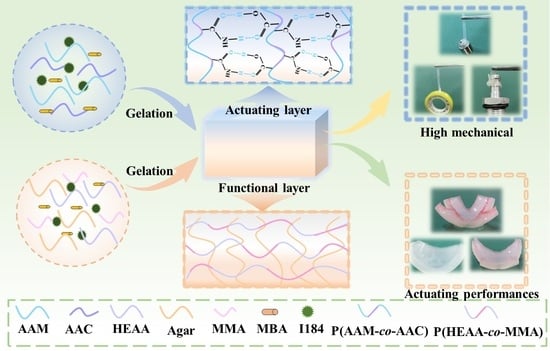
Bilayer Hydrogel Actuators with High Mechanical Properties and Programmable Actuation via the Synergy of Double-Network and Synchronized Ultraviolet Polymerization Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Hydrogel Actuator
2.2.1. Preparation of the MC Hydrogel Actuator
2.2.2. Preparation of the AHA Hydrogel Actuator
2.2.3. Preparation of the MC-AHA Bilayer Hydrogel Actuator
2.3. Scanning Electron Microscopy Characterization
2.4. Fourier Transform Infrared (FTIR) Spectroscopy Tests
2.5. Mechanical Property Tests
2.5.1. Tensile Property Tests
2.5.2. Compression Property Tests
2.5.3. Use of 90° Peeling Tests
2.5.4. Fatigue-Resistance Tests
2.6. Deswelling Tests
2.7. Actuating Performance Tests
3. Results and Discussion
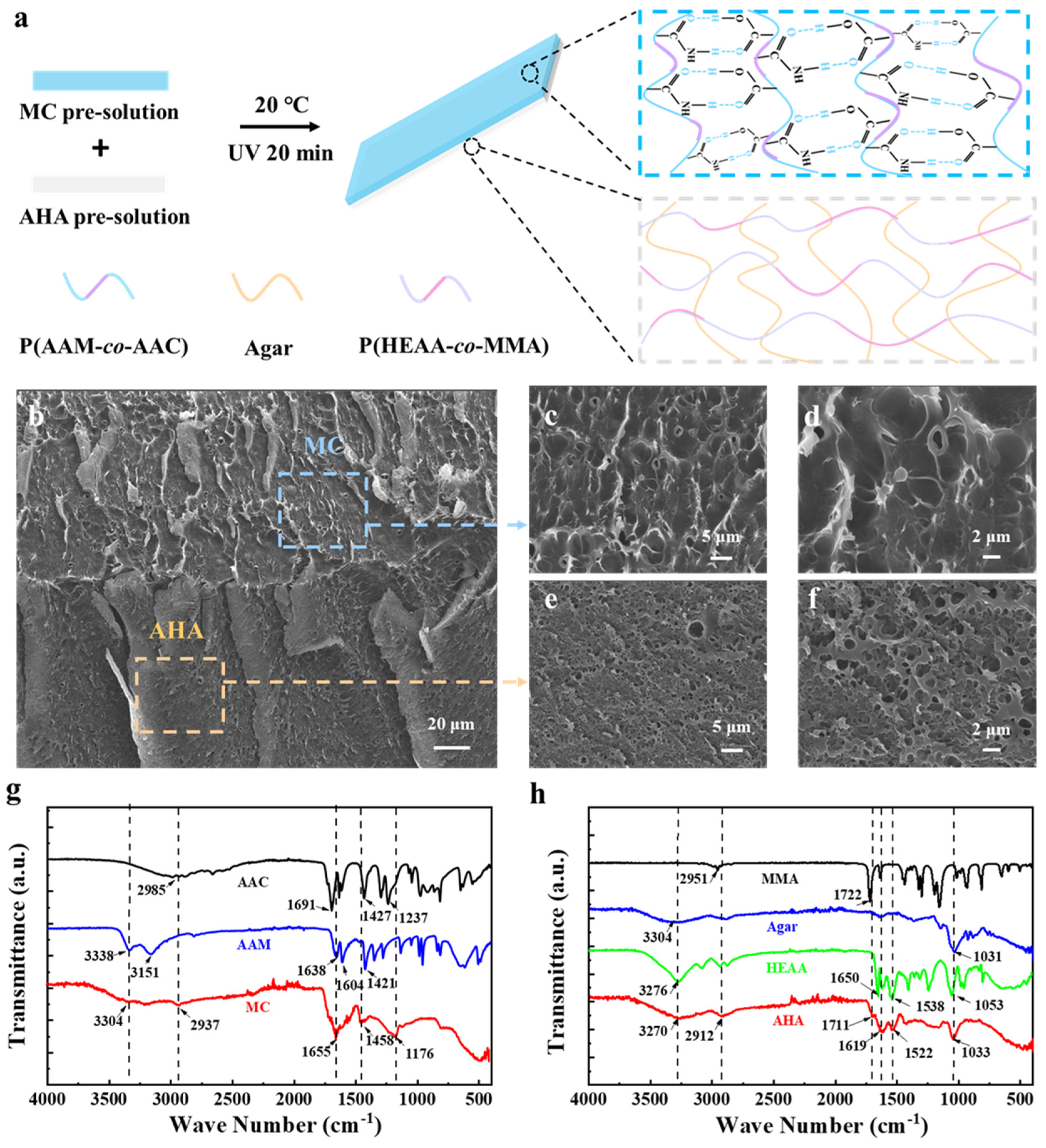
3.1. Fabrication of the MC-AHA Bilayer Hydrogel Actuator
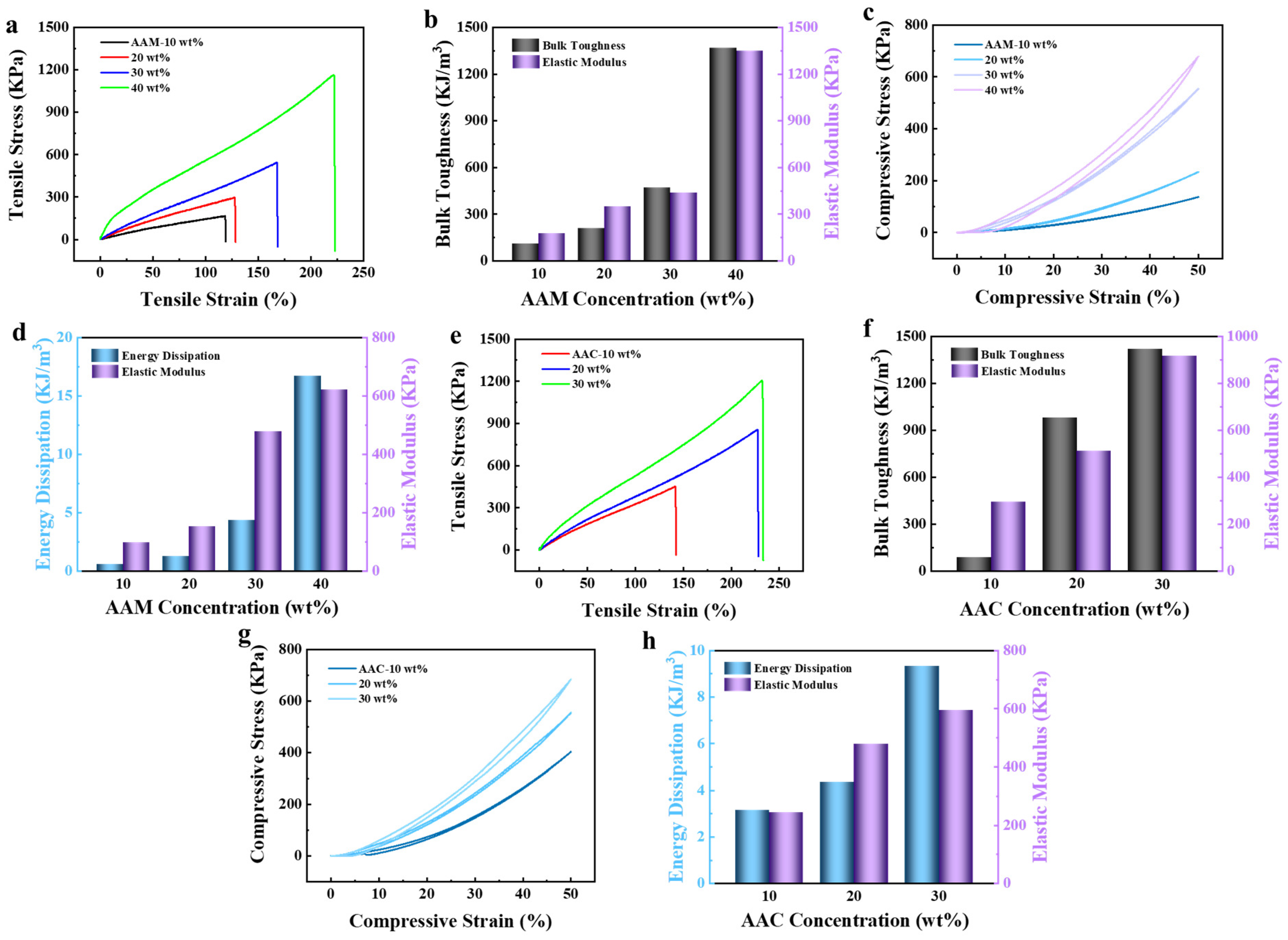
3.2. Mechanical Properties of the MC-AHA Bilayer Hydrogel Actuator
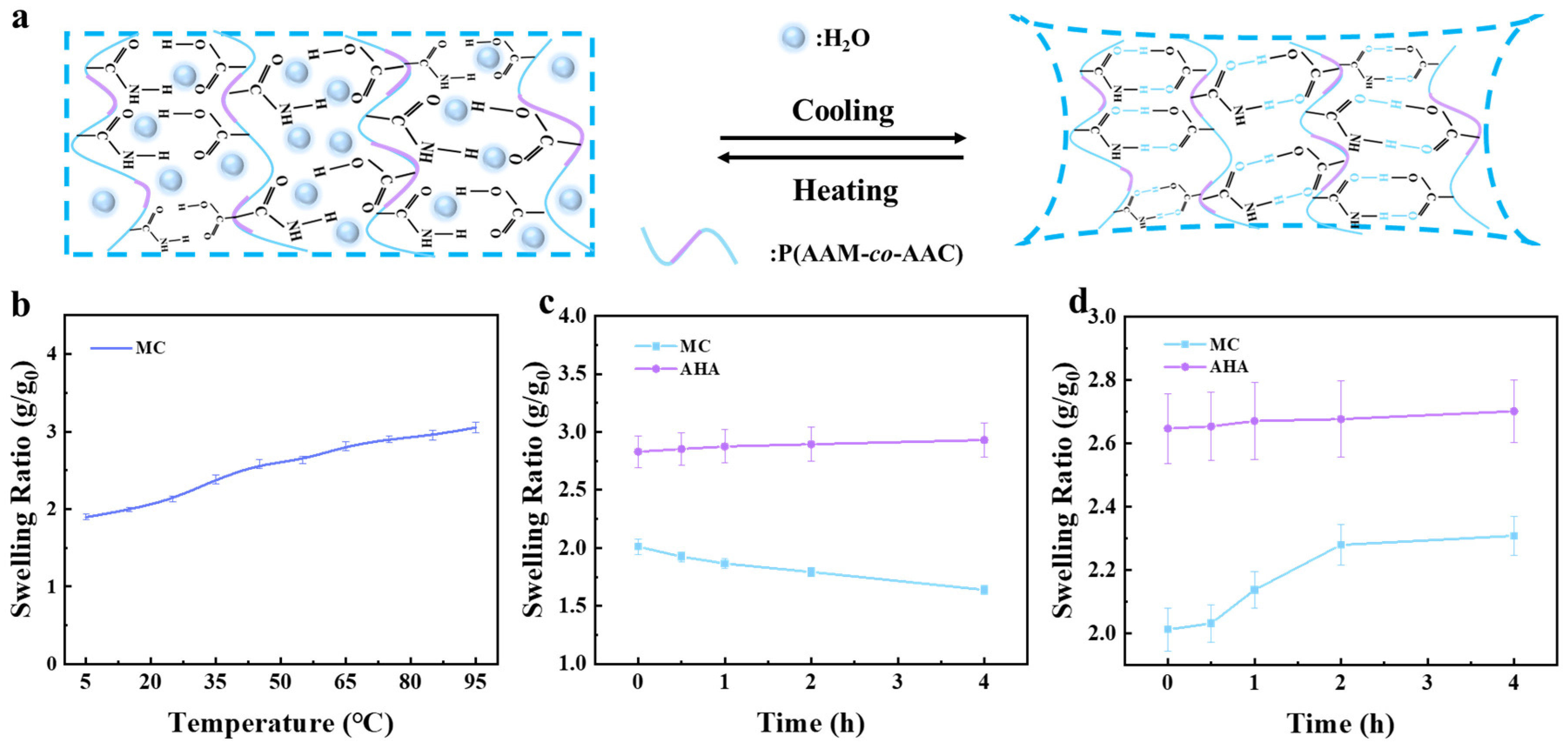
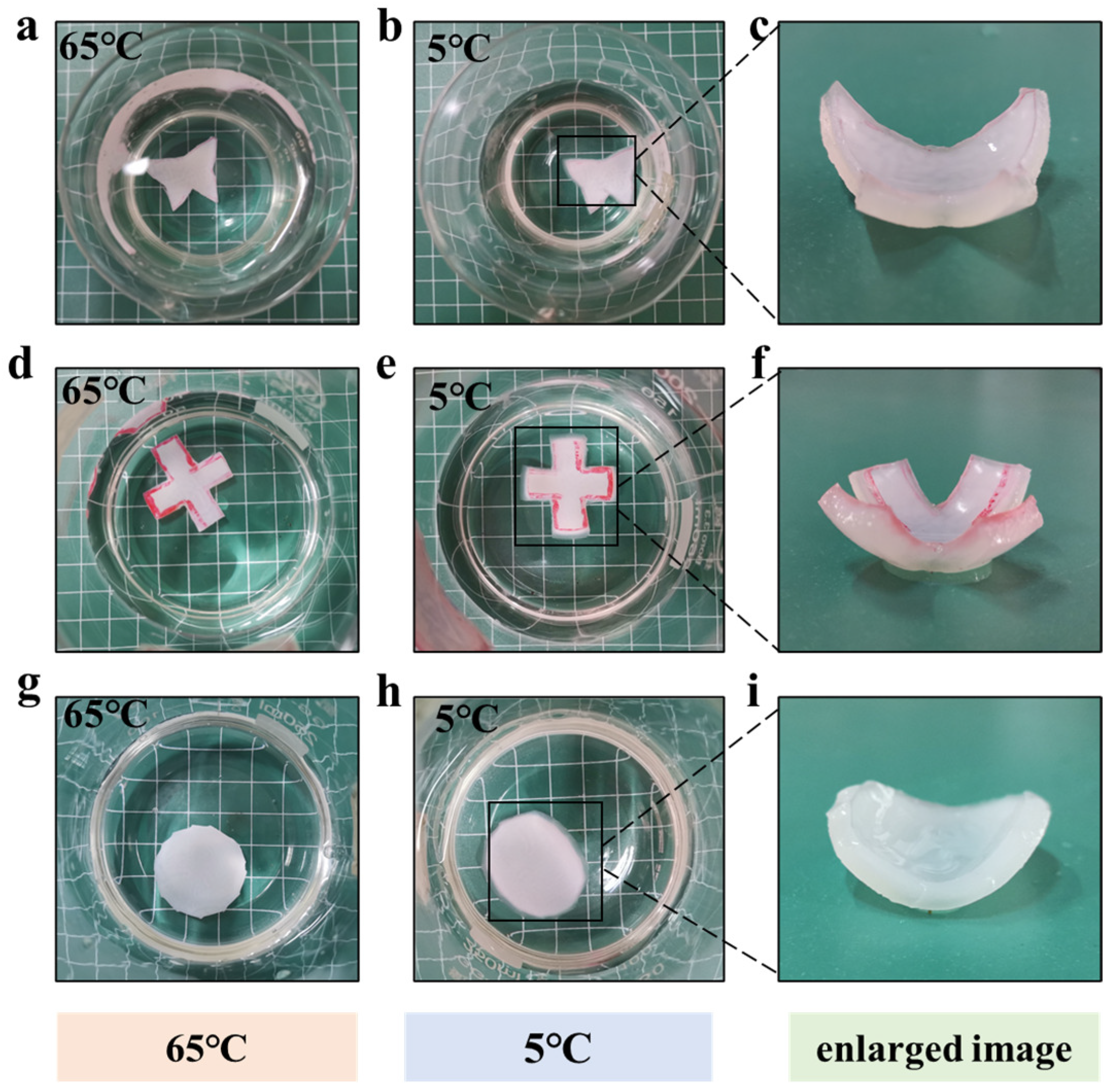
3.3. Temperature-Sensitive Behavior and Actuating Mechanism of the MC-AHA Bilayer Hydrogel Actuator
3.4. Actuating Performances of the MC-AHA Bilayer Hydrogel Actuator
