Polymer-Based Drug Delivery Systems for Cancer Therapeutics
Abstract
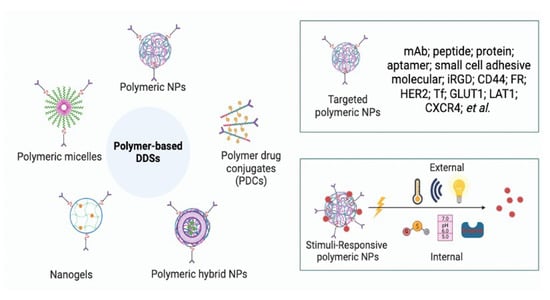
:1. Introduction
2. Barriers That Impact the Therapeutics Reaching the Tumor Sites
2.1. TME Barriers
2.2. Other Biological Barriers
3. Polymer-Based DDSs for Cancer Therapeutics
3.1. Natural Polymer-Based DDSs
3.1.1. Chitosan
3.1.2. Hyaluronic Acid
3.1.3. Alginate
3.1.4. Cellulose
3.1.5. Gelatin
3.1.6. Dextran
3.2. Synthetic Polymer-Based DDSs
3.2.1. Hydrophobic/Hydrophilic and Block Copolymers
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| HA-PLGA/PF68/PF127-NPs | Irinotecan (IRT) | Microfluidic-assisted nanoprecipitation process | Improved physicochemical behavior drug with high drug loading. | [141] |
| Dual receptor-targeted DTX-PLGA-NPs | DTX | Single emulsion solvent evaporation technique and further covalent conjugation | Micro- and nanosized carriers for imaging, chemotherapy, hyperthermia, and glioma. | [142] |
| PEG-coated PLGA-NPs | Curcumin | Solvent displacement method, PEGylation, and ligand conjugation | The ligands HA or FA conjugated with PLGA-PEG showed better in vitro efficacy and target ability. | [143] |
| CD56 antibody-conjugated PLGA-NPs | IRT and Stattic | Double emulsion solvent evaporation | Improved cellular uptake and in vitro efficacy and efficient active targeting of lung cancer cells. | [144] |
| CS-FA-PLGA-DTX | DTX | Nanoprecipitation approach | High drug-loading efficiency and controlled drug release, and high level of receptor-mediated internalization. | [145] |
| PLGA-NPs | Raloxifene hydrochloride (RAL) | Emulsion solvent diffusion evaporation | Improvement in stability at different temperatures and increase the in vitro efficacy at a lower concentration. | [146] |
| PLGA-NPs | Afatinib | Emulsification followed by solvent evaporation | Localized inhalational drug delivery for small lung cancer significantly improved cytotoxicity and cellular uptake. | [147] |
3.2.2. Dendrimers and Hyperbranched Polymers (HBPs)
3.2.3. pH-Responsive Polymers
3.2.4. Redox-Responsive Polymers
3.2.5. Other Stimuli-Responsive (Thermo-, Hypoxia-, and Enzyme-Responsive) Polymers
3.2.6. Targeting Ligands of Polymers
3.2.7. Fluorinated Polymers
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Common Cancer Sites. 2023. Available online: https://seer.cancer.gov/statfacts/html/common.html (accessed on 12 January 2023).
- Lin, A.; Giuliano, C.J.; Palladino, A.; John, K.M.; Abramowicz, C.; Yuan, M.L.; Sausville, E.L.; Lukow, D.A.; Liu, L.; Chait, A.R.; et al. Off-target Toxicity is A Common Mechanism of Action of Cancer Drugs Undergoing Clinical Trials. Sci. Transl. Med. 2019, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal antibodies in cancer therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Shen, Y.; Qian, C.; Oupicky, D.; Sun, M. Targeting pulmonary tumor microenvironment with CXCR4-inhibiting nanocomplex to enhance anti–PD-L1 immunotherapy. Sci. Adv. 2020, 6, eaaz9240. [Google Scholar] [CrossRef] [PubMed]
- Haag, R.; Kratz, F. Polymer therapeutics: Concepts and applications. Angew. Chem. Int. Ed. 2006, 45, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Le, T.M.D.; Yoon, A.-R.; Thambi, T.; Yun, C.-O. Polymeric systems for cancer immunotherapy: A review. Front. Immunol. 2022, 13, 826876. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.-u.; Jaber, F.; Dera, A.A.; Awwad, N.S.; Ibrahium, H.A. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 2023, 13, 23087–23121. [Google Scholar] [CrossRef]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-based nanoparticles for the treatment of cancer: Current strategies and perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Braatz, D.; Cherri, M.; Tully, M.; Dimde, M.; Ma, G.; Mohammadifar, E.; Reisbeck, F.; Ahmadi, V.; Schirner, M.; Haag, R. Chemical approaches to synthetic drug delivery systems for systemic applications. Angew. Chem. Int. Ed. 2022, 61, e202203942. [Google Scholar] [CrossRef]
- Molineux, G. The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta®). Curr. Pharm. Des. 2004, 10, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Aloss, K.; Hamar, P. Recent preclinical and clinical progress in liposomal doxorubicin. Pharmaceutics 2023, 15, 893. [Google Scholar] [CrossRef] [PubMed]
- Dirauf, M.; Muljajew, I.; Weber, C.; Schubert, U.S. Recent advances in degradable synthetic polymers for biomedical applications-Beyond polyesters. Prog. Polym. Sci. 2022, 129, 101547. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 2020, 6283796. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Louault, K.; Li, R.R.; DeClerck, Y.A. Cancer-Associated Fibroblasts: Understanding Their Heterogeneity. Cancers 2020, 12, 3108. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.J.; Kalliomaki, T.M.K.; Brown, A.; Yang, V.X.; Milosevic, M.; Hill, R.P. Interstitial fluid pressure, vascularity and metastasis in ectopic, orthotopic and spontaneous tumours. BMC Cancer 2008, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef]
- Kim, S.M.; Faix, P.H.; Schnitzer, J.E. Overcoming Key Biological Barriers to Cancer Drug Delivery and Efficacy. J. Control Release 2017, 267, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.u.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Mathaba, M.; Daramola, M.O. Effect of chitosan’s degree of deacetylation on the performance of pes membrane infused with chitosan during amd treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Multi-functional chitosan-based nanoparticles for drug delivery: Recent advanced insight into cancer therapy. Carbohydr. Polym. 2023, 315, 120972. [Google Scholar] [CrossRef] [PubMed]
- Rostaminejad, B.; Dinari, M.; Karimi, A.R.; Hadizadeh, M. Oxidative cross-linking of biocompatible chitosan injectable hydrogel by perylene-dopamine to boost phototoxicity of perylene on in vitro melanoma and breast cancer therapy. J. Mol. Liq. 2023, 386, 122553. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, M.; Rajeev, M.R. Modified chitosan-hyaluronic acid based hydrogel for the pH-responsive Co-delivery of cisplatin and doxorubicin. Int. J. Biol. Macromol. 2022, 201, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Rawat, S.G.; Manjit; Mishra, M.; Priya; Kumar, A.; Chawla, R. Dual targeting pH responsive chitosan nanoparticles for enhanced active cellular internalization of gemcitabine in non-small cell lung cancer. Int. J. Biol. Macromol. 2023, 249, 126057. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Z.; Feng, L.; Deng, L.; Fang, Z.; Liu, Z.; Li, Y.; Wu, X.; Qin, L.; Guo, R.; et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Carbohydr. Polym. 2021, 268, 118237. [Google Scholar] [CrossRef]
- Ghobadi-Oghaz, N.; Asoodeh, A.; Mohammadi, M. Fabrication, characterization and in vitro cell exposure study of zein-chitosan nanoparticles for co-delivery of curcumin and berberine. Int. J. Biol. Macromol. 2022, 204, 576–586. [Google Scholar] [CrossRef]
- Escareño, N.; Hassan, N.; Kogan, M.J.; Juárez, J.; Topete, A.; Daneri-Navarro, A. Microfluidics-assisted conjugation of chitosan-coated polymeric nanoparticles with antibodies: Significance in drug release, uptake, and cytotoxicity in breast cancer cells. J. Colloid Interface Sci. 2021, 591, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Jariyal, H.; Srivastava, A. Hyaluronic acid: More than a carrier, having an overpowering extracellular and intracellular impact on cancer. Carbohydr. Polym. 2023, 317, 121081. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- de Paula, M.C.; Carvalho, S.G.; Silvestre, A.L.P.; dos Santos, A.M.; Meneguin, A.B.; Chorilli, M. The role of hyaluronic acid in the design and functionalization of nanoparticles for the treatment of colorectal cancer. Carbohydr. Polym. 2023, 320, 121257. [Google Scholar] [CrossRef]
- Zhao, Y.-f.; Qiao, S.-p.; Shi, S.-l.; Yao, L.-f.; Hou, X.-l.; Li, C.-f.; Lin, F.-H.; Guo, K.; Acharya, A.; Chen, X.-b.; et al. Modulating Three-Dimensional Microenvironment with Hyaluronan of Different Molecular Weights Alters Breast Cancer Cell Invasion Behavior. ACS Appl. Mater. Interfaces 2017, 9, 9327–9338. [Google Scholar] [CrossRef]
- Chandra, J.; Molugulu, N.; Annadurai, S.; Wahab, S.; Karwasra, R.; Singh, S.; Shukla, R.; Kesharwani, P. Hyaluronic acid-functionalized lipoplexes and polyplexes as emerging nanocarriers for receptor-targeted cancer therapy. Environ. Res. 2023, 233, 116506. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Nishida, Y.; Knudson, W.; Knudson, C.B.; Arai, E.; Kozawa, E.; Futamura, N.; Wasa, J.; Ishiguro, N. Therapeutic potential of hyaluronan oligosaccharides for bone metastasis of breast cancer. J. Orthop. Res. 2012, 30, 662–672. [Google Scholar] [CrossRef]
