Chitosan-Enhanced pH-Sensitive Anthocyanin Indicator Film for the Accurate Monitoring of Mutton Freshness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Chitosan–BA Indicator Films
2.3. Color Measurement
2.4. pH Sensitivity
2.5. Ammonia Sensitivity
2.6. Characterisation of the Prepared Indicator Film
2.6.1. Thickness
2.6.2. Moisture Content
2.6.3. Water Solubility
2.6.4. Water Vapor Barrier Property
2.6.5. Mechanical Properties
2.6.6. Color Stability
2.6.7. Fourier Transformation Infrared Spectroscopy (FT-IR) Analysis
2.6.8. X-ray Diffraction (XRD) Analysis
2.7. Application of the Prepared Indicator Films for Monitoring Mutton Freshness
2.7.1. Fresh Mutton Spoilage Trial
2.7.2. TVB-N Analysis
2.7.3. TVC Analysis
2.8. Statistical Analysis
3. Results and Discussion
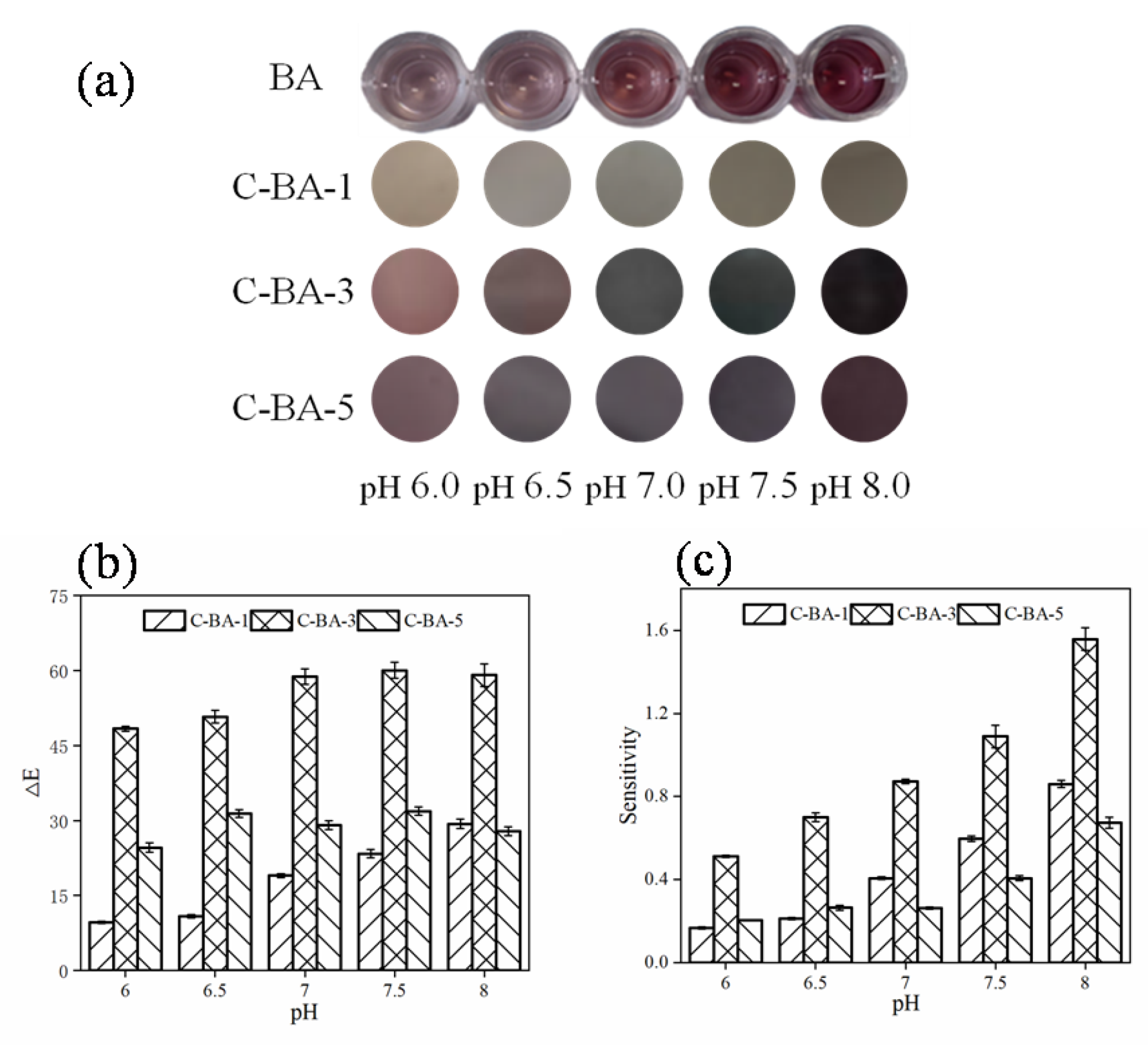
3.1. Determination of the BA Content in the C-BA Indicator Films
3.1.1. Ammonia Sensitivity
3.1.2. pH Sensitivity
3.2. Characterization of the C-BA-3 Indicator Film
3.2.1. Thickness, Moisture Content, and Water VAPOR Permeability Analysis
3.2.2. Mechanical Property Analysis
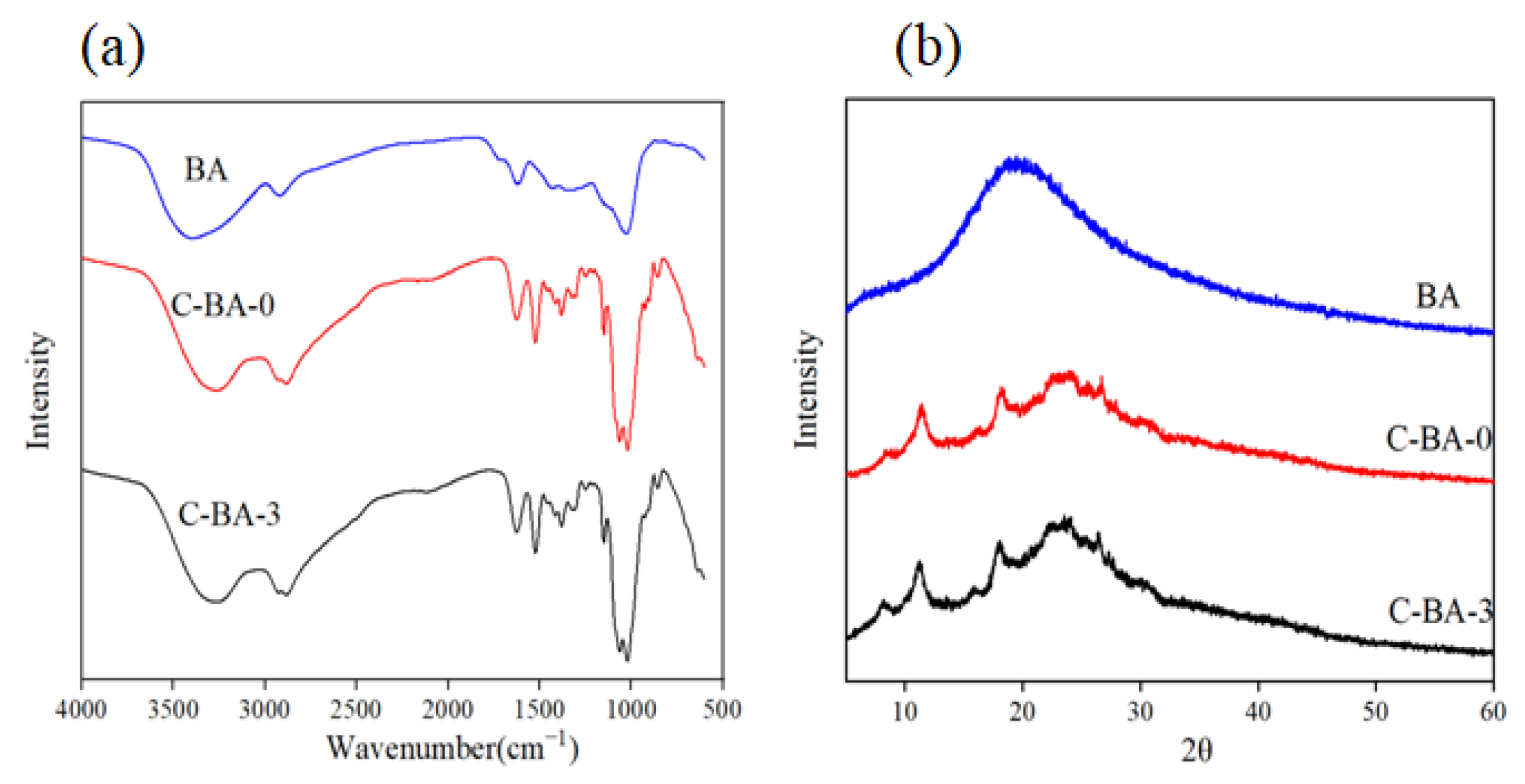
3.2.3. FT-IR Analysis
3.2.4. XRD Analysis
