Study on the Effect of Residual Polymer Superplasticizer on the Properties of Graphene–Cement Composites
Abstract
:1. Introduction
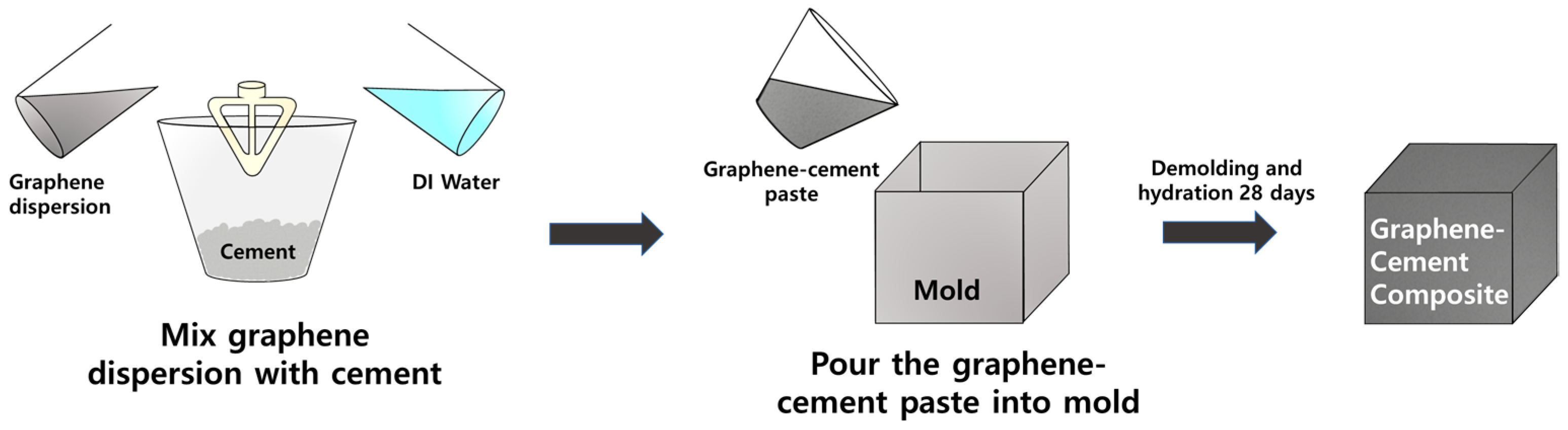
2. Experimental Section
2.1. Materials
2.2. Dispersions of Graphene/PCE
2.3. Contact Angle Measurement
2.4. Compressive Strength Measurement
2.5. Microstructure Analysis
3. Results and Discussion
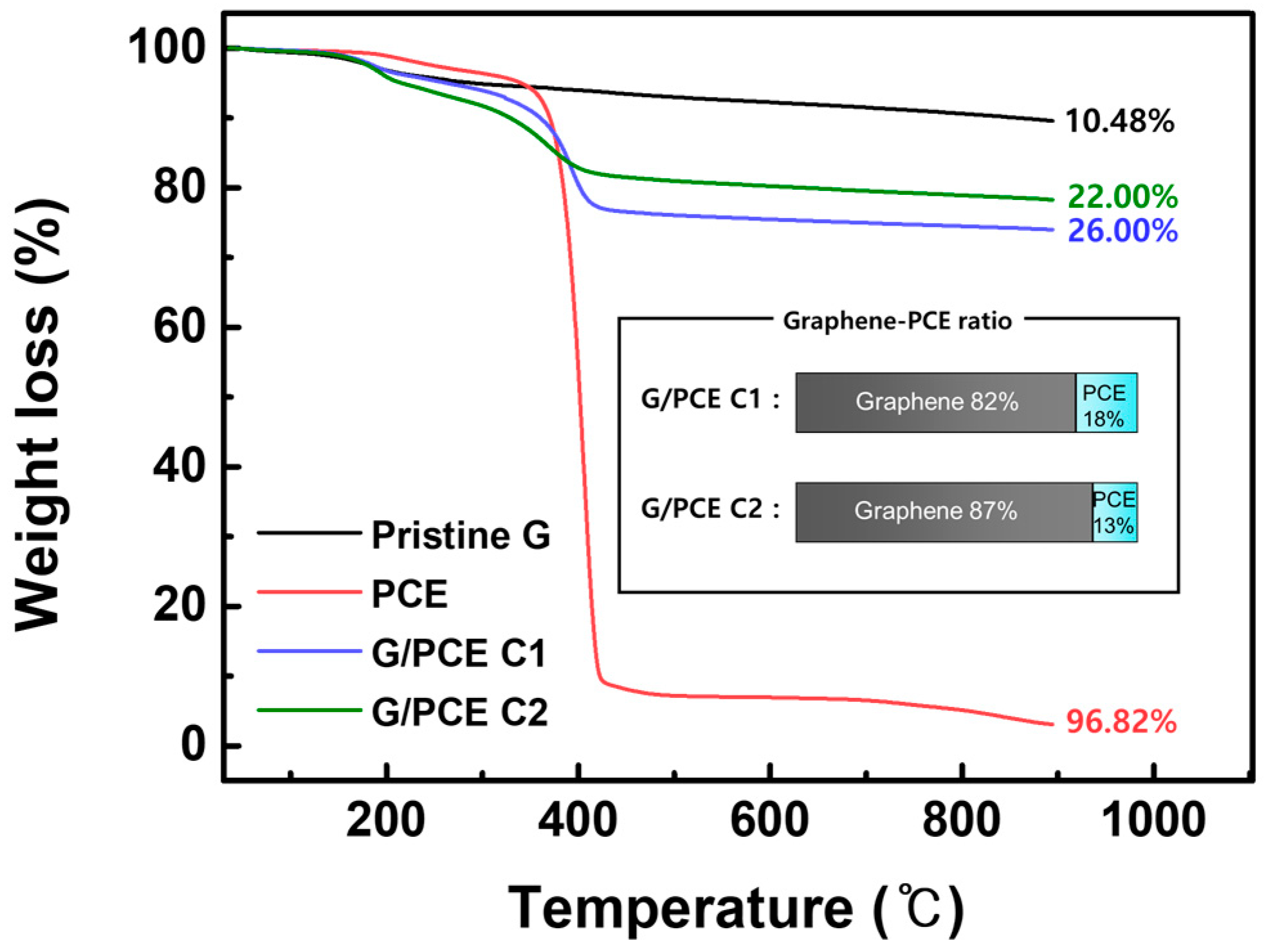
3.1. Thermogravimetric Analysis
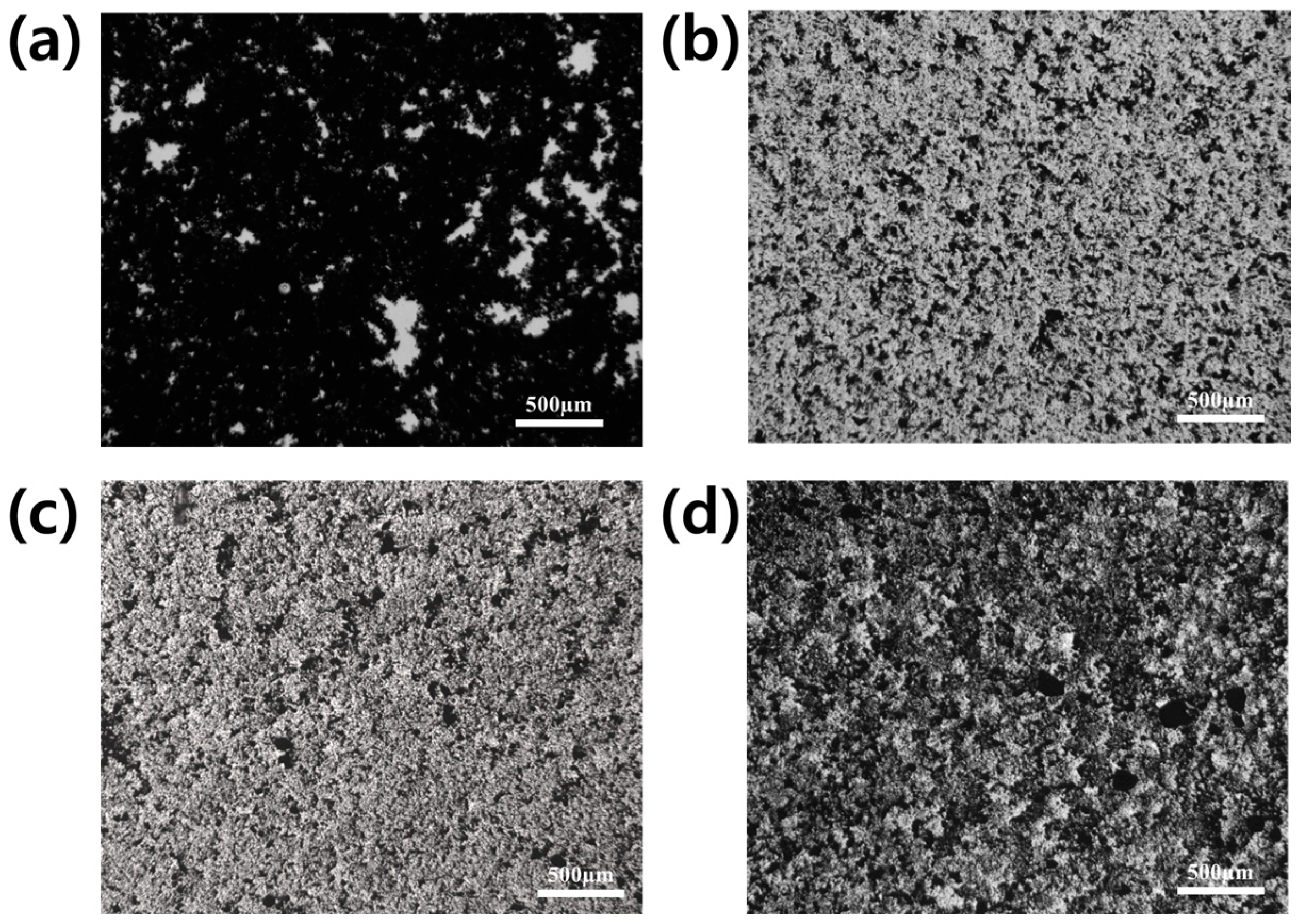
3.2. Optical Microscope Analysis
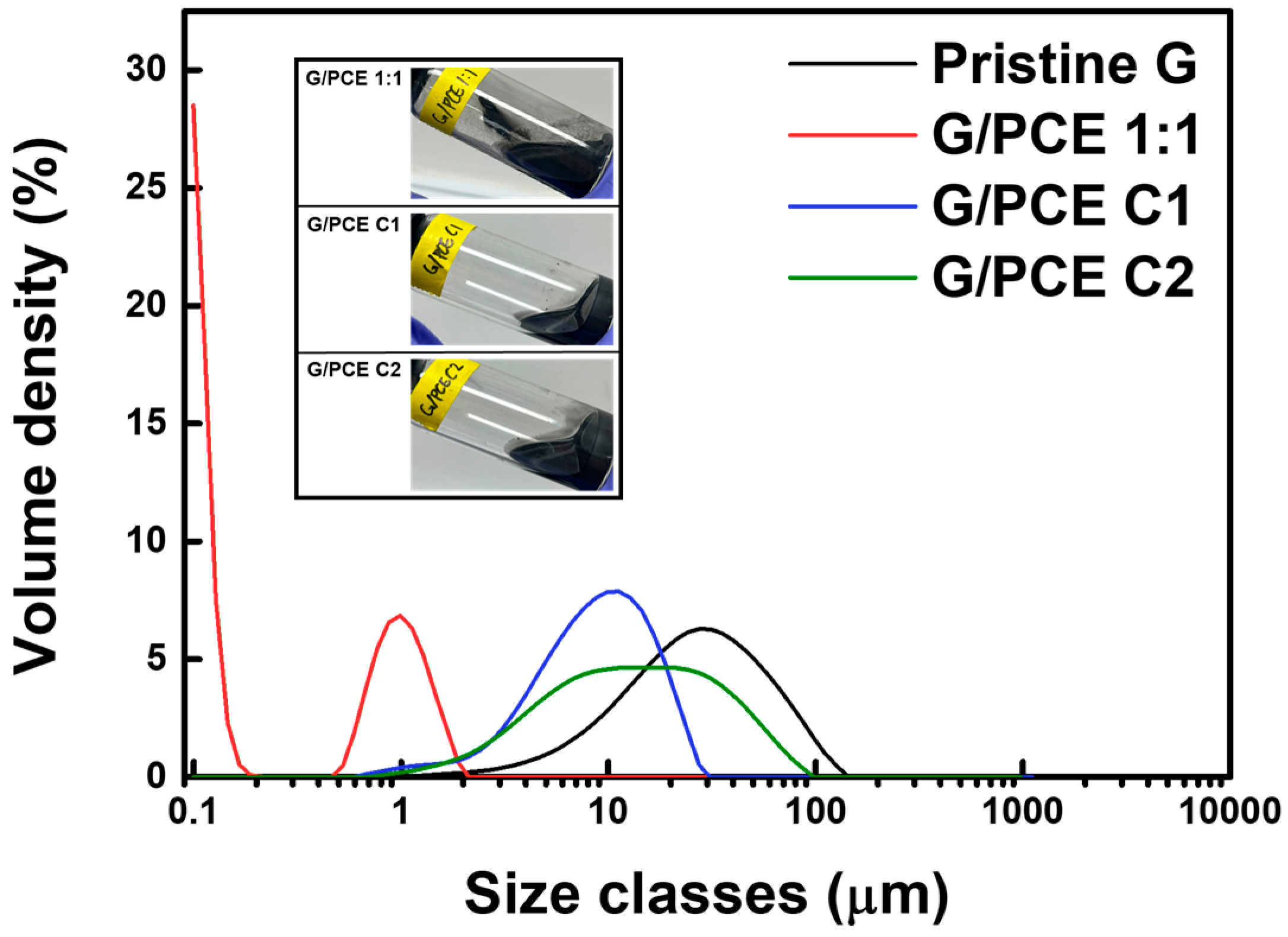
3.3. Particle Size Analysis
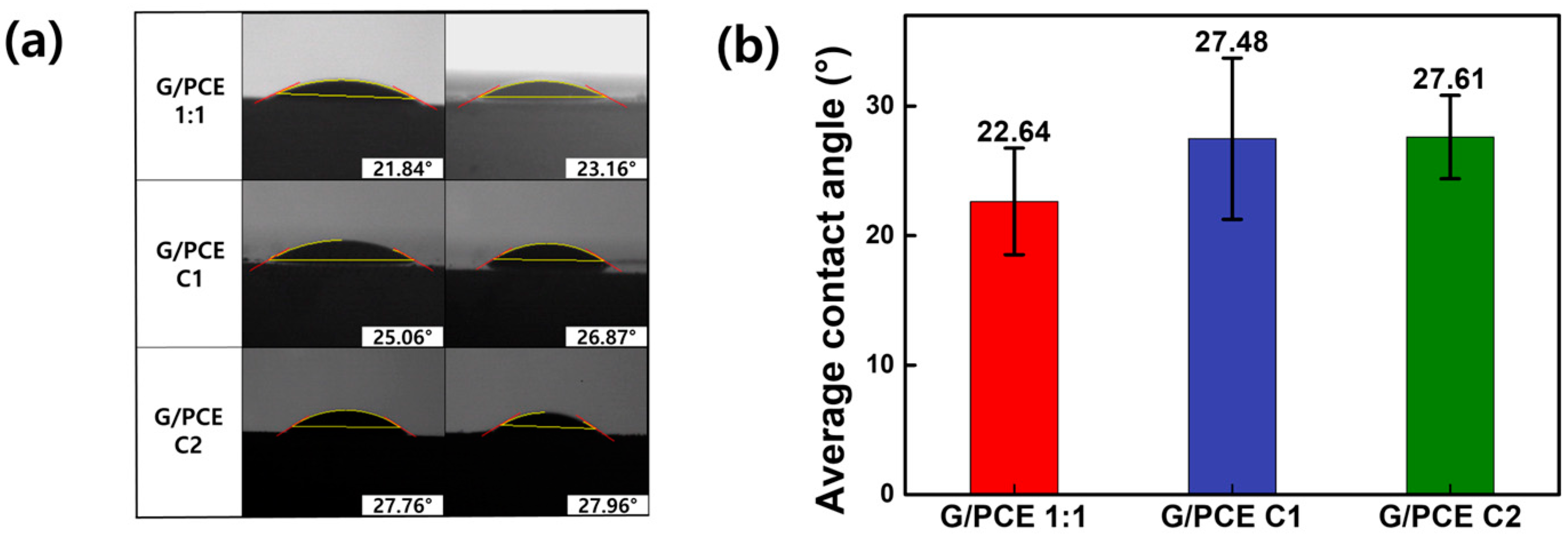
3.4. Contact Angle Measurements
3.5. Compressive Strength Test
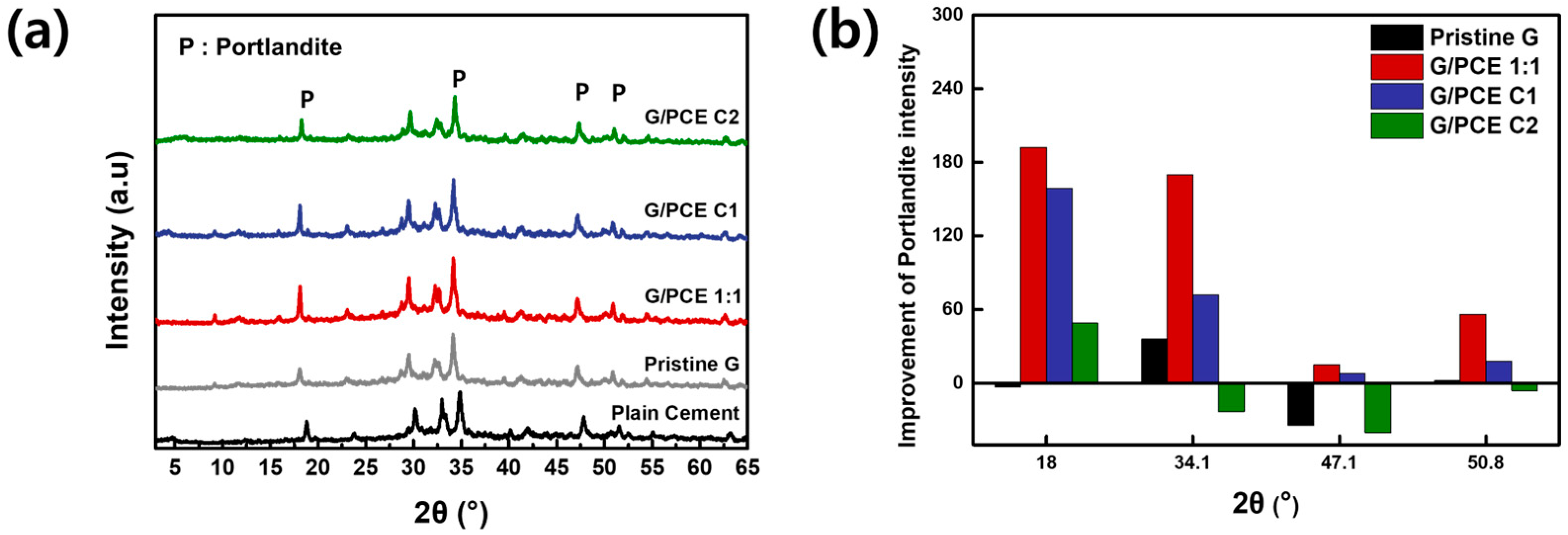
3.6. XRD Analysis of GCC
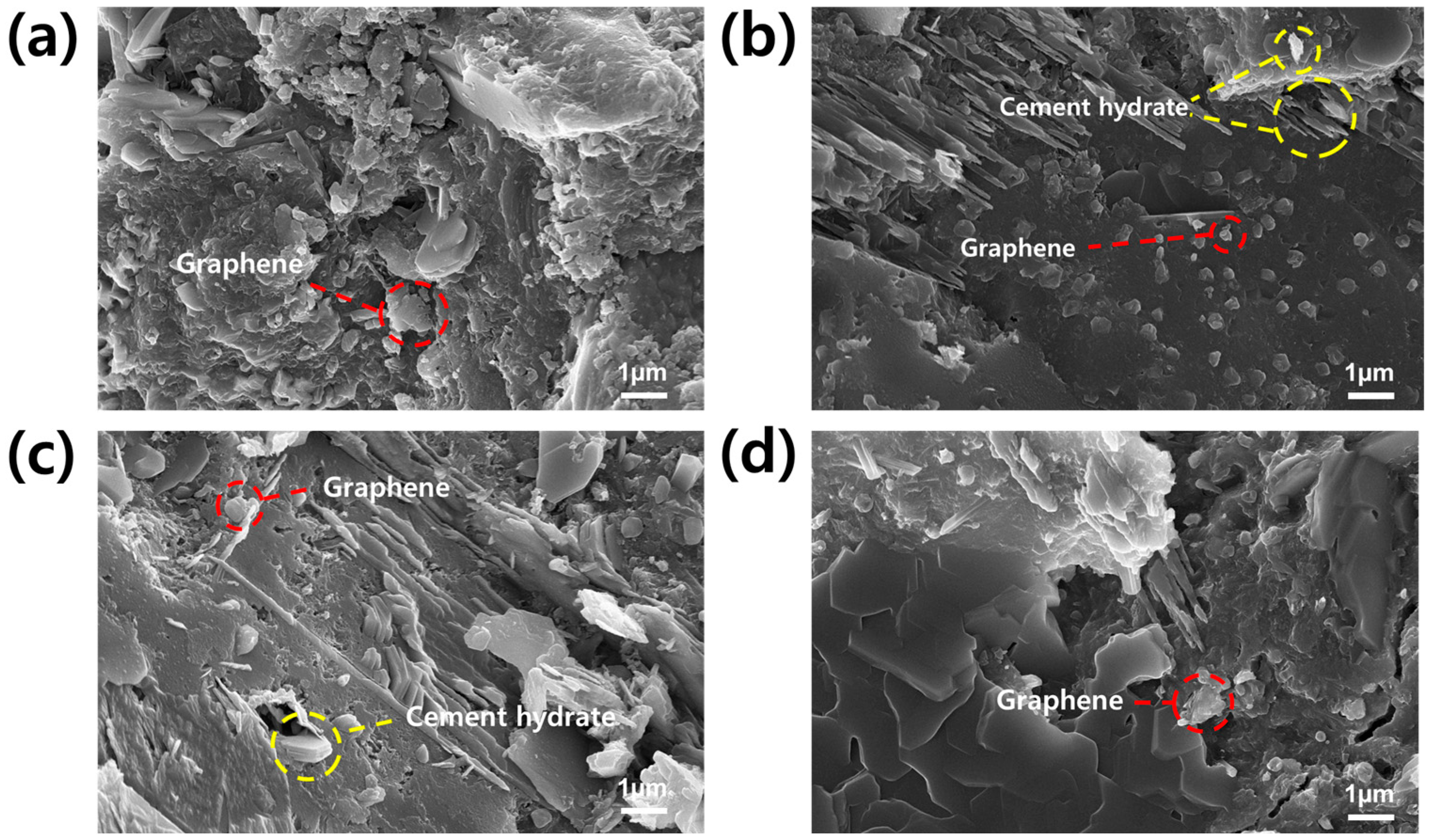
3.7. FE-SEM Analysis of GCC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Du, H. Graphene reinforced cement composites: A review. Constr. Build. Mater. 2020, 265, 120312. [Google Scholar] [CrossRef]