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Parashar, A. Atomistic Simulations of Pristine and Nanoparticle Reinforced Hydrogels: A Review. WIREs Comput. Mol. Sci. 2023, 13, e1655. [Google Scholar] [CrossRef]
- Yang, D. Recent Advances in Hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Wu, S.; Gong, C.; Wang, Z.; Xu, S.; Feng, W.; Qiu, Z.; Yan, Y. Continuous Spinning of High-Tough Hydrogel Fibers for Flexible Electronics by Using Regional Heterogeneous Polymerization. Adv. Sci. 2023, 10, e2305226. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tang, L.; Xu, Y.; Yao, J.; Tang, G.; Dai, B.; Wang, W.; Tang, J.; Gong, L. A Self-Powered Flexible Sensing System Based on A Super-Tough, High Ionic Conductivity Supercapacitor and A Rapid Self-recovering Fully Physically Crosslinked Double Network Hydrogel. J. Mater. Chem. C 2022, 10, 3027–3035. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent Progress of Collagen, Chitosan, Alginate and Other Hydrogels in Skin Repair and Wound Dressing Applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xue, Y.; Sun, Y.; Chen, L.; Zhang, C.; Wu, Q.; Peng, S.; Ma, C.; Liu, Z.; Jiang, S.; et al. A Robust Anisotropic Light-Responsive Hydrogel for Ultrafast and Complex Biomimetic Actuation Via Poly(pyrrole)-Coated Electrospun Nanofiber. Chem. Eng. J. 2023, 452, 139373–139386. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Z.; Zhang, B.; Tang, X.; Fan, F.; Fu, Y.; Zhang, J.; Wang, T.; Meng, F. Sponge-Like, Semi-Interpenetrating Self-sensory Hydrogel for Smart Photothermal-Responsive Soft Actuator with Biomimetic Self-Diagnostic Intelligence. Chem. Eng. J. 2023, 467, 143515. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, L.; Ma, H.; Yang, T.; Qian, L. pH and Temperature Dual-Responsive Hydrogel Actuator with Bidirectional Bending Behavior and Ultra Large Bending Angle. Eur. Polym. J. 2023, 197, 112296. [Google Scholar] [CrossRef]
- Liu, H.; Liu, R.; Chen, K.; Liu, Y.; Zhao, Y.; Cui, X.; Tian, Y. Bioinspired Gradient Structured Soft Actuators: From Fabrication to Application. Chem. Eng. J. 2023, 461, 141966. [Google Scholar] [CrossRef]
- Xiang, S.-L.; Su, Y.-X.; Yin, H.; Li, C.; Zhu, M.-Q. Visible-Light-Driven Isotropic Hydrogels as Anisotropic Underwater Actuators. Nano Energy 2021, 85, 105965. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar, S.; Safi, S.Z.; Singh, I.; Emran, T.B. Wound Dressings: Recent updates. Int. J. Surg. 2022, 104, 106793. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Zhao, X.Y.; Jiang, J.Q.; Liu, Z.T.; Liu, Z.W.; Li, G. Thermal-Responsive Hydrogel Actuators with Photo-Programmable Shapes and Actuating