- Wickens, J.M.; Alsaab, H.O.; Kesharwani, P.; Bhise, K.; Amin, M.C.I.M.; Tekade, R.K.; Gupta, U.; Iyer, A.K. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today 2017, 22, 665–680. [Google Scholar] [CrossRef]
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in hyaluronic acid for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef]
- Hou, X.; Zhong, D.; Chen, H.; Gu, Z.; Gong, Q.; Ma, X.; Zhang, H.; Zhu, H.; Luo, K. Recent advances in hyaluronic acid-based nanomedicines: Preparation and application in cancer therapy. Carbohydr. Polym. 2022, 292, 119662. [Google Scholar] [CrossRef]
- Cho, H.-J. Recent progresses in the development of hyaluronic acid-based nanosystems for tumor-targeted drug delivery and cancer imaging. J. Pharm. Investig. 2020, 50, 115–129. [Google Scholar] [CrossRef]
- Hejazi, M.; Arshadi, S.; Amini, M.; Baradaran, B.; Shahbazi-Derakhshi, P.; Sameti, P.; Soleymani, J.; Mokhtarzadeh, A.; Tavangar, S.M. Hyaluronic acid-functionalized gold nanoparticles as a cancer diagnostic probe for targeted bioimaging applications. Microchem. J. 2023, 193, 108953. [Google Scholar] [CrossRef]
- Maki, M.A.A.; Teng, M.S.; Tan, K.F.; Kumar, P.V. Polyamidoamine-stabilized and hyaluronic acid-functionalized gold nanoparticles for cancer therapy. OpenNano 2023, 13, 100182. [Google Scholar] [CrossRef]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent advantage of hyaluronic acid for anti-cancer application: A review of “3S” transition approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, M.; Velashjerdi, M.; Shaterabadi, Z.; Barati, A. One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 2020, 237, 116130. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Raei, M.; Hushmandi, K.; Zarrabi, A.; Voelcker, N.H.; Aref, A.R.; et al. Hyaluronic acid-based nanoplatforms for Doxorubicin: A review of stimuli-responsive carriers, co-delivery and resistance suppression. Carbohydr. Polym. 2021, 272, 118491. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Humeniuk, E.; Adamczuk, G.; Korga-Plewko, A. Hyaluronic Acid as a Modern Approach in Anticancer Therapy-Review. Int. J. Mol. Sci. 2022, 24, 103. [Google Scholar] [CrossRef]
- Lu, B.; Xiao, F.; Wang, Z.; Wang, B.; Pan, Z.; Zhao, W.; Zhu, Z.; Zhang, J. Redox-sensitive hyaluronic acid polymer prodrug nanoparticles for enhancing intracellular drug self-delivery and targeted cancer therapy. ACS Biomater. Sci. Eng. 2020, 6, 4106–4115. [Google Scholar] [CrossRef]
- Xiong, Q.; Cui, M.; Bai, Y.; Liu, Y.; Liu, D.; Song, T. A supramolecular nanoparticle system based on β-cyclodextrin-conjugated poly-l-lysine and hyaluronic acid for co-delivery of gene and chemotherapy agent targeting hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 155, 93–103. [Google Scholar] [CrossRef]
- Moustafa, M.A.; El-Refaie, W.M.; Elnaggar, Y.S.R.; El-Mezayen, N.S.; Awaad, A.K.; Abdallah, O.Y. Fucoidan/hyaluronic acid cross-linked zein nanoparticles loaded with fisetin as a novel targeted nanotherapy for oral cancer. Int. J. Biol. Macromol. 2023, 241, 124528. [Google Scholar] [CrossRef]
- Jeannot, V.; Gauche, C.; Mazzaferro, S.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Didier, C.; Vollaire, J.; Josserand, V.; Coll, J.-L.; et al. Anti-tumor efficacy of hyaluronan-based nanoparticles for the co-delivery of drugs in lung cancer. J. Control. Release 2018, 275, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Crawford, A.J.; Wojtynek, N.E.; Holmes, M.B.; Souchek, J.J.; Almeida-Porada, G.; Ly, Q.P.; Cohen, S.M.; Hollingsworth, M.A.; Mohs, A.M. Indocyanine green loaded hyaluronan-derived nanoparticles for fluorescence-enhanced surgical imaging of pancreatic cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, A.; Shiri, F.; Keikha, S.; Majd, M.H. Hyaluronan magnetic nanoparticle for mitoxantrone delivery toward CD44-positive cancer cells. Colloids Surf. B Biointerfaces 2018, 171, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, M.; Zhu, P.; Yan, C. Preparation, characterization and in vitro antitumor activity evaluation of hyaluronic acid-alendronate-methotrexate nanoparticles. Int. J. Biol. Macromol. 2021, 166, 71–79. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Shaikh, M.A.J.; Alharbi, K.S.; Almalki, W.H.; Imam, S.S.; Albratty, M.; Meraya, A.M.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Afzal, O.; et al. Sodium alginate based drug delivery in management of breast cancer. Carbohydr. Polym. 2022, 292, 119689. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2021, 170, 424–436. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.; Jiang, Z. Alginate oligosaccharides: Production, biological activities, and potential applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef]
- Lopes, M.; Abrahim, B.; Veiga, F.; Seiça, R.; Cabral, L.M.; Arnaud, P.; Andrade, J.C.; Ribeiro, A.J. Preparation methods and applications behind alginate-based particles. Expert. Opin. Drug Deliv. 2017, 14, 769–782. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Gujarathi, P.; Pansare, P.; Tripathi, S. A comprehensive review on alginate-based delivery systems for the delivery of chemotherapeutic agent: Doxorubicin. Carbohydr. Polym. 2021, 259, 117696. [Google Scholar] [CrossRef]
- Boi, S.; Rouatbi, N.; Dellacasa, E.; Di Lisa, D.; Bianchini, P.; Monticelli, O.; Pastorino, L. Alginate microbeads with internal microvoids for the sustained release of drugs. Int. J. Biol. Macromol. 2020, 156, 454–461. [Google Scholar] [CrossRef]
- Selvaraj, S.; Shanmugasundaram, S.; Maruthamuthu, M.; Venkidasamy, B.; Shanmugasundaram, S. Facile Synthesis and Characterization of Quercetin-Loaded Alginate Nanoparticles for Enhanced In Vitro Anticancer Effect Against Human Leukemic Cancer U937 Cells. J. Clust. Sci. 2021, 32, 1507–1518. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Samarakoon, S.R.; Soysa, P.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Chitosan-Alginate Nanoparticle System Efficiently Delivers Doxorubicin to MCF-7 Cells. J. Nanomater. 2016, 2016, 3178904. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438–445. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Ifeanafor, A.R.; Ifade, W.A.; Akinleye, M.O.; Patrojanasophon, P.; Silva, B.O.; Osuntoki, A.A. Formulation and evaluation of paclitaxel-loaded boronated chitosan/alginate nanoparticles as a mucoadhesive system for localized cervical cancer drug delivery. J. Drug Deliv. Sci. Technol. 2023, 87, 104810. [Google Scholar] [CrossRef]
- Abbasi, M.; Sohail, M.; Minhas, M.U.; Mahmood, A.; Shah, S.A.; Munir, A.; Kashif, M.-U.-R. Folic acid-decorated alginate nanoparticles loaded hydrogel for the oral delivery of diferourylmethane in colorectal cancer. Int. J. Biol. Macromol. 2023, 233, 123585. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef] [PubMed]
- Raghav, N.; Vashisth, C.; Mor, N.; Arya, P.; Sharma, M.R.; Kaur, R.; Bhatti, S.P.; Kennedy, J.F. Recent advances in cellulose, pectin, carrageenan and alginate-based oral drug delivery systems. Int. J. Biol. Macromol. 2023, 244, 125357. [Google Scholar] [CrossRef]
- Lugoloobi, I.; Maniriho, H.; Jia, L.; Namulinda, T.; Shi, X.; Zhao, Y. Cellulose nanocrystals in cancer diagnostics and treatment. J. Control. Release 2021, 336, 207–232. [Google Scholar] [CrossRef] [PubMed]
- Hosseinidoust, Z.; Alam, M.N.; Sim, G.; Tufenkji, N.; van de Ven, T.G. Cellulose nanocrystals with tunable surface charge for nanomedicine. Nanoscale 2015, 7, 16647–16657. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.P.F.; Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, J.; Kim, T.-i. Cholic Acid-Conjugated Methylcellulose-Polyethylenimine Nano-Aggregates for Drug Delivery Systems. Nanomaterials 2019, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, Y.; Su, H.; Luo, Y.; Wang, M.; Li, T.; Fu, Y. Delivery of curcumin in a carboxymethyl cellulose and hydroxypropyl methyl cellulose carrier: Physicochemical properties and biological activity. Int. J. Biol. Macromol. 2023, 239, 124203. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- He, P.; Dai, L.; Wei, J.; Zhu, X.; Li, J.; Chen, Z.; Ni, Y. Nanocellulose-based hydrogels as versatile drug delivery vehicles: A review. Int. J. Biol. Macromol. 2022, 222, 830–843. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M. Mucoadhesive curcumin crosslinked carboxy methyl cellulose might increase inhibitory efficiency for liver cancer treatment. Mater. Sci. Eng. C 2020, 116, 111119. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Lee-Kiun, M.S.; Teow, S.-Y.; Moeini, H.; Ali, R.R.; Kia, P.; Jie, C.J.; Abdullah, N.H. Chitosan coated magnetic cellulose nanowhisker as a drug delivery system for potential colorectal cancer treatment. Int. J. Biol. Macromol. 2023, 233, 123388. [Google Scholar] [CrossRef]