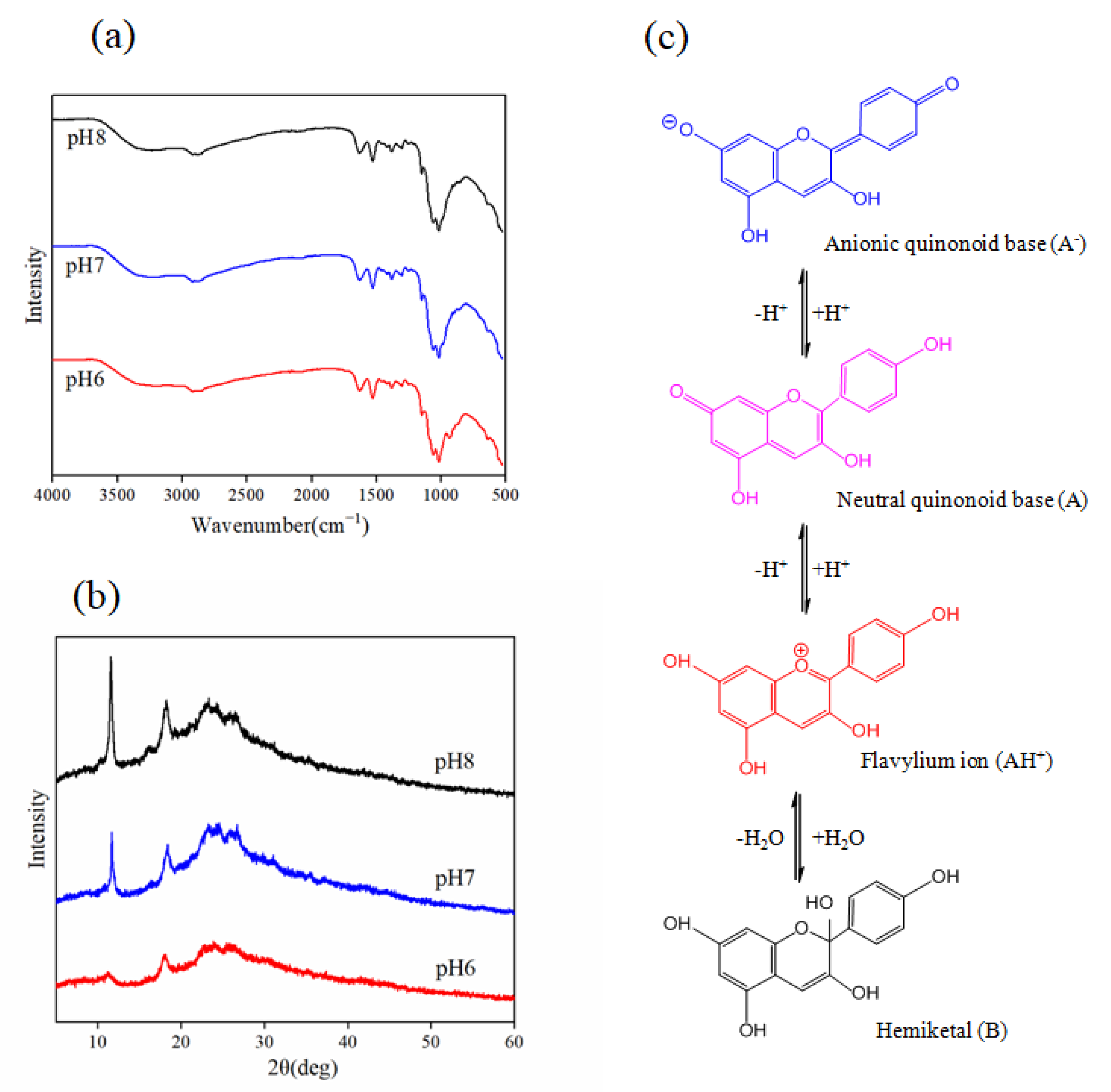
3.2.5. Possible Mechanism Underlying the Color Response Sensitivity Enhancement of Anthocyanin in the C-BA-3 Indicator Film
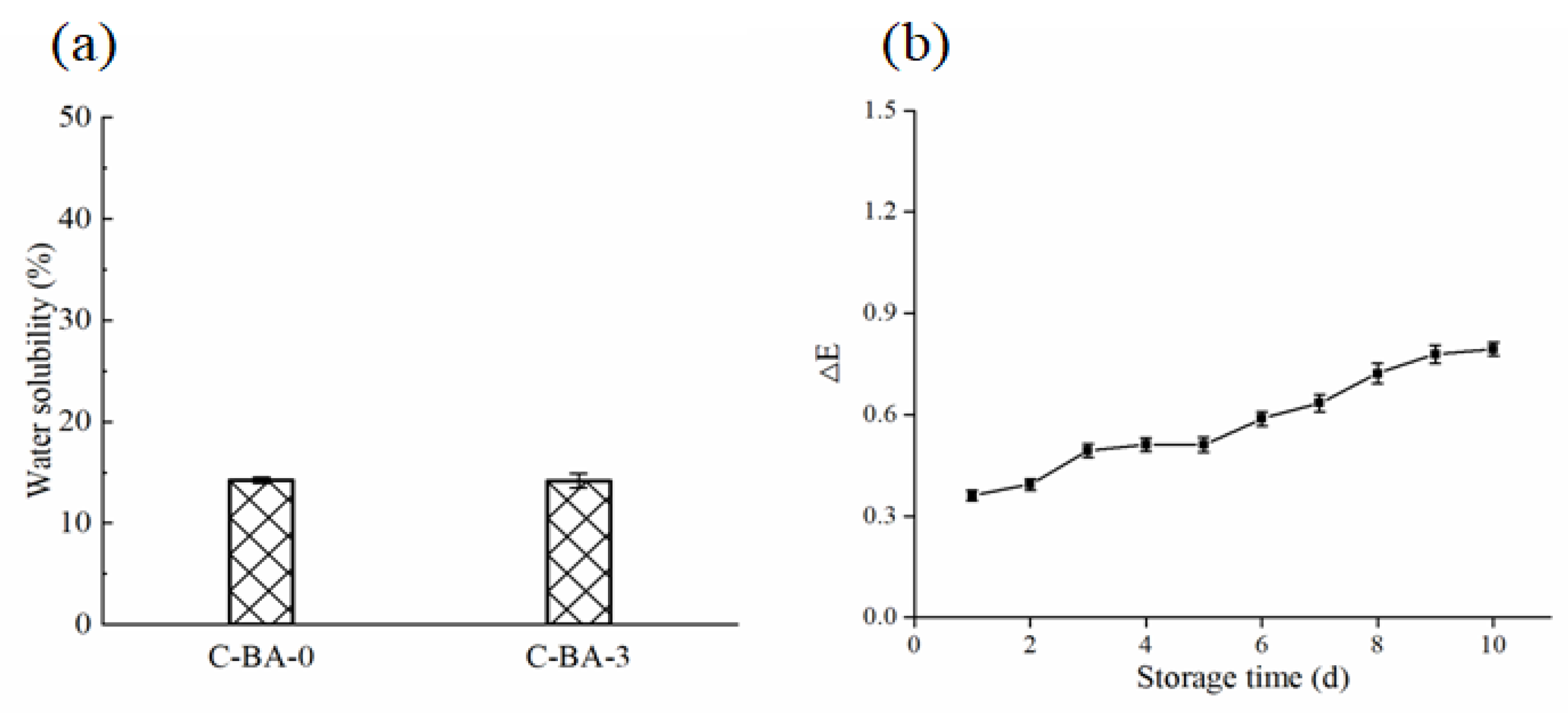
3.2.6. Water Solubility
3.2.7. Color Stability of the C-BA-3 Indicator Film
3.3. Application of the C-BA-3 Indicator Film in Monitoring Mutton Meat Freshness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, X.N.; Che, Y.X.; Qu, W.J.; Zhang, Y.M.; Lin, H.Q.; Wei, T.B. Design and fabricating biogenic amine-responsive platform based on self-assembly property of phenazine derivative for visual monitoring of meat spoilage. Sens. Actuators B Chem. 2021, 333, 129430. [Google Scholar] [CrossRef]
- Kanha, N.; Osiriphun, S.; Rakariyatham, K.; Klangpetch, W.; Laokuldilok, T. On-package indicator films based on natural pigments and polysaccharides for monitoring food quality: A review. J. Sci. Food Agric. 2022, 102, 6804–6823. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Ezati, P.; Rhim, J.W. Recent advances in intelligent food packaging applications using natural food colorants. ACS Food Sci. Technol. 2021, 1, 124–138. [Google Scholar] [CrossRef]
