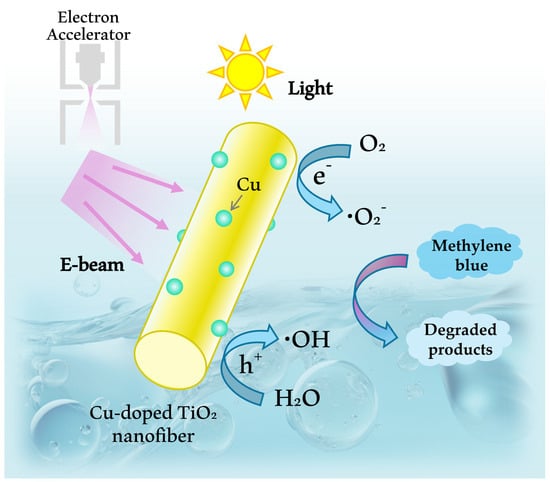
Fabrication and Characterization of Electrospun Cu-Doped TiO2 Nanofibers and Enhancement of Photocatalytic Performance Depending on Cu Content and Electron Beam Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. FE-SEM
2.2. Photocatalytic Efficiency Depending on Cu Content
2.3. XRD Analysis
2.4. Photocatalytic Efficiency According to E-Beam Irradiation
2.5. Raman Spectroscopy
2.6. XPS Analysis
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Cu-Doped and Bare TiO2 Nanofibers
3.3. Characterization
3.4. Photocatalytic MB Degradation
3.5. E-Beam Treatment Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omer, A.M. Energy, Environment and Sustainable Development. Renew. Sustain. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Appannagari, R.R. Environmental Pollution Causes and Consequences: A Study. North Asian Int. Res. J. Soc. Sci. Humanit. 2017, 3, 151–161. [Google Scholar]
- Singh, R.L.; Singh, P.K. Global Environmental Problems. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Singapore, 2017; pp. 13–41. [Google Scholar]
- Wasi, S.; Tabrez, S.; Ahmad, M. Toxicological Effects of Major Environmental Pollutants: An Overview. Environ. Monit. Assess. 2013, 185, 2585–2593. [Google Scholar] [CrossRef]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Aboulhassan, M.A.; Souabi, S.; Yaacoubi, A.; Baudu, M. Removal of Surfactant from Industrial Wastewaters by Coagulation Flocculation Process. Int. J. Environ. Sci. Technol. 2006, 3, 327–332. [Google Scholar] [CrossRef]
- Markiewics, R. Eco-Friendly Alternative for Water Treatment from Nanotechnology. Nano Sci. Technol. 2016, 4, 28–34. [Google Scholar]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Leiknes, T. The Effect of Coupling Coagulation and Flocculation with Membrane Filtration in Water Treatment: A Review. J. Environ. Sci. 2009, 21, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Park, S.-J. TiO2 Photocatalyst for Water Treatment Applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-T.; Na, K.-H.; Park, D.-C.; Yang, W.-H.; Choi, W.-Y. Photocatalytic Methylene Blue Degradation of Electrospun Ti–Zn Complex Oxide Nanofibers. Nanomaterials 2020, 10, 1311. [Google Scholar] [CrossRef]
- Shen, R.; Ren, D.; Ding, Y.; Guan, Y.; Ng, Y.H.; Zhang, P.; Li, X. Nanostructured CdS for Efficient Photocatalytic H2 Evolution: A Review. Sci. China Mater. 2020, 63, 2153–2188. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Lee, C.-G.; Na, K.-H.; Kim, W.-T.; Park, D.-C.; Yang, W.-H.; Choi, W.-Y. TiO2/ZnO Nanofibers Prepared by Electrospinning and Their Photocatalytic Degradation of Methylene Blue Compared with TiO2 Nanofibers. Appl. Sci. 2019, 9, 3404. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Hou, H.; Zeng, X.; Zhang, X. Production of Hydrogen Peroxide by Photocatalytic Processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, A. Introduction: Titanium Dioxide (TiO2) Nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent Progress in Defective TiO2 Photocatalysts for Energy and Environmental Applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Braun, J.H.; Baidins, A.; Marganski, R.E. TiO2 Pigment Technology: A Review. Prog. Org. Coat. 1992, 20, 105–138. [Google Scholar] [CrossRef]
- He, Z.; Tang, J.; Shen, J.; Chen, J.; Song, S. Enhancement of Photocatalytic Reduction of CO2 to CH4 over TiO2 Nanosheets by Modifying with Sulfuric Acid. Appl. Surf. Sci. 2016, 364, 416–427. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjines, R.; Schmid, P.E.; Levy, F. Electrical and Optical Properties of TiO2 Anatase Thin Films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Zhang, G.-W.; He, G.-H.; Xue, W.-L.; Xu, X.-F.; Liu, D.-N.; Xu, Y.-H. Enhanced Photocatalytic Performance of Titania Nanotubes Modified with Sulfuric Acid. J. Mol. Catal. A Chem. 2012, 363–364, 423–429. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Silva, C.G.; Dražić, G.; Faria, J.L. Ce-Doped TiO2 for Photocatalytic Degradation of Chlorophenol. Catal. Today 2009, 144, 13–18. [Google Scholar] [CrossRef]