- Kumari, P.; Seth, R.; Meena, A.; Sharma, D. Enzymatic synthesis of cellulose nanocrystals from lemongrass and its application in improving anti-cancer drug release, uptake and efficacy. Ind. Crops Prod. 2023, 192, 115933. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Chen, C.; Qi, H.; Huang, K.; Hu, S. Preparation and synergistic chemo-photothermal therapy of redox-responsive carboxymethyl cellulose/chitosan complex nanoparticles. Carbohydr. Polym. 2022, 275, 118714. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I.; Yadollahi, M. Doxorubicin-loaded carboxymethyl cellulose/Starch/ZnO nanocomposite hydrogel beads as an anticancer drug carrier agent. Int. J. Biol. Macromol. 2020, 160, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Araoye, E.; Pyram, D.; Hemraz, U.D.; Boluk, Y.; Ckless, K. Cellulose nanocrystal cationic derivative induces NLRP3 inflammasome-dependent IL-1β secretion associated with mitochondrial ROS production. Biochem. Biophys. Rep. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Liebert, T.; Kostag, M.; Wotschadlo, J.; Heinze, T. Stable cellulose nanospheres for cellular uptake. Macromol. Biosci. 2011, 11, 1387–1392. [Google Scholar] [CrossRef]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Tu, Z.; Jia, H.; Gou, X.; Ngai, T. Hierarchical Porous Protein Scaffold Templated from High Internal Phase Emulsion Costabilized by Gelatin and Gelatin Nanoparticles. Langmuir 2018, 34, 4820–4829. [Google Scholar] [CrossRef]
- Mahmoudi Saber, M. Strategies for surface modification of gelatin-based nanoparticles. Colloids Surf. B Biointerfaces 2019, 183, 110407. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Raza, F.; Siyu, L.; Zafar, H.; Kamal, Z.; Zheng, B.; Su, J.; Qiu, M. Recent advances in gelatin-based nanomedicine for targeted delivery of anti-cancer drugs. Curr. Pharm. Des. 2022, 28, 380–394. [Google Scholar] [CrossRef]
- Geh, K.J.; Hubert, M.; Winter, G. Optimisation of one-step desolvation and scale-up of gelatine nanoparticle production. J. Microencapsul. 2016, 33, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Vaghasiya, K.; Ray, E.; Singh, R.; Jadhav, K.; Sharma, A.; Khan, R.; Katare, O.P.; Verma, R.K. Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy. Mater. Sci. Eng. C 2021, 123, 112027. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Hasan, A.; Babadaei, M.M.N.; Bloukh, S.H.; Edis, Z.; Rasti, B.; Sharifi, M.; Falahati, M. Application of gelatin nanoconjugates as potential internal stimuli-responsive platforms for cancer drug delivery. J. Mol. Liq. 2020, 318, 114053. [Google Scholar] [CrossRef]
- Morán, M.C.; Rosell, N.; Ruano, G.; Busquets, M.A.; Vinardell, M.P. Gelatin-based nanoparticles as DNA delivery systems: Synthesis, physicochemical and biocompatible characterization. Colloids Surf. B Biointerfaces 2015, 134, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.; Mekhail, G.; Wettig, S.D. Modified gelatin nanoparticles for gene delivery. Int. J. Pharm. 2019, 554, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Selimovic, A.; Kara, G.; Denkbas, E.B. Magnetic gelatin nanoparticles as a biocompatible carrier system for small interfering RNA in human colorectal cancer: Synthesis, optimization, characterization, and cell viability studies. Mater. Today Commun. 2022, 33, 104616. [Google Scholar] [CrossRef]
- Chen, X.; Zou, J.; Zhang, K.; Zhu, J.; Zhang, Y.; Zhu, Z.; Zheng, H.; Li, F.; Piao, J.-G. Photothermal/matrix metalloproteinase-2 dual-responsive gelatin nanoparticles for breast cancer treatment. Acta Pharm. Sin. B 2021, 11, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Jing, W.; Yan, Z.; Che, J.; Xu, H.; Hu, X.; Zhang, R. Melanin-gelatin nanoparticles with both EPR effect and renal clearance for PA/MRI dual-modal imaging of tumors. Biomater. Adv. 2022, 134, 112718. [Google Scholar] [CrossRef]
- ElMasry, S.R.; Hathout, R.M.; Abdel-Halim, M.; Mansour, S. In Vitro transdermal delivery of sesamol using oleic acid chemically-modified gelatin nanoparticles as a potential breast cancer medication. J. Drug Deliv. Sci. Technol. 2018, 48, 30–39. [Google Scholar] [CrossRef]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A novel smart PEGylated gelatin nanoparticle for co-delivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Mater. Sci. Eng. C 2019, 97, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Metwally, A.A. Gelatin Nanoparticles. In Pharmaceutical Nanotechnology: Basic Protocols; Weissig, V., Elbayoumi, T., Eds.; Springer: New York, NY, USA, 2019; pp. 71–78. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Huang, H. Preparation and application of dextran and its derivatives as carriers. Int. J. Biol. Macromol. 2020, 145, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gowda, B.H.J.; Nasir, N.; Wahab, S.; Pichika, M.R.; Sahebkar, A.; Kesharwani, P. Advancements in dextran-based nanocarriers for treatment and imaging of breast cancer. Int. J. Pharm. 2023, 643, 123276. [Google Scholar] [CrossRef] [PubMed]
- Luanda, A.; Badalamoole, V. Past, present and future of biomedical applications of dextran-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 228, 794–807. [Google Scholar] [CrossRef]
- Huo, M.; Wang, H.; Zhang, Y.; Cai, H.; Zhang, P.; Li, L.; Zhou, J.; Yin, T. Co-delivery of silybin and paclitaxel by dextran-based nanoparticles for effective anti-tumor treatment through chemotherapy sensitization and microenvironment modulation. J. Control. Release 2020, 321, 198–210. [Google Scholar] [CrossRef]
- Su, H.; Zhang, W.; Wu, Y.; Han, X.; Liu, G.; Jia, Q.; Shan, S. Schiff base-containing dextran nanogel as pH-sensitive drug delivery system of doxorubicin: Synthesis and characterization. J. Biomater. Appl. 2018, 33, 170–181. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Wang, H.; Li, P.; Chen, Y.; Wang, C.; Zhou, C.; Song, S.; Chen, S.; Huang, G.; et al. Dual-grafted dextran based nanomicelles: Higher antioxidant, anti-inflammatory and cellular uptake efficiency for quercetin. Int. J. Biol. Macromol. 2023, 224, 1361–1372. [Google Scholar] [CrossRef]
- Shaki, H.; Ganji, F.; Kempen, P.J.; Dolatshahi-Pirouz, A.; Vasheghani-Farahani, E. Self-assembled amphiphilic-dextran nanomicelles for delivery of rapamycin. J. Drug Deliv. Sci. Technol. 2018, 44, 333–341. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Xu, R.; Li, S.; Hu, H.; Xiao, C.; Wu, H.; Zhu, L.; Ming, J.; Chu, Z. Self-assembly of folic acid dextran conjugates for cancer chemotherapy. Nanoscale 2018, 10, 17265–17274. [Google Scholar] [CrossRef]
- Tian, H.; Yu, L.; Zhang, M.; He, J.; Sun, X.; Ni, P. Dextran-doxorubicin prodrug nanoparticles conjugated with CD147 monoclonal antibody for targeted drug delivery in hepatoma therapy. Colloids Surf. B Biointerfaces 2023, 228, 113400. [Google Scholar] [CrossRef] [PubMed]
- Behnke, M.; Klemm, P.; Dahlke, P.; Shkodra, B.; Beringer-Siemers, B.; Czaplewska, J.A.; Stumpf, S.; Jordan, P.M.; Schubert, S.; Hoeppener, S.; et al. Ethoxy acetalated dextran nanoparticles for drug delivery: A comparative study of formulation methods. Int. J. Pharm. X 2023, 5, 100173. [Google Scholar] [CrossRef] [PubMed]
- Sagnella, S.M.; Duong, H.; MacMillan, A.; Boyer, C.; Whan, R.; McCarroll, J.A.; Davis, T.P.; Kavallaris, M. Dextran-Based Doxorubicin Nanocarriers with Improved Tumor Penetration. Biomacromolecules 2014, 15, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; You, D.G.; Han, H.S.; Deepagan, V.G.; Jeon, S.M.; Suh, Y.D.; Choi, K.Y.; Kim, K.; Kwon, I.C.; Yi, G.-R.; et al. Bioreducible Carboxymethyl Dextran Nanoparticles for Tumor-Targeted Drug Delivery. Adv. Healthc. Mater. 2014, 3, 1829–1838. [Google Scholar] [CrossRef]
- Wang, S.; Fontana, F.; Shahbazi, M.-A.; Santos, H.A. Acetalated dextran based nano-and microparticles: Synthesis, fabrication, and therapeutic applications. Chem. Commun. 2021, 57, 4212–4229. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Kang, J.S.; DeLuca, P.P.; Lee, K.C. Emerging pegylated drugs. Expert. Opin. Emerg. Drugs 2009, 14, 363–380. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Naloxegol: A review of its use in patients with opioid-induced constipation. Drugs 2015, 75, 419–425. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: Current evidence and suggested approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; de Araújo Júnior, R.F.; Cavalcante, R.S.; Yu, Z.; Schomann, T.; Gu, Z.; Eich, C.; Cruz, L.J. Effective breast cancer therapy based on palmitic acid-loaded PLGA nanoparticles. Biomater. Adv. 2023, 145, 213270. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhou, J.; Sun, R.; Chen, Y.; Pan, D.; Wang, Q.; Chen, Y.; Gong, Z.; Du, Q. Dual-targeting of artesunate and chloroquine to tumor cells and tumor-associated macrophages by a biomimetic PLGA nanoparticle for colorectal cancer treatment. Int. J. Biol. Macromol. 2023, 244, 125163. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Öztürk, A.A.; Santos-Oliveira, R.; İlem-Özdemir, D. The use of lamivudine-loaded PLGA nanoparticles in the diagnosis of lung cancer: Preparation, characterization, radiolabeling with 99mTc and cell binding. J. Drug Deliv. Sci. Technol. 2022, 69, 103139. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front. Pharmacol. 2022, 13, 990505. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Fabozzi, A.; Barretta, M.; Valente, T.; Borzacchiello, A. Preparation and optimization of hyaluronic acid decorated irinotecan-loaded poly (lactic-co-glycolic acid) nanoparticles by microfluidics for cancer therapy applications. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131790. [Google Scholar] [CrossRef]