- Neves, D.; Andrade, P.B.; Videira, R.A.; de Freitas, V.; Cruz, L. Anthocyanin-based films in smart food packaging: A mini-review. Food Hydrocoll. 2022, 133, 107885. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.R.; Zhang, Y.H.; Wang, X.P.; Wang, J.Y.; Ning, Z.X.; Ruan, Q.J.; Zhang, Y.S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Yoon, H.J.; Lee, G.; Kim, J.T.; Yoo, J.Y.; Luan, H.; Cheng, S.; Kang, S.; Huynh, H.L.T.; Kim, H.; Park, J.; et al. Biodegradable, three-dimensional colorimetric fliers for environmental monitoring. Sci. Adv. 2022, 8, eade3201. [Google Scholar] [CrossRef]
- Bao, Y.; Cui, H.; Tian, J.; Ding, Y.; Tian, Q.; Zhang, W.; Wang, M.; Zang, Z.; Sun, X.; Li, D.; et al. Novel pH sensitivity and colorimetry-enhanced anthocyanin indicator films by chondroitin sulfate co-pigmentation for shrimp freshness monitoring. Food Control 2022, 131, 108441. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zou, X.; Shi, J.; Zhai, X.; Liu, L.; Li, Z.; Holmes, M.; Gong, Y.; Povey, M.; et al. A visual indicator based on curcumin with high stability for monitoring the freshness of freshwater shrimp, Macrobrachium rosenbergii. J. Food Eng. 2020, 292, 110290. [Google Scholar] [CrossRef]