Trajectories. ACS Appl. Mater. Interfaces 2022, 14, 51244–51252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; Cao, Y.; Chen, Y.; Akkus, O.; Liu, H.; Cao, C. Bio-Inspired Anisotropic Hydrogels and Their Applications in Soft Actuators and Robots. Matter 2023, 6, 1–35. [Google Scholar] [CrossRef]
- Ling, Y.; Fan, H.; Wang, K.; Lu, Z.; Wang, L.; Hou, C.; Zhang, Q.; Li, Y.; Li, K.; Wang, H. Electrochemical Actuators with Multicolor Changes and Multidirectional Actuation. Small 2022, 18, e2107778. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, L.; Liu, A.; He, S.; Shao, W. Ultrafast Thermo-Responsive Bilayer Hydrogel Actuator Assisted by Hydrogel Microspheres. Sens. Actuators B Chem. 2022, 357, 131434–131443. [Google Scholar] [CrossRef]
- Qian, C.; Li, Y.; Chen, C.; Han, L.; Han, Q.; Liu, L.; Lu, Z. A Stretchable and Conductive Design Based on Multi-Responsive Hydrogel for Self-Sensing Actuators. Chem. Eng. J. 2023, 454, 140263–140274. [Google Scholar] [CrossRef]
- Sun, W.-J.; Guan, Y.; Jia, L.-C.; Li, Y.; Huang, H.-D.; Wang, Y.-Y.; Tang, J.-H.; Yan, D.-X.; Li, Z.-M. Low-Voltage and Controllable-Developed Actuator with Bilayer Structure Based on Triple-Shape Actuation. Compos. Sci. Technol. 2022, 222, 109399–109407. [Google Scholar] [CrossRef]