- Litter, M.I. Heterogeneous Photocatalysis: Transition Metal Ions in Photocatalytic Systems. Appl. Catal. B 1999, 23, 89–114. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Heo, Y.-J.; Park, S.-J. Advanced Design and Synthesis of Composite Photocatalysts for the Remediation of Wastewater: A Review. Catalysts 2019, 9, 122. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Jiang, G.; Zhang, Y.; Huang, H.; Wu, Z.; Lee, S.C.; Dong, F. Unraveling the Mechanisms of Visible Light Photocatalytic NO Purification on Earth-Abundant Insulator-Based Core–Shell Heterojunctions. Environ. Sci. Technol. 2018, 52, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wang, H.; Kim, J.K.; Wang, J. Rational Design of Metal Oxide-based Heterostructure for Efficient Photocatalytic and Photoelectrochemical Systems. Adv. Funct. Mater. 2021, 31, 2008247. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Kim, W.-T.; Kim, I.-H.; Choi, W.-Y. Fabrication of TiO2 Nanotube Arrays and Their Application to a Gas Sensor. J. Nanosci. Nanotechnol. 2015, 15, 8161–8165. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Sigmund, W.; Yuh, J.; Park, H.; Maneeratana, V.; Pyrgiotakis, G.; Daga, A.; Taylor, J.; Nino, J.C. Processing and Structure Relationships in Electrospinning of Ceramic Fiber Systems. J. Am. Ceram. Soc. 2006, 89, 395–407. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Barakat, N.A.M.; Sheikh, F.A.; Baek, W.; Khil, M.S.; Kim, H.Y. Effects of Silver Content and Morphology on the Catalytic Activity of Silver-Grafted Titanium Oxide Nanostructure. Fibers Polym. 2010, 11, 700–709. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Kanjwal, M.A.; Chronakis, I.S.; Kim, H.Y. Influence of Temperature on the Photodegradation Process Using Ag-Doped TiO2 Nanostructures: Negative Impact with the Nanofibers. J. Mol. Catal. A Chem. 2013, 366, 333–340. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, K. A Novel Z-Scheme Visible Light Driven Cu2O/Cu/g-C3N4 Photocatalyst Using Metallic Copper as a Charge Transfer Mediator. Mol. Catal. 2017, 432, 187–195. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-Doped TiO2 Systems with Improved Photocatalytic Activity. Appl. Catal. B 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Latthe, S.S.; An, S.; Jin, S.; Yoon, S.S. High Energy Electron Beam Irradiated TiO2 Photoanodes for Improved Water Splitting. J. Mater. Chem. A Mater. 2013, 1, 13567–13575. [Google Scholar] [CrossRef]
- Gallegos, E.; Muñoz Bisesti, F.; Vaca-Escobar, K.; Santacruz, C.; Fernández, L.; Debut, A.; Espinoza-Montero, P.J. Degradation of Direct Blue 1 through Heterogeneous Photocatalysis with TiO2 Irradiated with E-Beam. Processes 2020, 8, 1181. [Google Scholar] [CrossRef]
- Ghasemi, S.; Rahimnejad, S.; Setayesh, S.R.; Rohani, S.; Gholami, M.R. Transition Metal Ions Effect on the Properties and Photocatalytic Activity of Nanocrystalline TiO2 Prepared in an Ionic Liquid. J. Hazard. Mater. 2009, 172, 1573–1578. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Abdelkareem, M.A.; El-Newehy, M.; Kim, H.Y. Influence of the Nanofibrous Morphology on the Catalytic Activity of NiO Nanostructures: An Effective Impact toward Methanol Electrooxidation. Nanoscale Res. Lett. 2013, 8, 402. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Li, B.; Pan, W. Diameter-dependent Photocatalytic Activity of Electrospun TiO2 Nanofiber. J. Am. Ceram. Soc. 2010, 93, 2503–2506. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-Demethylation of Methylene Blue in Aqueous TiO2 Dispersions under UV Irradiation. J. Photochem. Photobiol. A Chem. 2001, 140, 163–172. [Google Scholar] [CrossRef]