- Kang, S.; Wang, H.; Xia, L.; Chen, M.; Li, L.; Cheng, J.; Li, X.; Jiang, S. Colorimetric film based on polyvinyl alcohol/okra mucilage polysaccharide incorporated with rose anthocyanins for shrimp freshness monitoring. Carbohydr. Polym. 2020, 229, 115402. [Google Scholar] [CrossRef]
- GB 5009.228–2016; National Food Safety Standard, Determination of Volatile Base Nitrogen in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 4789.2–2016; National Standard for Food Safety Food Microbiology Examination Aerobic Plate Count. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Sadeghi, K.; Yoon, J.Y.; Seo, J. Chromogenic polymers and their packaging applications: A review. Polym. Rev. 2020, 60, 442–492. [Google Scholar] [CrossRef]
- Tan, C.; Celli, G.B.; Abbaspourrad, A. Copigment-polyelectrolyte complexes (PECs) composite systems for anthocyanin stabilization. Food Hydrocoll. 2018, 81, 371–379. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; He, X.; Duan, C.; He, F. Acetylation of Malvidin-3-O-glucoside impedes intermolecular copigmentation: Experimental and theoretical investigations. J. Agric. Food Chem. 2021, 69, 7733–7741. [Google Scholar] [CrossRef]
- Aljawish, A.; Muniglia, L.; Klouj, A.; Jasniewski, J.; Scher, J.; Desobry, S. Characterization of fflms based on enzymatically modiffed chitosan derivatives with phenol compounds. Food Hydrocoll. 2016, 60, 551–558. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT–Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Yang, K.; Dang, H.; Liu, L.; Hu, X.; Li, X.; Ma, Z.; Wang, X.; Ren, T. Effect of syringic acid incorporation on the physical, mechanical, structural and antibacterial properties of chitosan film for quail eggs preservation. Int. J. Biol. Macromol. 2019, 141, 876–884. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ling, Z.; Zhang, X.; Zhang, X.; Ramaswamy, S.; Xu, F. Smart colorimetric sensing films with high mechanical strength and hydrophobic properties for visual monitoring of shrimp and pork freshness. Sens. Actuators B Chem. 2020, 309, 127752. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.G.; Liu, S.; Kan, J.; Jin, C. Preparation and characterization of protocatechuic acid grafted chitosan fflms with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- GB 9961–2008; Fresh and Frozen Mutton Carcass. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008.