- Emami, F.; Duwa, R.; Banstola, A.; Woo, S.M.; Kwon, T.K.; Yook, S. Dual receptor specific nanoparticles targeting EGFR and PD-L1 for enhanced delivery of docetaxel in cancer therapy. Biomed. Pharmacother. 2023, 165, 115023. [Google Scholar] [CrossRef]
- Prabhuraj, R.; Bomb, K.; Srivastava, R.; Bandyopadhyaya, R. Selection of superior targeting ligands using PEGylated PLGA nanoparticles for delivery of curcumin in the treatment of triple-negative breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101722. [Google Scholar] [CrossRef]
- Arslan, F.B.; Öztürk, K.; Tavukçuoğlu, E.; Öztürk, S.C.; Esendağlı, G.; Çalış, S. A novel combination for the treatment of small cell lung cancer: Active targeted irinotecan and stattic co-loaded PLGA nanoparticles. Int. J. Pharm. 2023, 632, 122573. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Altawabeyeh, R.M.; Darweesh, R.S. Preparation and characterization of docetaxel-PLGA nanoparticles coated with folic acid-chitosan conjugate for cancer treatment. J. Pharm. Sci. 2022, 111, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Maddiboyina, B.; Roy, H.; Nakkala, R.K.; Gandhi, S.; Kavisri, M.; Moovendhan, M. Formulation, optimization and characterization of raloxifene hydrochloride loaded PLGA nanoparticles by using Taguchi design for breast cancer application. Chem. Biol. Drug Des. 2023, 102, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Elbatanony, R.S.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Chauhan, G.; Kunda, N.K.; Gupta, V. Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—Development and in-vitro efficacy. Drug Deliv. Transl. Res. 2021, 11, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Bal-Öztürk, A.; Tietilu, S.D.; Yücel, O.; Erol, T.; Akgüner, Z.P.; Darıcı, H.; Alarcin, E.; Emik, S. Hyperbranched polymer-based nanoparticle drug delivery platform for the nucleus-targeting in cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 81, 104195. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G. Applications and limitations of dendrimers in biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A new race of pharmaceutical nanocarriers. Biomed. Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Synthesis and functionalization of hyperbranched polymers for targeted drug delivery. J. Control. Release 2020, 321, 285–311. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Rastogi, V.; Yadav, P.; Porwal, M.; Sur, S.; Verma, A. Dendrimer as nanocarrier for drug delivery and drug targeting therapeutics: A fundamental to advanced systematic review. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 310–332. [Google Scholar] [CrossRef]
- Sarma, K.; Akther, M.H.; Ahmad, I.; Afzal, O.; Altamimi, A.S.; Alossaimi, M.A.; Jaremko, M.; Emwas, A.-H.; Gautam, P. Adjuvant Novel Nanocarrier-Based Targeted Therapy for Lung Cancer. Molecules 2024, 29, 1076. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.D.; Bigham, A.; Esmaeili, Y.; Ashrafizadeh, M.; Moghaddam, F.D.; Tan, S.C.; Yousefiasl, S.; Sharma, S.; Maleki, A.; Rabiee, N. Dendrimers as nanoscale vectors: Unlocking the bars of cancer therapy. Semin. Cancer Biol. 2022, 86, 396–419. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur. Polym. J. 2021, 158, 110683. [Google Scholar] [CrossRef]
- Cruz, A.; Barbosa, J.; Antunes, P.; Bonifácio, V.D.; Pinto, S.N. A Glimpse into Dendrimers Integration in Cancer Imaging and Theranostics. Int. J. Mol. Sci. 2023, 24, 5430. [Google Scholar] [CrossRef] [PubMed]
- Franiak-Pietryga, I.; Ziemba, B.; Messmer, B.; Skowronska-Krawczyk, D. Dendrimers as drug nanocarriers: The future of gene therapy and targeted therapies in cancer. Dendrimers Fundam. Appl. 2018, 25, 7. [Google Scholar]
- Amreddy, N.; Babu, A.; Panneerselvam, J.; Srivastava, A.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Munshi, A.; Ramesh, R. Chemo-biologic combinatorial drug delivery using folate receptor-targeted dendrimer nanoparticles for lung cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 373–384. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Sobierajska, E.; Chworos, A.; Klajnert-Maculewicz, B. Multicomponent conjugates of anticancer drugs and monoclonal antibody with PAMAM dendrimers to increase efficacy of HER-2 positive breast cancer therapy. Pharm. Res. 2019, 36, 154. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Gajek, A.; Ksiezak, M.; Dzialak, P.; Klajnert-Maculewicz, B. Molecular mechanisms of antitumor activity of PAMAM dendrimer conjugates with anticancer drugs and a monoclonal antibody. Polymers 2019, 11, 1422. [Google Scholar] [CrossRef]
- Pearce, A.K.; Simpson, J.D.; Fletcher, N.L.; Houston, Z.H.; Fuchs, A.V.; Russell, P.J.; Whittaker, A.K.; Thurecht, K.J. Localised delivery of doxorubicin to prostate cancer cells through a PSMA-targeted hyperbranched polymer theranostic. Biomaterials 2017, 141, 330–339. [Google Scholar] [CrossRef]
- Kianamiri, S.; Dinari, A.; Sadeghizadeh, M.; Rezaei, M.; Daraei, B.; Bahsoun, N.E.-H.; Nomani, A. Mitochondria-targeted polyamidoamine dendrimer–curcumin construct for hepatocellular cancer treatment. Mol. Pharm. 2020, 17, 4483–4498. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Sobierajska, E.; Stanczyk, M.; Janaszewska, A.; Chworos, A.; Klajnert-Maculewicz, B. Conjugate of PAMAM dendrimer, doxorubicin and monoclonal antibody—Trastuzumab: The new approach of a well-known strategy. Polymers 2018, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic polyglycerol-derived nano-architectures as delivery platforms of gemcitabine for pancreatic cancer. Macromol. Biosci. 2019, 19, 1900073. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Liu, P. Facile synthesis of GSH-triggered skeleton-cleavable hyperbranched polymer prodrug as unimolecular micelles for tumor-specific camptothecin delivery. Eur. Polym. J. 2023, 196, 112279. [Google Scholar] [CrossRef]
- Liaw, K.; Zhang, F.; Mangraviti, A.; Kannan, S.; Tyler, B.; Kannan, R.M. Dendrimer size effects on the selective brain tumor targeting in orthotopic tumor models upon systemic administration. Bioeng. Transl. Med. 2020, 5, e10160. [Google Scholar] [CrossRef]
- Huang, J.; Zhuang, C.; Chen, J.; Chen, X.; Li, X.; Zhang, T.; Wang, B.; Feng, Q.; Zheng, X.; Gong, M. Targeted Drug/Gene/Photodynamic Therapy via a Stimuli-Responsive Dendritic-Polymer-Based Nanococktail for Treatment of EGFR-TKI-Resistant Non-Small-Cell Lung Cancer. Adv. Mater. 2022, 34, 2201516. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-responsive polymer nanomaterials for tumor therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Abd El-Sattar, N.E.; El-Hddad, S.E.S.; Ghobashy, M.M.; Zaher, A.A.; El-Adl, K. Nanogel-mediated drug delivery system for anticancer agent: pH stimuli responsive poly (ethylene glycol/acrylic acid) nanogel prepared by gamma irradiation. Bioorganic Chem. 2022, 127, 105972. [Google Scholar] [CrossRef]
- Guo, S.; Qiu, L.; Chen, Y.; Wang, X.; Ma, B.; Qu, C.; Cui, J.; Zhang, H.; Xing, C.; Zhan, Y. TMEM16A-inhibitor loaded pH-responsive nanoparticles: A novel dual-targeting antitumor therapy for lung adenocarcinoma. Biochem. Pharmacol. 2020, 178, 114062. [Google Scholar] [CrossRef]
- Li, Z.; Wan, W.; Bai, Z.; Peng, B.; Wang, X.; Cui, L.; Liu, Z.; Lin, K.; Yang, J.; Hao, J. Construction of pH-responsive nanoplatform from stable magnetic nanoparticles for targeted drug delivery and intracellular imaging. Sens. Actuators B Chem. 2023, 375, 132869. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Du, Z.; Pu, X.; Zhu, B. 131I labeled pH-responsive gold nanoparticles for bimodal tumor diagnosis. Mater. Lett. 2023, 330, 133202. [Google Scholar] [CrossRef]
- Hayati, M.; Rezanejade Bardajee, G.; Ramezani, M.; Mizani, F. Temperature/pH/magnetic triple sensitive nanogel for doxorubicin anticancer drug delivery. Inorg. Nano-Met. Chem. 2020, 50, 1189–1200. [Google Scholar] [CrossRef]
- Heragh, B.K.; Taherinezhad, H.; Mahdavinia, G.R.; Javanshir, S.; Labib, P.; Ghasemsolb, S. pH-responsive co-delivery of doxorubicin and saffron via cross-linked chitosan/laponite RD nanoparticles for enhanced-chemotherapy. Mater. Today Commun. 2023, 34, 104956. [Google Scholar] [CrossRef]