- Grosjean, M.; Ouedraogo, S.; Dejean, S.; Garric, X.; Luchnikov, V.; Ponche, A.; Mathieu, N.; Anselme, K.; Nottelet, B. Bioresorbable Bilayered Elastomer/Hydrogel Constructs with Gradual Interfaces for the Fast Actuation of Self-Rolling Tubes. ACS Appl. Mater. Interfaces 2022, 14, 43719–43731. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, Q.; Chen, L.; Sun, Y.; Chen, L.; Zhang, C.; Li, S.; Ma, C.; Jiang, S. Remotely Controlled Light/Electric/Magnetic Multiresponsive Hydrogel for Fast Actuations. ACS Appl. Mater. Interfaces 2023, 15, 10030–10043. [Google Scholar] [CrossRef]
- Liu, W.; Geng, L.; Wu, J.; Huang, A.; Peng, X. Highly Strong and Sensitive Bilayer Hydrogel Actuators Enhanced by Cross-Oriented Nanocellulose Networks. Compos. Sci. Technol. 2022, 225, 109494. [Google Scholar] [CrossRef]
- Gregg, A.; De Volder, M.F.L.; Baumberg, J.J. Light-Actuated Anisotropic Microactuators from CNT/Hydrogel Nanocomposites. Adv. Opt. Mater. 2022, 10, 2200180. [Google Scholar] [CrossRef]
- Han, W.J.; Lee, J.H.; Lee, J.-K.; Choi, H.J. Remote-Controllable, Tough, Ultrastretchable, and Magneto-Sensitive Nanocomposite Hydrogels with Homogeneous Nanoparticle Dispersion as Biomedical Actuators, and Their Tuned Structure, Properties, and Performances. Compos. Part B Eng. 2022, 236, 109802–109817. [Google Scholar] [CrossRef]