- Langan, B.W.; Weng, K.; Ward, M.A. Effect of silica fume and fly ash on heat of hydration of Portland cement. Cem. Concr. Res. 2002, 32, 1045–1051. [Google Scholar] [CrossRef]
- Senff, L.; Labrincha, J.A.; Ferreira, V.M.; Hotza, D.; Repette, W.L. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Constr. Build. Mater. 2009, 23, 2487–2491. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide—Cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, Y.; Dai, Z.; Corr, D.J.; Shah, S.P. Effect of interfacial transition zone on the Young’s modulus of carbon nanofiber reinforced cement concrete. Cem. Concr. Res. 2018, 107, 49–63. [Google Scholar] [CrossRef]
- Nochaiya, T.; Chaipanich, A. Behavior of multi-walled carbon nanotubes on the porosity and microstructure of cement-based materials. Appl. Surf. Sci. 2011, 257, 1941–1945. [Google Scholar] [CrossRef]
- Gholampour, A.; Kiamahalleh, M.V.; Tran, D.N.; Ozbakkaloglu, T. From graphene oxide to reduced graphene oxide: Impact on the physiochemical and mechanical properties of graphene–cement composites. ACS Appl. Mater. Interfaces 2017, 9, 43275–43286. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J. Hydration, reinforcing mechanism, and macro performance of multi-layer graphene-modified cement composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.; Koratkar, N. Enhanced mechanical properties of nanocomposites at low graphene content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Azpeitia, J.; Otero-Irurueta, G.; Palacio, I.; Martinez, J.I.; del Árbol, N.R.; Santoro, G.; Gutiérrez, A.; Aballe, L.; Foerster, M.; Kalbac, M.; et al. High-quality PVD graphene growth by fullerene decomposition on Cu foils. Carbon 2017, 119, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Narula, U.; Tan, C.M.; Lai, C.S. Growth mechanism for low temperature PVD graphene synthesis on copper using amorphous carbon. Sci. Rep. 2017, 7, 44112. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G. Graphene CVD growth on copper and nickel: Role of hydrogen in kinetics and structure. Phys. Chem. Chem. Phys. 2011, 13, 20836–20843. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Fu, L.; Peng, H.; Liu, Z. Designed CVD growth of graphene via process engineering. Acc. Chem. Res. 2013, 46, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Baomin, W.; Shuang, D. Effect and mechanism of graphene nanoplatelets on hydration reaction, mechanical properties and microstructure of cement composites. Constr. Build. Mater. 2019, 228, 116720. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, R.; Wu, Z. Investigation of the mechanical properties and microstructure of graphene nanoplatelet-cement composite. Nanomaterials 2016, 61, 200. [Google Scholar] [CrossRef]
- Qureshi, T.S.; Panesar, D.K. Nano reinforced cement paste composite with functionalized graphene and pristine graphene nanoplatelets. Compos. B Eng. 2020, 197, 108063. [Google Scholar] [CrossRef]
- Qureshi, T.S.; Panesar, D.K.; Sidhureddy, B.; Chen, A.; Wood, P.C. Nano-cement composite with graphene oxide produced from epigenetic graphite deposit. Compos. B Eng. 2019, 159, 248–258. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Boutsioukou, S.; Amenta, M. Dispersion of Multi-Walled Carbon Nanotubes into White Cement Mortars: The Effect of Concentration and Surfactants. Nanomaterials 2022, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, I.; de Souza, L.R.; Litina, C.; Al-Tabbaa, A. Investigation of the dispersion of multi-layer graphene nanoplatelets in cement composites using different superplasticiser treatments. Constr. Build. Mater. 2021, 293, 123543. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Kong, X.M.; Lu, Z.B.; Lu, Z.C.; Hou, S.S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Liebscher, M.; Lange, A.; Schröfl, C.; Fuge, R.; Mechtcherine, V.; Plank, J.; Leonhardt, A. Impact of the molecular architecture of polycarboxylate superplasticizers on the dispersion of multi-walled carbon nanotubes in aqueous phase. J. Mater. Sci. 2017, 52, 2296–2307. [Google Scholar] [CrossRef]
- An, S.H.; Kim, K.Y.; Chung, C.W.; Lee, J.U. Development of cement nanocomposites reinforced by carbon nanotube dispersion using superplasticizers. Carbon Lett. 2024. [Google Scholar] [CrossRef]
