-Blown Nonwoven Fabric with Superhydrophilicity and Antibacterial Properties for Face Masks. Polymers 2023, 15, 4335. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.K.; Ke, Q.N.; Bai, J.C.; Luo, F.S.; Zhang, J.C.; Ding, Y.F.; Chen, J.; Liu, P.; Wang, S.; et al. Preparation of Rechargeable Antibacterial Polypropylene/N-Halamine Materials Based on Melt Blending and Surface Segregation. ACS Appl. Mater. Inter. 2023, 15, 47531–47540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ou, Y.; Lei, W.-X.; Wan, L.-S.; Ji, J.; Xu, Z.-K. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem. Int. Ed. 2016, 55, 3054–3057. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhang, Y.; Deng, Y.; Zhang, Q.; Li, J.; Wang, K.; Li, J.; Tan, H.; Fu, Q. Effects of interaction between a polycation and a nonionic polymer on their cross-assembly into mixed micelles. Soft Matter 2015, 11, 4197–4207. [Google Scholar] [CrossRef] [PubMed]
- Tsuang, Y.H.; Sun, J.S.; Huang, Y.C.; Lu, C.H.; Chang, W.H.; Wang, C.C. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif. Organs 2008, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- de Avila, E.D.; Lima, B.P.; Sekiya, T.; Torii, Y.; Ogawa, T.; Shi, W.; Lux, R. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials 2015, 67, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Marie, H.; Barrere, A.; Schoentstein, F.; Chavanne, M.-H.; Grosgogeat, B.; Mora, L. PEM Anchorage on Titanium Using Catechol Grafting. PLoS ONE 2012, 7, e50326. [Google Scholar] [CrossRef]
- Wang, X.H.; Yuan, S.S.; Shi, D.; Yang, Y.K.; Jiang, T.; Yan, S.J.; Shi, H.C.; Luan, S.F.; Yin, J.H. Integrated antifouling and bactericidal polymer membranes through bioinspired polydopamine/poly(N-vinyl pyrrolidone) coating. Appl. Surf. Sci. 2016, 375, 9–18. [Google Scholar] [CrossRef]
- Thakur, V.K.; Vennerberg, D.; Kessler, M.R. Green Aqueous Surface Modification of Polypropylene for Novel Polymer Nanocomposites. ACS Appl. Mater. Inter. 2014, 6, 9349–9356. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; Zhu, L.P.; Zhu, L.J.; Zhang, H.T.; Zhu, B.K.; Xu, Y.Y. Antifouling and Antimicrobial Polymer Membranes Based on Bioinspired Polydopamine and Strong Hydrogen-Bonded Poly(N-vinyl pyrrolidone). ACS Appl. Mater. Inter. 2013, 5, 12895–12904. [Google Scholar] [CrossRef] [PubMed]
- Shalev, T.; Gopin, A.; Bauer, M.; Stark, R.W.; Rahimipour, S. Non-leaching antimicrobial surfaces through polydopamine bio-inspired coating of quaternary ammonium salts or an ultrashort antimicrobial lipopeptide. J. Mater. Chem. 2012, 22, 2026–2032. [Google Scholar] [CrossRef]
- Yang, J.Z.; Qian, H.C.; Wang, J.P.; Ju, P.F.; Lou, Y.T.; Li, G.L.; Zhang, D.W. Mechanically durable antibacterial nanocoatings based on zwitterionic copolymers containing dopamine segments. J. Mater. Sci. Technol. 2021, 89, 233–241. [Google Scholar] [CrossRef]
- Chang, C.C.; Nogan, J.; Yang, Z.P.; Kort-Kamp, W.J.M.; Ross, W.; Luk, T.S.; Dalvit, D.A.R.; Azad, A.; Chen, H.T. Highly Plasmonic Titanium Nitride by Room-Temperature Sputtering. Sci. Rep. 2019, 9, 15287. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.S.; Tu, R.; Goto, T. High-speed deposition of titanium carbide coatings by laser-assisted metal-organic CVD. Mater. Res. Bull. 2013, 48, 2766–2770. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Sim, U.; Kim, J.K. Biopolymer-Inspired N-Doped Nanocarbon Using Carbonized Polydopamine: A High-Performance Electrocatalyst for Hydrogen-Evolution Reaction. Polymers 2020, 12, 912. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Toh, W.S.; Yap, A.; Lim, S.Y. In Vitro Biocompatibility of Contemporary Bulk-fill Composites. Oper. Dent. 2015, 40, 644–652. [Google Scholar] [CrossRef]
- Demirel, G.; Gür, G.; Demirsoy, F.F.; Altuntaş, E.G.; Yener-Ilce, B.; Kiliçarslan, M.A. Cytotoxic effects of contemporary bulk-fill dental composites: A real-time cell analysis. Dent. Mater. J. 2020, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.B.; Tian, Y.; Shi, X.L.; Liu, Y.; You, J.Q.; Yang, Z.; Wu, Y.C.; Chu, S.L. Overview of strategies to improve the antibacterial property of dental implants. Front. Bioeng. Biotechnol. 2023, 11, 1267128. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Shi, Q.; Chen, M.L.; Yu, W.Y.; Yang, T.T. Antibacterial-Based Hydrogel Coatings and Their Application in the Biomedical Field-A Review. J. Funct. Biomater. 2023, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef]