- Ma, Y.; Du, P.; Wang, S.; Hu, P.; Han, Q.; Wen, L.; Liu, Y.; Wang, W. Construction of freshness evaluation model for mutton at 4 °C storage. Food Sci. Technol. 2023, 48, 100–105. [Google Scholar] [CrossRef]


| Film | C-BA-1 | C-BA-3 | C-BA-5 |
|---|---|---|---|
| photograph |  |  |  |
| L* | 65.68 ± 0.94 c | 41.46 ± 0.11 a | 49.43 ± 0.24 b |
| a* | 6.17 ± 0.25 a | 35.53 ± 0.05 c | 19.88 ± 0.18 b |
| b* | 0.63 ± 0.028 b | 5.23 ± 0.22 c | −3.96 ± 0.02 a |
| Film | Thickness | Moisture Content | Water Vapor Permeability | Tensile Strength | Elongation at Break |
|---|---|---|---|---|---|
| μm | % | 10−11 g m−1 s −1 Pa−1 | Mpa | % | |
| C-BA-0 | 100.00 ± 1.25 | 28.59 ± 2.16 | 16.29 ± 0.23 | 16.20 ± 0.56 | 34.83 ± 1.00 |
| C-BA-3 | 100.10 ± 1.13 | 27.67 ± 2.55 | 15.97 ± 0.51 | 19.03 ± 0.25 | 25.37 ± 1.12 |
| Storage Time (Day) | TVB-N (mg/100 g) | TVC (lg (CFU/g)) | ΔE |
|---|---|---|---|
| 0 | 8.82 ± 0.18 | 3.60 ± 0.01 | — |
| 1 | 8.91 ± 0.30 | 3.68 ± 0.01 | 10.72 ± 0.31 |
| 2 | 10.24 ± 0.62 | 3.98 ± 0.02 | 19.72 ± 0.42 |
| 3 | 10.26 ± 0.58 | 5.04 ± 0.08 | 20.69 ± 0.23 |
| 4 | 11.16 ± 0.36 | 5.48 ± 0.02 | 29.22 ± 0.34 |
| 5 | 12.09 ± 0.10 | 5.62 ± 0.02 | 32.71 ± 0.47 |
| 6 | 14.34 ± 0.70 | 5.70 ± 0.02 | 33.82 ± 0.5 |
| 7 | 15.53 ± 0.37 | 6.94 ± 0.02 | 51.79 ± 0.11 |
| TVB-N | TVC | ΔE | |
|---|---|---|---|
| TVB-N | 1 | ||
| TVC | 0.713 ** | 1 | |
| ΔE | 0.921 ** | 0.731 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wen, L.; Liu, Y.; Du, P.; Hu, P.; Cao, J.; Wang, W. Chitosan-Enhanced pH-Sensitive Anthocyanin Indicator Film for the Accurate Monitoring of Mutton Freshness. Polymers 2024, 16, 849. https://doi.org/10.3390/polym16060849
Ma Y, Wen L, Liu Y, Du P, Hu P, Cao J, Wang W. Chitosan-Enhanced pH-Sensitive Anthocyanin Indicator Film for the Accurate Monitoring of Mutton Freshness. Polymers. 2024; 16(6):849. https://doi.org/10.3390/polym16060849
Chicago/Turabian StyleMa, Yanli, Lei Wen, Yaobo Liu, Pengfei Du, Peng Hu, Jianfang Cao, and Weiting Wang. 2024. "Chitosan-Enhanced pH-Sensitive Anthocyanin Indicator Film for the Accurate Monitoring of Mutton Freshness" Polymers 16, no. 6: 849. https://doi.org/10.3390/polym16060849