- Wang, Q.; Zhan, D.F.; Qi, G.D.; Wang, Y.; Zheng, H.Y. Impact of the microstructure of polycarboxylate superplasticizers on the dispersion of graphene. New Carbon Mater. 2020, 35, 547–558. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P. Handbook of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2008; Volume 2, pp. 738–765. [Google Scholar]
- Wang, Y.; Shu, X.; Yang, Y.; Ran, Q.; Liu, J. Polymer physics and PCE superplasticizers revisited. In Proceedings of the 12th International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Beijing, China, 28–31 October 2018; Volume 1. [Google Scholar]
- Sun, J.; Shi, H.; Qian, B.; Xu, Z.; Li, W.; Shen, X. Effects of synthetic CSH/PCE nanocomposites on early cement hydration. Constr. Build. Mater. 2017, 140, 282–292. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yang, S.M.; Lee, J.U.; Kim, J.H.; Yang, S.J. Magnetic alignment of electrochemically exfoliated graphite in epoxy as a thermal interface material with high through-plane thermal conductivity. Carbon Lett. 2022, 32, 1433–1439. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, L.; Xu, N.; Jin, M.; Jiang, S. Enhancement of mechanical and electrical properties of graphene/cement composite due to improved dispersion of graphene by addition of silica fume. Constr. Build. Mater. 2018, 164, 433–441. [Google Scholar] [CrossRef]