- Na, H.; Kang, Y.-W.; Park, C.S.; Jung, S.; Kim, H.-Y.; Sun, J.-Y. Hydrogel-Based Strong and Fast Actuators by Electroosmotic Turgor Pressure. Science 2022, 376, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Kadla, J.F. Effect of Nanofillers on Carboxymethyl Cellulose/Hydroxyethyl Cellulose Hydrogels. Appl. Polym. 2009, 114, 1664–1669. [Google Scholar] [CrossRef]
- Zeng, W.; Jiang, C.; Wu, D. Heterogeneity Regulation of Bilayer Polysaccharide Hydrogels for Integrating pH- and Humidity-Responsive Actuators and Sensors. ACS Appl. Mater. Interfaces 2023, 15, 16097–16108. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly(vinyl alcohol)/cellulose nanowhiskers nanocomposite hydrogels for potential wound dressings. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 54–61. [Google Scholar] [CrossRef]
- Zhao, K.; Lee, J.W.; Yu, Z.G.; Jiang, W.; Oh, J.W.; Kim, G.; Han, H.; Kim, Y.; Lee, K.; Lee, S.; et al. Humidity-Tolerant Moisture-Driven Energy Generator with MXene Aerogel-Organohydrogel Bilayer. ACS Nano 2023, 17, 5472–5485. [Google Scholar] [CrossRef]
- Xu, W.; Dong, P.; Lin, S.; Kuang, Z.; Zhang, Z.; Wang, S.; Ye, F.; Cheng, L.; Wu, H.; Liu, A. Bioinspired Bilayer Hydrogel-Based Actuator with Rapidly Bidirectional Actuation, Programmable Deformation and Devisable Functionality. Sens. Actuators B Chem. 2022, 359, 131547–131557. [Google Scholar] [CrossRef]