- Ghazimoradi, M.; Tarlani, A.; Alemi, A.; Hamishehkar, H.; Ghorbani, M. pH-responsive, magnetic-luminescent core/shell carriers for co-delivery of anticancer drugs (MTX & DOX) for breast cancer treatment. J. Alloys Compd. 2023, 936, 168257. [Google Scholar]
- Tao, J.; Diao, L.; Chen, F.; Shen, A.; Wang, S.; Jin, H.; Cai, D.; Hu, Y. pH-sensitive nanoparticles codelivering docetaxel and dihydroartemisinin effectively treat breast cancer by enhancing reactive oxidative species-mediated mitochondrial apoptosis. Mol. Pharm. 2020, 18, 74–86. [Google Scholar] [CrossRef]
- Ibrahim, B.; Mady, O.Y.; Tambuwala, M.M.; Haggag, Y.A. pH-sensitive nanoparticles containing 5-fluorouracil and leucovorin as an improved anti-cancer option for colon cancer. Nanomedicine 2022, 17, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Swetha, K.L.; Paul, M.; Maravajjala, K.S.; Kumbham, S.; Biswas, S.; Roy, A. Overcoming drug resistance with a docetaxel and disulfiram loaded pH-sensitive nanoparticle. J. Control. Release 2023, 356, 93–114. [Google Scholar] [CrossRef]
- Li, D.; Zhang, R.; Liu, G.; Kang, Y.; Wu, J. Redox-Responsive Self-Assembled Nanoparticles for Cancer Therapy. Adv. Healthc. Mater. 2020, 9, 2000605. [Google Scholar] [CrossRef]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319. [Google Scholar] [CrossRef]
- Alsehli, M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery. Saudi Pharm. J. 2020, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.F.; Travanut, A.; Conte, C.; Alexander, C. Reduction-responsive polymers for drug delivery in cancer therapy—Is there anything new to discover? WIREs Nanomed. Nanobiotechnol. 2021, 13, e1678. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Rempson, C.M.; Puche, V.; Zhao, B.; Zhang, F. Construction of disulfide containing redox-responsive polymeric nanomedicine. Methods 2022, 199, 67–79. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Jia, Y.; Li, J.; Xiao, C. Reduction-responsive dextran-based Pt(IV) nano-prodrug showed a synergistic effect with doxorubicin for effective melanoma treatment. Int. J. Biol. Macromol. 2023, 233, 123277. [Google Scholar] [CrossRef]
- Meng, L.; Liu, F.; Du, C.; Zhu, J.; Xiong, Q.; Li, J.; Sun, W. Glucosamine-modified reduction-responsive polymeric micelles for liver cancer therapy. Molecules 2023, 28, 3824. [Google Scholar] [CrossRef]
- Maruf, A.; Milewska, M.; Lalik, A.; Student, S.; Wandzik, I. A simple synthesis of reduction-responsive acrylamide-type nanogels for miRNA delivery. Molecules 2023, 28, 761. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, W.; Kang, G.; Li, Q.; Tao, L.; Wang, X.; Yin, J. Synergistic chemo-photo anticancer therapy by using reversible Diels-Alder dynamic covalent bond mediated polyprodrug amphiphiles and immunoactivation investigation. Biomater. Sci. 2023, 11, 5819–5830. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Y.; Sun, J.; Chen, H.; Liu, Z.; Lin, K.; Ma, P.; Zhang, W.; Zhen, Y.; Zhang, S.; et al. pH/reduction dual-responsive hyaluronic acid-podophyllotoxin prodrug micelles for tumor targeted delivery. Carbohydr. Polym. 2022, 288, 119402. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, D.; Ma, X.; Liang, M.; Hou, S.; Qiu, W.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. Reduction-Responsive Chemo-Capsule-Based Prodrug Nanogel for Synergistic Treatment of Tumor Chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 8940–8951. [Google Scholar] [CrossRef]
- Cai, Q.; Jiang, J.; Zhang, H.; Ge, P.; Yang, L.; Zhu, W. Reduction-responsive anticancer nanodrug using a full poly(ethylene glycol) carrier. ACS Appl. Mater. Interfaces 2021, 13, 19387–19397. [Google Scholar] [CrossRef]
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Zhaleh, H.; Cruz, L.J. Enhanced intracellular delivery of curcumin by chitosan-lipoic acid as reduction-responsive nanoparticles. Curr. Pharm. Biotechnol. 2021, 22, 622–635. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-isopropylacrylamide)-based hydrogels for biomedical applications: A review of the state-of-the-art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Lupu, A.; Gradinaru, L.M.; Rusu, D.; Bercea, M. Self-healing of pluronic® F127 hydrogels in the presence of various polysaccharides. Gels 2023, 9, 719. [Google Scholar] [CrossRef]
- Chen, N.; Wei, X.; Zhao, G.; Jia, Z.; Fu, X.; Jiang, H.; Xu, X.; Zhao, Z.; Singh, P.; Lessard, S. Single dose thermoresponsive dexamethasone prodrug completely mitigates joint pain for 15 weeks in a murine model of osteoarthritis. Nanomed. Nanotechnol. Biol. Med. 2024, 57, 102735. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Duan, S.; Han, C.; Jing, C.; Xiao, Z.; Li, C. Hypoxia-responsive nanomaterials for tumor imaging and therapy. Front. Oncol. 2022, 12, 1089446. [Google Scholar] [CrossRef]
- Mamnoon, B.; Feng, L.; Froberg, J.; Choi, Y.; Sathish, V.; Mallik, S. Hypoxia-responsive, polymeric nanocarriers for targeted drug delivery to estrogen receptor-positive breast cancer cell spheroids. Mol. Pharm. 2020, 17, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, J.; Zhang, T.; Gao, A.; Sun, C. Application of tumor pH/hypoxia-responsive nanoparticles for combined photodynamic therapy and hypoxia-activated chemotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1197404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, Y.; Yan, Z.; Ding, Y.; Liang, F. Hypoxia-responsive polymeric nanoprodrugs for combo photodynamic and chemotherapy. ACS Omega 2023, 9, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels—Synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-responsive hydrogels as potential drug delivery systems—State of knowledge and future prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kwak, C. Prostate-specific membrane antigen-mediated theragnostics in prostate cancer. Investig. Clin. Urol. 2021, 62, 497–499. [Google Scholar] [CrossRef]
- Li, M.; Zhao, G.; Su, W.K.; Shuai, Q. Enzyme-responsive nanoparticles for anti-tumor drug delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.-C. Thermo-sensitive poly (N-isopropylacrylamide-co-polyacrylamide) hydrogel for pH-responsive therapeutic delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Houzong, R.; Fu, J.; Shao, K.; Wang, L.; Ma, Y.; Shi, J. Application of a novel thermo-sensitive injectable hydrogel in therapy in situ for drug accurate controlled release. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3200–3216. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wang, H.; Long, S.; Zhang, T.; Guo, X.; Chen, S.; Kakuchi, T.; Duan, Q.; Zhao, D. Thermo- and light-responsive polymer-coated magnetic nanoparticles as potential drug carriers. Front. Bioeng. Biotechnol. 2022, 10, 931830. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Onodera, S.; Takemoto, H.; Harun, N.F.C.; Nomoto, T.; Matsui, M.; Tomoda, K.; Sun, Y.; Miura, Y.; Nishiyama, N. Thermo-responsive polymer-siRNA conjugates enabling artificial control of gene silencing around body Ttemperature. Pharm. Res. 2023, 40, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Guo, C.; Guo, H.; Su, Y.; Chen, Q.; Sun, C.; Liu, Q.; Chen, D.; Mu, H. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022, 29, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Liu, X.; Huang, X.; Lu, M.; Wu, X.; Weng, L.; Chen, Q.; Wang, X.; Zhu, L.; Chen, Z. Alendronate-functionalized hypoxia-responsive polymeric micelles for targeted therapy of bone metastatic prostate cancer. J. Control Release 2021, 334, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Peng, Y.Y.; Diaz-Dussan, D.; White, J.; Duan, W.; Kong, L.; Narain, R.; Hao, X. Zwitterionic block copolymer prodrug micelles for pH responsive drug delivery and hypoxia-specific chemotherapy. Mol. Pharm. 2022, 19, 1766–1777. [Google Scholar] [CrossRef]
- Kang, Y.; Lim, J.; Saravanakumar, G.; Kim, J.; Park, M.; Im, S.; Kim, W.J. Immunostimulation of tumor microenvironment by targeting tumor-associated macrophages with hypoxia-responsive nanocomplex for enhanced anti-tumor therapy. J. Control Release 2022, 343, 78–88. [Google Scholar] [CrossRef]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020, 113, 501–511. [Google Scholar] [CrossRef]