- Egghe, T.; Morent, R.; Hoogenboom, R.; De Geyter, N. Substrate-independent and widely applicable deposition of antibacterial coatings. Trends Biotechnol. 2023, 41, 63–76. [Google Scholar] [CrossRef]
- Ma, T.; Wang, C.X.; Ge, X.Y.; Zhang, Y. Applications of Polydopamine in Implant Surface Modification. Macromol. Biosci. 2023, 23, e2300067. [Google Scholar] [CrossRef]

| Sample (X = Ti, PP) | Conc. of PQA-C8 | Conc. of Dopamine | Oxidants and Coating Duration |
|---|---|---|---|
| Bare X | Cleaning only | ||
| 10:0-X | 10 mg/mL | - | Nil The coating duration was 24 h |
| 10:0.5-X | 10 mg/mL | 0.5 mg/mL | |
| 10:1-X | 10 mg/mL | 1 mg/mL | |
| 10:0-X (CuSO4/H2O2) | 10 mg/mL | - | CuSO4/H2O2 The coating duration was reduced to 2 h |
| 10:0.5-X (CuSO4/H2O2) | 10 mg/mL | 0.5 mg/mL | |
| 10:1-X (CuSO4/H2O2) | 10 mg/mL | 1 mg/mL | |
| Sample | Treatment |
|---|---|
| Bare PP | Cleaning only |
| DA-PP | Cleaning followed by immersion in 2 mg/mL of dopamine solution (10 mM tris buffer) for 4 h |
| PQA-DA-PP | The DA-PP was further immersed in 10 mg/mL of PQA-C8 solution (ethanol–tris buffer; 1:1 volume ratio, 10 mM pH = 8.5) for 24 h |
| Sample | Ti2p | C1s | N1s | O1s | Cu2p | N+ |
|---|---|---|---|---|---|---|
| Bare Ti | 18.8% | 30.7% | 2.9% | 47.6% | - | 0% |
| 10:0-Ti | 8.1% | 51.3% | 3.7% | 36.9% | - | 2.34% |
| 10:0.5-Ti | 1.6% | 69.5% | 5.8% | 23.2% | - | 2.87% |
| 10:1-Ti | 0.7% | 71.7% | 6.1% | 21.5% | - | 3.06% |
| 10:0-Ti (CuSO4/H2O2) | 11.9% | 41.3% | 2.8% | 40.4% | 3.6% | 1.77% |
| 10:0.5-Ti (CuSO4/H2O2) | 8.9% | 50.6% | 3.3% | 35.0% | 2.2% | 1.65% |
| 10:1-Ti (CuSO4/H2O2) | 5.9% | 58.0% | 4.2% | 29.1% | 2.7% | 2.81% |
| Sample | C1s | N1s | O1s | Cu2p | N+ |
|---|---|---|---|---|---|
| Bare PP | 93.7% | 0.7% | 5.6% | - | 0% |
| 10:0-PP | 82.1% | 0.8% | 17.1% | - | 0.56% |
| 10:0.5-PP | 80.0% | 3.5% | 16.6% | - | 2.47% |
| 10:1-PP | 79.7% | 4.1% | 16.2% | - | 2.24% |
| 10:0-PP (CuSO4/H2O2) | 88.6% | 1.7% | 9.3% | 0.5% | 1.31% |
| 10:0.5-PP (CuSO4/H2O2) | 76.7% | 3.9% | 19.0% | 0.4% | 2.93% |
| 10:1-PP (CuSO4/H2O2) | 79.1% | 3.3% | 17.5% | 0.2% | 2.65% |
| PQA-DA-PP | 76.2% | 4.1% | 19.6% | - | 2.67% |
| Sample | Bacterial Reduction (%) | |
|---|---|---|
| S. aureus (ATCC 21351) | E. coli (ATCC 23501) | |
| 10:0-Ti | 97.7 ± 2.4 | 72.28 ± 13.8 |
| 10:0.5-Ti | 64.8 ± 12.5 | ----- |
| 10:1-Ti | 46.0 ± 4.7 | ----- |
| 10:0-Ti (CuSO4/H2O2) | 98.1 ± 0.5 | 98.49 ± 0.6 |
| 10:0.5-Ti (CuSO4/H2O2) | 98.4 ± 0.6 | 98.23 ± 0.8 |
| 10:1-Ti (CuSO4/H2O2) | 98.0 ± 1.5 | 99.25 ± 0.8 |
| 10:0-PP | 49.8 ± 4.5 | ----- |
| 10:0.5-PP | 68.5 ± 5.8 | ----- |
| 10:1-PP | 54.1 ± 6.5 | ----- |
| 10:0-PP (CuSO4/H2O2) | 97.78 ± 0.3 | <30 |
| 10:0.5-PP (CuSO4/H2O2) | 95.05 ± 0.7 | <30 |
| 10:1-PP (CuSO4/H2O2) | 80.26 ± 12.9 | ----- |
| PQA-DA-PP | 99.9 | 96.17 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-H.; Zeng, X.-Z.; Chiu, W.-Y.; Lin, J.-C. A Facile Surface Modification Scheme for Medical-Grade Titanium and Polypropylene Using a Novel Mussel-Inspired Biomimetic Polymer with Cationic Quaternary Ammonium Functionalities for Antibacterial Application. Polymers 2024, 16, 503. https://doi.org/10.3390/polym16040503
Cheng C-H, Zeng X-Z, Chiu W-Y, Lin J-C. A Facile Surface Modification Scheme for Medical-Grade Titanium and Polypropylene Using a Novel Mussel-Inspired Biomimetic Polymer with Cationic Quaternary Ammonium Functionalities for Antibacterial Application. Polymers. 2024; 16(4):503. https://doi.org/10.3390/polym16040503
Chicago/Turabian StyleCheng, Chi-Hui, Xiang-Zhen Zeng, Wen-Yuan Chiu, and Jui-Che Lin. 2024. "A Facile Surface Modification Scheme for Medical-Grade Titanium and Polypropylene Using a Novel Mussel-Inspired Biomimetic Polymer with Cationic Quaternary Ammonium Functionalities for Antibacterial Application" Polymers 16, no. 4: 503. https://doi.org/10.3390/polym16040503