- Tang, L.; Xu, Y.; Liu, F.; Liu, S.; Chen, Z.; Tang, J.; Wu, S. Synchronous Ultraviolet Polymerization Strategy to Improve the Interfacial Toughness of Bilayer Hydrogel Actuators. Macromolecules 2023, 56, 6199–6207. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Wu, X.; Xu, Y.; Li, Y.; Wu, S.; Gong, L.; Tang, J. Bilayer Hydrogel Actuators with High Mechanical Properties and Programmable Actuation via the Synergy of Double-Network and Synchronized Ultraviolet Polymerization Strategies. Polymers 2024, 16, 840. https://doi.org/10.3390/polym16060840
Tang L, Wu X, Xu Y, Li Y, Wu S, Gong L, Tang J. Bilayer Hydrogel Actuators with High Mechanical Properties and Programmable Actuation via the Synergy of Double-Network and Synchronized Ultraviolet Polymerization Strategies. Polymers. 2024; 16(6):840. https://doi.org/10.3390/polym16060840
Chicago/Turabian StyleTang, Li, Xuemei Wu, Yue Xu, Youwei Li, Shaoji Wu, Liang Gong, and Jianxin Tang. 2024. "Bilayer Hydrogel Actuators with High Mechanical Properties and Programmable Actuation via the Synergy of Double-Network and Synchronized Ultraviolet Polymerization Strategies" Polymers 16, no. 6: 840. https://doi.org/10.3390/polym16060840