- Guo, F.; Jiao, Y.; Du, Y.; Luo, S.; Hong, W.; Fu, Q.; Li, A.; Wang, G.; Yang, G. Enzyme-responsive nano-drug delivery system for combined antitumor therapy. Int. J. Biol. Macromol. 2022, 220, 1133–1145. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, Q.; Yin, Y.; Mei, Y.; Li, J.; Xu, L.; Gao, H.; Wang, W. An enzyme-responsive and NIR-triggered lipid-polymer hybrid nanoplatform for synergistic photothermal/chemo cancer therapy. Biomater. Sci. 2022, 10, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.Y.; Zhu, Y.X.; Jia, H.R.; Guo, Y.; Zhang, X.; Gu, R.; Li, C.; Wu, F.G. Platinum-Coordinated Dual-Responsive Nanogels for Universal Drug Delivery and Combination Cancer Therapy. Small 2022, 18, e2203260. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Thummarati, P. Innovative design of targeted nanoparticles: Polymer-drug conjugates for enhanced cancer therapy. Pharmaceutics 2023, 15, 2216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Li, J.; Peng, Z.H.; Sheinin, Y.; Zhou, J.; Oupický, D. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, J.; Jin, X.; Liu, L.; Tian, X. Folate receptor targeting and cathepsin B-sensitive drug delivery system for selective cancer cell death and imaging. ACS Med. Chem. Lett. 2020, 11, 1514–1520. [Google Scholar] [CrossRef]
- Radford, D.C.; Yang, J.; Doan, M.C.; Li, L.; Dixon, A.S.; Owen, S.C.; Kopeček, J. Multivalent HER2-binding polymer conjugates facilitate rapid endocytosis and enhance intracellular drug delivery. J. Control. Release 2020, 319, 285–299. [Google Scholar] [CrossRef]
- Gyimesi, G.; Hediger, M.A. Transporter-mediated drug delivery. Molecules 2023, 28, 1151. [Google Scholar] [CrossRef]
- Winkler, E.A.; Nishida, Y.; Sagare, A.P.; Rege, S.V.; Bell, R.D.; Perlmutter, D.; Sengillo, J.D.; Hillman, S.; Kong, P.; Nelson, A.R.; et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015, 18, 521–530. [Google Scholar] [CrossRef]
- Ma, P.; Wei, G.; Chen, J.; Jing, Z.; Wang, X.; Wang, Z. GLUT1 targeting and hypoxia-activating polymer-drug conjugate-based micelle for tumor chemo-thermal therapy. Drug Deliv. 2021, 28, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Huttunen, J.; Auriola, S.; Huttunen, K.M. L-type amino acid transporter 1 utilizing prodrugs of ferulic acid revealed structural features supporting the design of prodrugs for brain delivery. Eur. J. Pharm. Sci. 2019, 129, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xu, W.; Nomoto, T.; Kanamori, K.; Voon, Y.M.; Honda, Y.; Yamada, N.; Takemoto, H.; Matsui, M.; Nishiyama, N. Polymeric ligands comprising sulfur-containing amino acids for targeting tumor-associated amino acid transporters. Biomaterials 2023, 293, 121987. [Google Scholar] [CrossRef] [PubMed]
- Conger, K.O.; Chidley, C.; Ozgurses, M.E.; Zhao, H.; Kim, Y.; Semina, S.E.; Burns, P.; Rawat, V.; Sheldon, R.; Ben-Sahra, I.; et al. ASCT2 is the primary serine transporter in cancer cells. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zhou, P.; Liang, X.; Zhou, C.; Qin, J.; Hou, C.; Zhu, Z.; Zhang, W.; Wang, S.; Zhong, D. Glutamine-β-cyclodextrin for targeted doxorubicin delivery to triple-negative breast cancer tumors via the transporter ASCT2. J. Mater. Chem. B 2019, 7, 5363–5375. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Tang, S.; Ding, L.; Foley, J.; Tang, W.; Jia, H.; Panja, S.; Holbert, C.E.; Hang, Y.; Stewart, T.M.; et al. Hyaluronate-coated perfluoroalkyl polyamine prodrugs as bioactive siRNA delivery systems for the treatment of peritoneal cancers. Biomater. Adv. 2022, 136, 212755. [Google Scholar] [CrossRef]
- Xiao, D.; Hu, X.; Zhang, J. Tumour targeted polymer nanoparticles co-loaded with docetaxel and siCCAT2 for combination therapy of lung cancer. J. Drug Target. 2022, 30, 534–543. [Google Scholar] [CrossRef]
- Zhang, H.T.; Peng, R.; Chen, S.; Shen, A.; Zhao, L.; Tang, W.; Wang, X.H.; Li, Z.Y.; Zha, Z.G.; Yi, M.; et al. Versatile nano-PROTAC-induced epigenetic reader degradation for efficient lung cancer therapy. Adv. Sci. 2022, 9, e2202039. [Google Scholar] [CrossRef]
- Yadav, B.; Chauhan, M.; Shekhar, S.; Kumar, A.; Mehata, A.K.; Nayak, A.K.; Dutt, R.; Garg, V.; Kailashiya, V.; Muthu, M.S.; et al. RGD-decorated PLGA nanoparticles improved effectiveness and safety of cisplatin for lung cancer therapy. Int. J. Pharm. 2023, 633, 122587. [Google Scholar] [CrossRef]
- Kazemi, M.; Parhizkar, E.; Samani, S.M.; Firuzi, O.; Sadeghpour, H.; Ahmadi, F.; Dehshahri, A. Targeted co-delivery of paclitaxel and anti P-gp shRNA by low molecular weight PEI decorated with L-3,4-dihydroxyphenylalanine. Biotechnol. Prog. 2023, 39, e3310. [Google Scholar] [CrossRef]
- Jin, J.; Yuan, P.; Yu, W.; Lin, J.; Xu, A.; Xu, X.; Lou, J.; Yu, T.; Qian, C.; Liu, B.; et al. Mitochondria-targeting polymer micelle of dichloroacetate induced pyroptosis to enhance osteosarcoma immunotherapy. ACS Nano 2022, 16, 10327–10340. [Google Scholar] [CrossRef] [PubMed]
- Kanjilal, P.; Singh, K.; Das, R.; Matte, J.; Thayumanavan, S. Antibody polymer conjugates (APCs) for active targeted therapeutic delivery. Biomacromolecules 2023, 24, 3638–3646. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef]
- Chandra, G.; Singh, D.V.; Mahato, G.K.; Patel, S. Fluorine-a small magic bullet atom in the drug development: Perspective to FDA approved and COVID-19 recommended drugs. Chem. Pap. 2023, 7, 4085–4106. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Han, J.; Fustero, S.; Medio-Simon, M.; Sedgwick, D.M.; Santi, C.; Ruzziconi, R.; Soloshonok, V.A. Fluorine-containing drugs approved by the FDA in 2018. Chemistry 2019, 25, 11797–11819. [Google Scholar] [CrossRef]
- Song, T.; Gao, Y.; Song, M.; Qian, J.; Zhang, H.; Zhou, J.; Ding, Y. Fluoropolymers-mediated efficient biomacromolecule drug delivery. Med. Drug Discov. 2022, 14, 100123. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.; Ameduri, B.; Chen, M. Fluoropolymer nanoparticles synthesized via reversible-deactivation radical polymerizations and their applications. Chem. Rev. 2023, 123, 12431–12470. [Google Scholar] [CrossRef]
- Yang, S.; Wong, K.H.; Hua, P.; He, C.; Yu, H.; Shao, D.; Shi, Z.; Chen, M. ROS-responsive fluorinated polyethyleneimine vector to co-deliver shMTHFD2 and shGPX4 plasmids induces ferroptosis and apoptosis for cancer therapy. Acta Biomater. 2022, 140, 492–505. [Google Scholar] [CrossRef]
- Shi, L.; Jin, Y.; Lai, S.; Bai, L.; Zhou, R.; Zhou, Y.; Shang, X. Redox-responsive carrier based on fluorinated gemini amphiphilic polymer for combinational cancer therapy. Colloids Surf. B Biointerfaces 2022, 216, 112551. [Google Scholar] [CrossRef]
- Li, G.; Lei, Q.; Wang, F.; Deng, D.; Wang, S.; Tian, L.; Shen, W.; Cheng, Y.; Liu, Z.; Wu, S. Fluorinated polymer mediated transmucosal peptide delivery for intravesical instillation therapy of bladder cancer. Small 2019, 15, e1900936. [Google Scholar] [CrossRef]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z.; et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef]
- Yang, Z.; Tao, D.; Zhong, W.; Liu, Z.; Feng, L.; Chen, M. Perfluorocarbon loaded fluorinated covalent organic polymers with effective sonosensitization and tumor hypoxia relief enable synergistic sonodynamic-immunotherapy. Biomaterials 2022, 280, 121250. [Google Scholar] [CrossRef]
- Zhan, Y.R.; He, X.; Huang, Z.Y.; Chen, P.; Tian, M.M.; Li, G.H.; Yu, X.Q.; Song, X.R.; Zhang, J. A novel fluoropolymer as a protein delivery vector with robust adjuvant effect for cancer immunotherapy. J. Mater. Chem. B 2023, 11, 8933–8942. [Google Scholar] [CrossRef]
- Zhu, W.; Chao, Y.; Jin, Q.; Chen, L.; Shen, J.J.; Zhu, J.; Chai, Y.; Lu, P.; Yang, N.; Chen, M.; et al. Oral delivery of therapeutic antibodies with a transmucosal polymeric carrier. ACS Nano 2023, 17, 4373–4386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, R.; Zhang, H.; Yang, W.; Tian, Y. Fluoropolymer coated DNA nanoclews for volumetric visualization of oligonucleotides delivery and near infrared light activated anti-angiogenic oncotherapy. Adv. Sci. 2023, 10, e2304633. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, S.; Ma, M.; Zhang, Y. Fluorinated PEG-PEI coated magnetic nanoparticles for siRNA delivery and CXCR4 knockdown. Nanomaterials 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yu, W.; Zhang, W.; Wang, C.; Liu, Y.; Yuan, W.E.; Feng, Y. A novel fluorinated polyethyleneimine with microRNA-942-5p-sponges polyplex gene delivery system for non-small-cell lung cancer therapy. J. Colloid Interface Sci. 2023, 648, 287–298. [Google Scholar] [CrossRef]






| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| Chitosan hydrogel | Dopamine-conjugated perylene | Covalent cross-linking | Biocompatible, elastic, and photosensitive gel significantly enhanced the phototoxicity of perylene compared to the free drug. | [36] |
| Nitrosalicylaldehyde; Aldehyde HA; Chitosan hydrogel | DOX and cisplatin | Covalent cross-linking | Sustained release of the drugs from the hydrogel at a physiological and slightly acidic pH value demonstrated antiproliferative effects and biodegradable properties. | [37] |
| Sialic acid; Cetuximab; Chitosan NPs | Gemcitabine | Ionic gelation | Increased bioavailability and reduced clearance along with enhanced antiproliferative activity and cell internalization of the targeted chitosan NPs. | [38] |