- Jeon, H.W.; Jeong, M.G.; An, B.Y.; Hong, M.S.; Seong, S.H.; Lee, G.D. Photocatalytic Degradation of Rhodamine B, Methyl Orange and Methylene Blue with CdS and CdZnS/ZnO Catalysts under Visible Light Irradiation. Clean Technol. 2020, 26, 311–320. [Google Scholar]
- Karakitsou, K.E.; Verykios, X.E. Effects of Altervalent Cation Doping of Titania on Its Performance as a Photocatalyst for Water Cleavage. J. Phys. Chem. 1993, 97, 1184–1189. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Aguilar, T.; Hernández, N.C.; de los Santos, D.M.; Sánchez-Márquez, J.; Zorrilla, D.; Fernández-Lorenzo, C.; Alcántara, R.; Martín-Calleja, J. Experimental and Theoretical Study of the Electronic Properties of Cu-Doped Anatase TiO2. Phys. Chem. Chem. Phys. 2014, 16, 3835–3845. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hegde, M.S.; Madras, G. Structure and Photocatalytic Activity of Ti1−xMxO2±δ (M = W, V, Ce, Zr, Fe, and Cu) Synthesized by Solution Combustion Method. J. Phys. Chem. B 2004, 108, 20204–20212. [Google Scholar] [CrossRef]
- Kumaravel, V.; Rhatigan, S.; Mathew, S.; Michel, M.C.; Bartlett, J.; Nolan, M.; Hinder, S.J.; Gascó, A.; Ruiz-Palomar, C.; Hermosilla, D. Mo Doped TiO2: Impact on Oxygen Vacancies, Anatase Phase Stability and Photocatalytic Activity. J. Phys. Mater. 2020, 3, 025008. [Google Scholar] [CrossRef]
- Xin, B.; Wang, P.; Ding, D.; Liu, J.; Ren, Z.; Fu, H. Effect of Surface Species on Cu-TiO2 Photocatalytic Activity. Appl. Surf. Sci. 2008, 254, 2569–2574. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Rybak-Wilusz, E.; Cholewa, M.; Wisz, G.; Yavorskyi, R. Review of the Development of Copper Oxides with Titanium Dioxide Thin-Film Solar Cells. AIP Adv. 2020, 10, 010701. [Google Scholar] [CrossRef]
- Raguram, T.; Rajni, K.S. Synthesis and Characterisation of Cu—Doped TiO2 Nanoparticles for DSSC and Photocatalytic Applications. Int. J. Hydrogen Energy 2022, 47, 4674–4689. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Martin, D.J.; Wang, W.; Tang, J.; Huang, W. CuOx–TiO2 Junction: What Is the Active Component for Photocatalytic H2 Production? Phys. Chem. Chem. Phys. 2013, 15, 14956–14960. [Google Scholar] [CrossRef]
- Shu-Xin, W.; Zhi, M.; Yong-Ning, Q.; Fei, H.; Li Shan, J.; Yan-Jun, Z. XPS Study of Cooper Dopping TiO2 Photocatalyst. Acta Phys. Chim. Sin. 2003, 19, 967–969. [Google Scholar] [CrossRef]
- Doong, R.; Chang, S.; Tsai, C. Enhanced Photoactivity of Cu-Deposited Titanate Nanotubes for Removal of Bisphenol A. Appl. Catal. B 2013, 129, 48–55. [Google Scholar] [CrossRef]
- Ward, M.D.; Bard, A.J. Photocurrent Enhancement via Trapping of Photogenerated Electrons of Titanium Dioxide Particles. J. Phys. Chem. 1982, 86, 3599–3605. [Google Scholar] [CrossRef]
- Brezová, V.; Blažková, A.; Borošová, E.; Čeppan, M.; Fiala, R. The Influence of Dissolved Metal Ions on the Photocatalytic Degradation of Phenol in Aqueous TiO2 Suspensions. J. Mol. Catal. A Chem. 1995, 98, 109–116. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 2002, 98, 13669–13679. [Google Scholar] [CrossRef]
- Rajamannan, B.; Mugundan, S.; Viruthagiri, G.; Praveen, P.; Shanmugam, N. Linear and Nonlinear Optical Studies of Bare and Copper Doped TiO2 Nanoparticles via Sol Gel Technique. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 651–656. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, Y.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef]