- Lootens, D.; Bentz, D.P. On the relation of setting and early-age strength development to porosity and hydration in cement-based materials. Cem. Concr. Res. 2016, 68, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Khatib, J.M.; Jones, A. Relative strength, pozzolanic activity and cement hydration in superplasticised metakaolin concrete. Cem. Concr. Res. 1996, 26, 1537–1544. [Google Scholar] [CrossRef]
- Wang, F.; Kong, X.; Jiang, L.; Wang, D. The acceleration mechanism of nano-CSH particles on OPC hydration. Constr. Build. Mater. 2020, 249, 118734. [Google Scholar] [CrossRef]


| W/C (%) | Graphene Content (wt.% to Cement) | Cement (g) | D.I. Water (g) | Water in Graphene Dispersion (g) | Addition of D.I. Water (g) | Graphene (g) | PCE (g) | |
|---|---|---|---|---|---|---|---|---|
| Plain | 0.3 | - | 1400 | 420 | - | - | - | - |
| Pristine graphene | 0.3 | 0.01 | 1400 | 420 | 13.86 | 406.14 | 0.14 | - |
| G/PCE 1:1 | 0.3 | 0.01 | 1400 | 420 | 13.86 | 406.14 | 0.14 | 0.14 |
| G/PCE C1 | 0.3 | 0.01 | 1400 | 420 | 13.86 | 406.14 | 0.14 | 0.03 |
| G/PCE C2 | 0.3 | 0.01 | 1400 | 420 | 13.86 | 406.14 | 0.14 | 0.02 |
| Specific Surface Area (m2/kg) | D (3,2) (μm) | Dv (50) (μm) | |
|---|---|---|---|
| Pristine Graphene | 333.1 | 18.0 | 28.2 |
| G/PCE 1:1 | 32,110 | 0.187 | 0.136 |
| G/PCE C1 | 908 | 6.61 | 9.42 |
| G/PCE C2 | 701 | 8.56 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.Y.; An, S.H.; Lee, J.U. Study on the Effect of Residual Polymer Superplasticizer on the Properties of Graphene–Cement Composites. Polymers 2024, 16, 956. https://doi.org/10.3390/polym16070956
Kim KY, An SH, Lee JU. Study on the Effect of Residual Polymer Superplasticizer on the Properties of Graphene–Cement Composites. Polymers. 2024; 16(7):956. https://doi.org/10.3390/polym16070956
Chicago/Turabian StyleKim, Ki Yun, Seok Hwan An, and Jea Uk Lee. 2024. "Study on the Effect of Residual Polymer Superplasticizer on the Properties of Graphene–Cement Composites" Polymers 16, no. 7: 956. https://doi.org/10.3390/polym16070956