| HA dialdehyde; Chitosan NPs | siRNA | Ionic gelation | Targeted accumulation, inhibiting tumor growth by silencing the oncogene, and good blood compatibility. | [39] |
| T7 peptide; Carboxymethyl chitosan NPs | Docetaxel (DTX) and curcumin | Ionic gelation | Enhanced in vitro and in vivo antitumor effects compared to monotherapy and good biosafety. | [40] |
| Zein; Chitosan NPs | Curcumin and berberine | Anti-solvent precipitation method | Biocompatible, redispersible, and stable NPs demonstrated improved cytotoxicity, cell internalization, and apoptosis with anti-inflammatory properties. | [41] |
| PLGA; Chitosan NPs | DOX | Anti-solvent precipitation method | Sustained pharmacodynamics of DOX. | [42] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| Fucoidan, Zein, HA NPs | Fisetin | Anti-solvent precipitation | Targeted delivery with significantly higher cytotoxicity. | [62] |
| L-glutamate, HA NPs | Gefitinib and vorinostat | Nanoprecipitation | Targeted therapy, reduced systemic toxicity, and substantial tumor growth inhibition. | [63] |
| HA NPs | Indocyanine green (ICG) | Self-assembly | Accumulation at the target site compared to healthy cells. | [64] |
| PEG HA NPs | Mitoxantrone | Anti-solvent precipitation | Significantly higher cytotoxicity to CD44-positive cells and apoptosis. | [65] |
| Alendronate sodium; HA NPs | Methotrexate | Self-assembly | Reduced off-target effects and improved antitumor activity. | [66] |
| β-cyclodextrin; Poly-l-lysine; HA NPs | DOX and oligo-RNA | Self-assembly and anti-solvent precipitation | CD44-mediated delivery of therapeutics and accumulation in tumors. | [61] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| Alginate microbeads | DOX | Microencapsulation | Reduction in the initial burst release and increase in the encapsulation efficiency. | [75] |
| Alginate NPs | Quercetin (leukemia) | Cold precipitation | Long shelf-life, high drug entrapment, sustained release, and improved cytotoxicity. | [76] |
| Chitosan; Alginate NPs | DOX | Ionic gelation | Sustained release and improved cytotoxicity. | [77] |
| Ferrous oxide, Gelatin, Oxidized alginate hydrogel | DOX | Physical mixing | pH-dependent release profile, and higher encapsulation efficiency. | [78] |
| Boronated chitosan; Sodium alginate NPs | PTX | Ionotropic gelation | Conjugation improved the mucoadhesive properties, very high encapsulation and drug-loading capacity, and sustained release. | [79] |
| Folic acid; Sodium alginate NPs | Diferourylmethane | Emulsion solvent evaporation method | Folic acid (FA) conjugation allowed sustained release and a higher cellular uptake. | [80] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| Carboxymethyl cellulose conjugation | Curcumin | Cross-linking | Enhanced permeation and anti-proliferation. | [91] |
| Chitosan; Ferrous oxide; Cellulose nanowhiskers | 5-Fluorouracil (5-FU) | Co-precipitation and ionic gelation | pH-dependent release, higher anticancer effect, and low cost. | [92] |
| Cellulose nanocrystals | Curcumin | Nanoprecipitation | Sustained drug release, biocompatible, and improved cytotoxicity. | [93] |
| Chitosan; Disulfide cross-linked carboxymethyl cellulose NPs | 5-FU and polypyrrole | Emulsification | Drug release upon acidic and redox stimuli, improved cellular uptake, and synergistic tumor growth inhibition. | [94] |
| Sodium carboxymethyl cellulose hydrogel | DOX | Cross-linking | Improved drug loading, controlled and prolonged release, and biocompatiblity. | [95] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| Iron oxide; Gelatin NPs | siRNA | Desolvation and cross-linking | Improved shelf-life, encapsulation efficiency, cytocompatibility, and anticancer activity. | [108] |
| Melanin; Gelatin NPs | Photoacoustic tumor imaging | Desolvation | Tumor-targeted accumulation, biocompatiblity, and substrate MMP degradation. | [110] |
| Oleic acid; Gelatin NPs | Sesamol | Desolvation and cross-linking | Significantly higher permeation after transdermal delivery and reduced IC50. | [111] |
| Concanavalin A; Gelatin NPs | Cisplatin | Desolvation and cross-linking | On-demand release system, targeted released of the drug due to interaction with MMPs, biocompatibility, and improved endocytosis. | [104] |
| PEGylated gelatin NPs | DOX; Betanin | Desolvation | pH-responsive controlled drug release, improved cellular uptake, cytotoxicity, and apoptosis. | [112] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| Oxidized dextran NPs | DOX and CD147 | Prodrug and self-assembly | Sustained and acid-sensitive release of the drug, prolonged blood half-life, and significant tumor growth inhibition. | [123] |
| Aldehyded dextran nanogel | DOX | Inverse microemulsion | Uptake by tumor cells and pH-sensitive drug release. | [119] |
| Deoxycholic acid; Dextran NPs | Silybin and PTX | Self-assembly | Passive targeting, tumor accumulation, and tumor growth inhibition. | [118] |
| Aldehyded dextran carrier | DOX | Prodrug conjugation | Internalization of particles, delayed drug release, and substantially high tumor penetration. | [125] |
| Lithocholic acid; Carboxymethyl dextran NPs | DOX | Self-assembly | Accumulation at the tumor site, rapid release of the drug in the reductive tumor environment, and extremely low release at a physiological pH. | [126] |
| Ethoxy acetalated dextran NPs | BRP-187 | Microfluidics, emulsification, and nanoprecipitation | Enhanced encapsulation efficiency, and nanoprecipitation was the method of choice. | [124] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| Mitochondrial-targeted PAMAM dendrimers | Curcumin | Chemical conjugation | Selectively induced potent apoptosis and cell cycle arrest at G2/M with improved solubility. | [164] |
| FA-conjugated PAMAM dendrimers | siRNA and CDDP | Covalent conjugation to G4 dendrimer using PEI and PEG | Improved the therapeutic effects of HuR siRNA and CDDP against H1299 lung cancer cells. | [159] |
| Trastuzumab-conjugated PAMAM dendrimers | DOX and mAb | PAMAM and DOX were conjugated by using cis-Aconitic anhydride (CAA) | High toxicity of PAMAM-DOX-trastuzumab conjugates against HER-2-positive (SKBR-3) and -negative (MCF-7) breast cancer cells. | [165] |
| PG-co-PCL dendritic nano-structure | Gemcitabine | Copolymerization of the monomer mixture composed of glycidol and ε-caprolactone | Improved pH-dependent release with a better toxicity for both non-covalent- and covalent-conjugated gemcitabine against pancreatic cancer. | [166] |
| GSH-triggered HBP-based micelles | Camptothecin | Self-condensing vinyl polymerization strategy via the atom transfer radical polymerization (ATRP) of drug-contained monomers and hydrophilic macromolecular monomers | Superior stability with a high release and improved tumor cell growth inhibition. | [167] |
| Cy3-labeled G4 (G4-Cy3) and Cy5-labeled G6 (PAMAM) dendrimers (G6-Cy5) | Fluorescent dye Cy3 and Cy5 | Surface-modified into amine-terminated bifunctional dendrimers | G6 dendrimer demonstrated a high delivery efficacy compared to G4. | [168,169] |
| Stimuli-eesponsive dendritic polymer-based nanococktail | Gefitinib and YAP-siRNA | Chemical conjugation and electric condensation | Induced tumor cell apoptosis through PDT and improved antitumor efficacy I cell line-derived xenograft and patient-derived xenograft tumor models. | [169] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| pH-Responsive triple-sensitive nanogel | DOX | Sensitive monomer grafted onto sodium alginate | Targeted and controlled release of DOX in vitro. | [175] |
| pH-Responsive cross-linked chitosan/laponite RD NPs | DOX and Sorafenib (SF) | Cross-linking | pH stimulated the simultaneous in vitro release of DOX and SF; higher cytotoxicity against breast cancer cell lines. | [176] |
| pH-Responsive poly (MAA-co-IA) NPs | DOX and Methotrexate | One-pot biphase stratification approach | Improved tumor inhibition compared to plan DOX and methotrexate. | [177] |
| pH-Sensitive NPs | DTX | DTX and dihydroartemisinin conjugated with 4-arm-PEG via a hydrazone bond | Significantly increased the apoptosis of 4T1 cells and inhibited lung metastasis due to a synergistic effect. | [178] |
| pH-Sensitive NPs | 5-FU and Leucovorin | Double emulsion and solvent evaporation | Showed a pH-responsive drug release and exhibited a significantly higher cytotoxic action. | [179] |
| pH-Sensitive NPs | DTX and Disulfiram | Nanoprecipitation method using microfluidics | Increased in vivo antitumor efficacy against a mouse orthotopic breast cancer model, while decreasing P-gp expression and preventing lung metastasis. | [180] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| ROS-responsive dextran-based Pt nanoprodrug (PDPN) | DOX | One-pot chemical coupling of carbonylated methoxy PEG, dextran, and the cross-linking agent cisPt | PDPN-DOX displayed the reduction-responsive release of DOX and Pt with synergistic anticancer effects. | [186] |