- Tuschel, D. Raman Spectroscopy and Polymorphism. Spectroscopy 2019, 34, 10–21. [Google Scholar]
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Understanding the Effect of Surface/Bulk Defects on the Photocatalytic Activity of TiO2: Anatase versus Rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978–10988. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Bhorde, A.; Bhopale, S.; Waykar, R.; Nair, S.; Borate, H.; Pandharkar, S.; Funde, A.; More, M.; Jadkar, S. Field Emission Investigations of Solvothermal Synthesized and Soaked Rutile-TiO2 Nanostructures. J. Mater. Sci. Mater. Electron. 2019, 30, 4920–4930. [Google Scholar] [CrossRef]
- Jun, J.; Dhayal, M.; Shin, J.-H.; Kim, J.-C.; Getoff, N. Surface Properties and Photoactivity of TiO2 Treated with Electron Beam. Radiat. Phys. Chem. 2006, 75, 583–589. [Google Scholar] [CrossRef]
- Kim, M.; Jo, W.; Lee, D.; Baeck, S.-H.; Shin, J.; Lee, B.-C. Enhanced Photocatalytic Activity of TiO2 Modified by E-Beam Irradiation. Bull. Korean Chem. Soc. 2013, 34, 1397–1400. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kim, C.H.; Yang, K.S.; Lee, B.C.; Woo, H.-G. Electron Beam Irradiation Effect on the Photocatalytic Activity of TiO2 on Carbon Nanofibers. J. Nanosci. Nanotechnol. 2011, 11, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Kyu Kim, J.; Kim, Y.; Seung Choi, J.; Young Jeong, K. Operation of Industrial-Scale Electron Beam Wastewater Treatment Plant. Radiat. Phys. Chem. 2012, 81, 1475–1478. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Critical Perspective on Advanced Treatment Processes for Water and Wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 2020, 10, 4549. [Google Scholar] [CrossRef]
- Xu, G.; Bu, T.; Wu, M.; Zheng, J.; Liu, N.; Wang, L. Electron Beam Induced Degradation of Clopyralid in Aqueous Solutions. J. Radioanal. Nucl. Chem. 2011, 288, 759–764. [Google Scholar] [CrossRef]













| TNF0 | TNF1 | TNF2 | TNF3 |
|---|---|---|---|
| 76.48 | 80.75 | 86.87 | 61.30 |
| Sample | Anatase (%) | Rutile (%) |
|---|---|---|
| TNF0 | 20 | 80 |
| TNF1 | 26 | 74 |
| TNF2 | 41 | 59 |
| TNF3 | 59 | 41 |
| 0 kGy | 5.3 kGy | 50.0 kGy |
|---|---|---|
| 86.87 | 90.87 | 95.80 |
| Sample | PVP | EtOH | Cu Acetate | TTIP | ACAC | Molar Ratio Cu/Ti (%) |
|---|---|---|---|---|---|---|
| TNF0 | 40 | 300 | 0 | 50 | 50 | 0 |
| TNF1 | 40 | 300 | 0.007 | 50 | 50 | 0.02 |
| TNF2 | 40 | 300 | 0.018 | 50 | 50 | 0.05 |
| TNF3 | 40 | 300 | 0.035 | 50 | 50 | 0.10 |
| Acceleration Voltage (MeV) | Beam Current (mA) | Processing Number | Radiation Exposure (kGy) |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 0.8 | 4 | 1 | 5.3 |
| 1.2 | 5 | 5 | 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Na, K.-H.; Kim, J.-Y.; Yoon, H.-S.; Han, H.; Choi, W.-Y. Fabrication and Characterization of Electrospun Cu-Doped TiO2 Nanofibers and Enhancement of Photocatalytic Performance Depending on Cu Content and Electron Beam Irradiation. Polymers 2024, 16, 694. https://doi.org/10.3390/polym16050694
Lee S-H, Na K-H, Kim J-Y, Yoon H-S, Han H, Choi W-Y. Fabrication and Characterization of Electrospun Cu-Doped TiO2 Nanofibers and Enhancement of Photocatalytic Performance Depending on Cu Content and Electron Beam Irradiation. Polymers. 2024; 16(5):694. https://doi.org/10.3390/polym16050694
Chicago/Turabian StyleLee, So-Hyeon, Kyeong-Han Na, Jae-Yoon Kim, Han-Sol Yoon, HyukSu Han, and Won-Youl Choi. 2024. "Fabrication and Characterization of Electrospun Cu-Doped TiO2 Nanofibers and Enhancement of Photocatalytic Performance Depending on Cu Content and Electron Beam Irradiation" Polymers 16, no. 5: 694. https://doi.org/10.3390/polym16050694