| ROS-responsive and active-targeting drug delivery systems (AG-PEG-SS-PCL) | SF | Thin-film hydration method | Excellent antitumor effects and better tolerance. | [187] |
| Acrylamide-based NPs containing ROS-sensitive cross-linkers | microRNA | Electrostatic interaction between positively charged NPs and negatively charged microRNA | Stable dispersions were formed in biological media and enhanced microRNA release in the presence of GSH. | [188] |
| Thermo- and reduction-responsive copolymers | CPT | Self-assembly | GSH could trigger the release of CPT drugs and was promoted by NIR light-induced photothermal therapy. | [189] |
| pH/Reduction dual-responsive HA prodrug | Podophyllotoxin (PPT) | Self-assembly | Due to HA receptor-mediated endocytosis, HA-S-S-PPT accumulated at the tumor site and achieved excellent antitumor effects. | [190] |
| Reduction-responsive chemo-capsule-based prodrug | 10-hydroxy camptothecin and DOX | Two-in-one cross-linking strategy to prepare the stimuli-responsive prodrug by virtue of delivery chemotherapeutics | Drug released from the prodrug in response to the reduction in the tumor microenvironment, enhancing tumor growth inhibition. | [191] |
| Reduction-responsive PEG nanodrug | PTX | Self-assembly | Nanodrug was selectively disassociated in the intratumor reduction microenvironment via the reduction of disulfide bonds to release PTX, and excellent in vivo antitumor efficacy while avoiding side effects was observed. | [192] |
| Chitosan-lipoic acid reduction-responsive (CS-LANPs) | Curcumin | Self-assembly | Increased tumor accumulation with a better tumor inhibitory activity in vitro. | [193] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| Thermo- and pH-responsive copolymer hydrogels | Curcumin | Radical polymerization and swelling diffusion | Both temperature- and pH-responsive behavior with good biocompatibility. | [205] |
| Chitosan thermo-sensitive hydrogel | Gemcitabine hydrochloride, levofloxacin, 5-FU | Self-assembling | Precisely regulated the gelling time with potential for drug delivery and chemotherapy. | [206] |
| PNIPAM-b-PAzoMA | Iron oxide | RAFT radical polymerization | Shown excellent thermo-sensitivity and photosensitivity. | [207] |
| Thermo-responsive copolymer | siRNA | Chemical conjugation | The LCST of siRNA-conjugated thermo-copolymer was 38 °C with excellent cellular uptake and gene silencing. | [208] |
| Hypoxia-responsive polymer micelles (AA/ASP-AZO-Fc, AAAF) | Curcumin | Self-assembling | Hypoxia-responsive drug release with improved cellular uptake and the inhibition of the proliferation of HepG2 cells. | [209] |
| Hypoxia-responsive polymeric micelles | DOX | Self-assembly using PEG and poly-l-lysine copolymer with an azobenzene linker | High affinity to metastatic bones and response to hypoxia bone metastasis for rapid drug release with prolonged survival time. | [210] |
| Dual pH- and hypoxia-responsive copolymer prodrug micelles | TPZ | Self-assembling and conjugation | Higher cytotoxicity to hypoxic cancer cells. | [211] |
| Hypoxia-responsive nanocomplex | Double-stranded RNA | Hypoxia-cleavable polymer PEG-azo-PLL was synthesized and self-assembled into a nanocomplex | Significant in vivo antitumor effect with prolonged survival time. | [212] |
| Enzyme-responsive biodegradable targeted polymeric micelle | Cabazitaxel | Self-assembling | Enzyme-responsive peptides are cleavable with MMP-2. Higher cellular uptake and excellent in vivo antitumor efficacy. | [213] |
| Enzyme-responsive PEG peptides and star-shaped polyester NPs | Curcumin | Static electricity | MMP-responsive NPs showed higher drug loading, good biocompatibility, enhanced cellular uptake, and antitumor efficacy. | [214] |
| Enzyme-responsive NIR-triggered lipid polymer hybrid NPs | ICG and dichloroacetate | Chemical conjugation with self-assembling | Higher drug loading and prolonged blood circulation with synergistic photothermal/chemotherapy effects. | [215] |
| Hyaluronidase (HAase)- and GSH-responsive responsive nanogel | Cisplatin | Self-assembling | Stimuli-responsive nanogel possessed excellent drug- and protein-loading and intracellular delivery capabilities. | [216] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| HA-coated perfluoroalkyl polyamine NPs | siRNA | Bioactive polycationic prodrug (F-PaP) based on the anticancer polyamine analogue bisethylnorspermine modified with perfluoroalkyl moieties, following the encapsulation of siRNA, and then coated with HA | The HA-coated NPs facilitated tumor accumulation and contributed to strong tumor inhibition and the favorable modulation of the tumor immune microenvironment in an orthotopic pancreatic cancer model. | [229] |
| Tf-coated PLGA NPs | siRNA and DTX | DTX-conjugated PLGA copolymer and further modified with Tf peptides on the surface | Excellent antitumor effect. | [230] |
| Nano-PROTAC pH/GSH-responsive polymer | BRD4-targeted PROTAC (dBET6) | Self-assembly | Improved dBET6 release with remodeling of the tumor microenvironment. | [231] |
| RGD-decorated PLGA NPs | Cisplatin | Double emulsification method | Low systemic toxicity, high biocompatiblity, and safety, with promising anticancer effect. | [232] |
| GLUT1-targeted micelle | PTX and IR808 | Self-assembly | Higher cytotoxicity, apoptosis rate, and metastasis inhibition both in vitro and in vivo. | [223] |
| LAT1-targeted PEI NPs | PTX and anti P-gp shRNA | Self-assembly and electrostatic interaction | Targeted delivery into the cells overexpressing the LAT-1 transporter. | [233] |
| Mitochondria-targeting polymer micelle (OPDEA-PDCA) | Dichloroacetate (DCA) | RAFT polymerization and self-assembly | Induced the secretion of PD-L1 and enhanced antitumor efficacy in combination with immunotherapy. | [234] |
| Antibody polymer conjugates (ADCs) | Antibody | Linker conjugation and drug conjugation | Exhibited cell targetability and selective cell killing in multiple cell lines expressing disease-relevant antigens, viz, HER2 and EGFR. | [235] |
| Delivery System | Drug | Methods | Inference | Ref. |
|---|---|---|---|---|
| ROS-responsive F-PEI and coated with HA | Plasmids | Thioketal-cross-linked F-PEI was further coated with HA for the delivery of plasmids | Improved the release of plasmids, reduced toxicity, and enhances the antitumor efficacy. | [241] |
| Fluoropolymer | Ovalbumin (OVA) | A novel fluoropolymer was developed via ring-opening polymerization and construct a fluoropolymer-based nanovaccine | Better efficacy in both pre-cancer prevention and tumor treatment and increased the proportion of CD4+ T and CD8+ T cells. | [246] |
| Fluorocarbon-modified chitosan (FCS) | Anti-programed cell death protein-1 (aPD-1) | Therapeutic proteins were mixed with FCS to form NPs, lyophilized with the appropriate excipients, and then filled into enteric capsules for oral administration | Promoted the transmucosal delivery and 5-fold dose oral delivery of a-PD1. | [247] |
| Fluorinated zwitterionic polymer-coated DNA nanoclews (FNCs) | DNA | Sequence-specific binding and coated with fluorinated zwitterionic polymer | Loaded by FNCs, an oligonucleotide can effectively silence the target miRNA when activated by NIR light and inhibit angiogenesis inside the tumor, leading to the complete ablation of the cancer. | [248] |
| Fluorinated PEG-PEI-coated magnetic NPs | siRNA | Heptafluorobutyryl-PEG-PEI (FPP) was first prepared and then used to coat magnetic nanoparticles (MNPs) to obtain the magnetic nanocarriers FPP@MNPs | Cellular uptake was significantly increased, and the transfection ability was increased to reach more than 90%. | [249,250] |
| Fluorinated gemini amphiphilic polymer (G-Fn) | CPT | Self-assembly | Excellent physical and chemical properties as well as good therapeutics. | [242] |
| Fluorinated covalent conjugate polymers | Sonosensitizer | Synthesized | Multifunctional nano-sonosensitizers with suppressing tumor growth ability and tumor recurrence by priming the host’s antitumor immunity. | [245] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Agrawal, P.; Singh, S.K.; Chhonker, Y.S.; Sun, J.; Murry, D.J. Polymer-Based Drug Delivery Systems for Cancer Therapeutics. Polymers 2024, 16, 843. https://doi.org/10.3390/polym16060843
Ding L, Agrawal P, Singh SK, Chhonker YS, Sun J, Murry DJ. Polymer-Based Drug Delivery Systems for Cancer Therapeutics. Polymers. 2024; 16(6):843. https://doi.org/10.3390/polym16060843
Chicago/Turabian StyleDing, Ling, Prachi Agrawal, Sandeep K. Singh, Yashpal S. Chhonker, Jingjing Sun, and Daryl J. Murry. 2024. "Polymer-Based Drug Delivery Systems for Cancer Therapeutics" Polymers 16, no. 6: 843. https://doi.org/10.3390/polym16060843