Characterization of Biodegradable Polymers for Porous Structure: Further Steps toward Sustainable Plastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Characterization
2.2. Thermal Characterization
2.3. Mechanical Characterization
2.4. Porous Polymer Processing and Analysis
3. Results
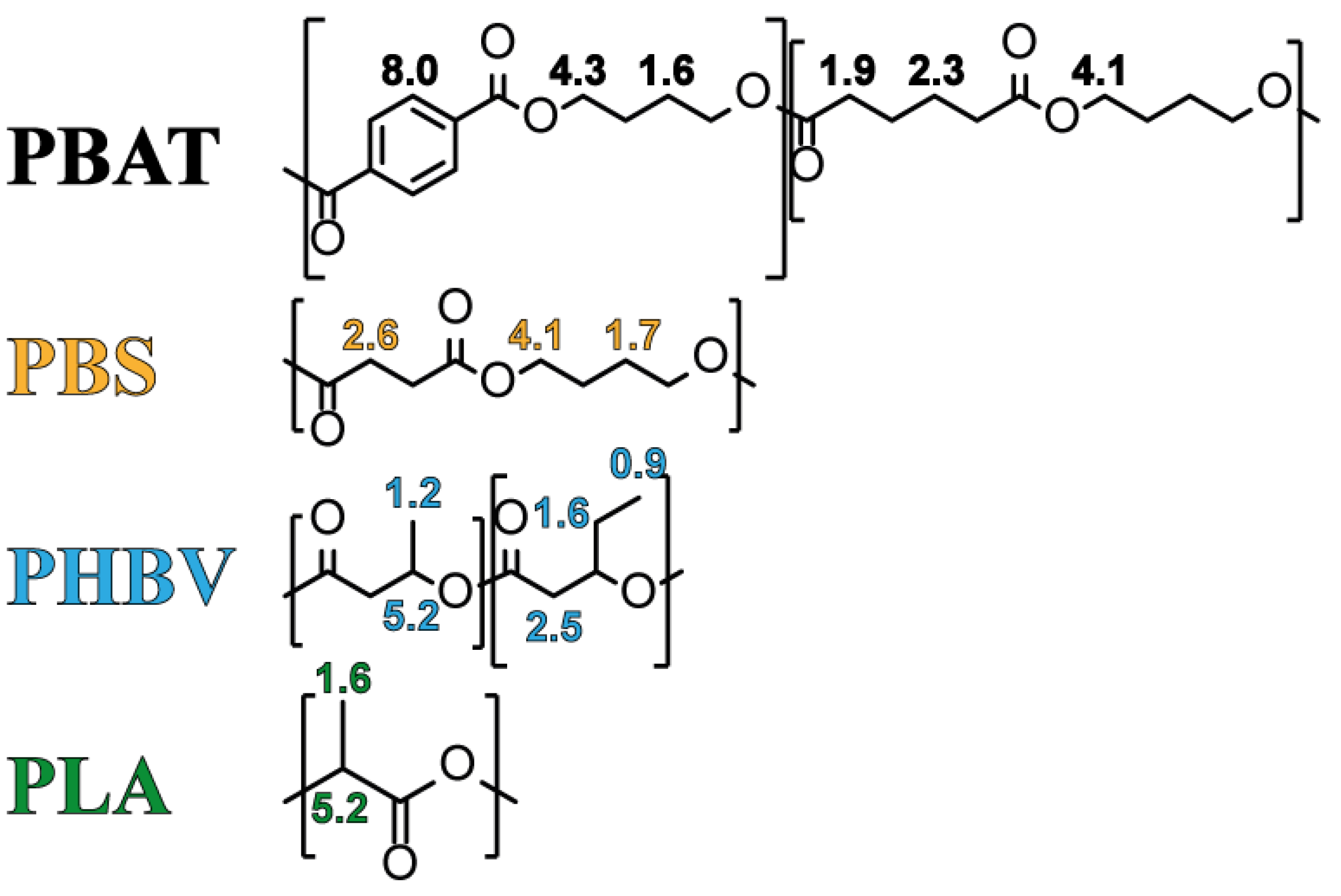
3.1. Chemical Characterization
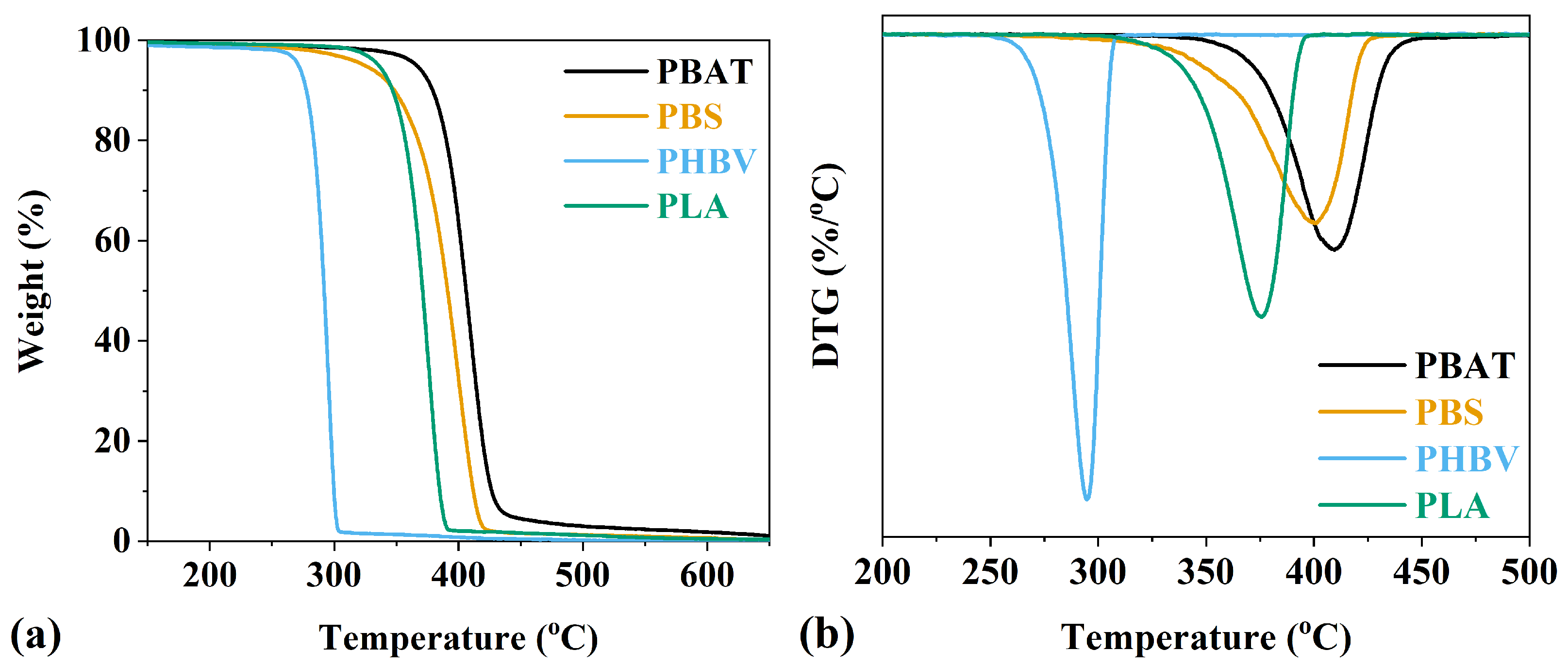
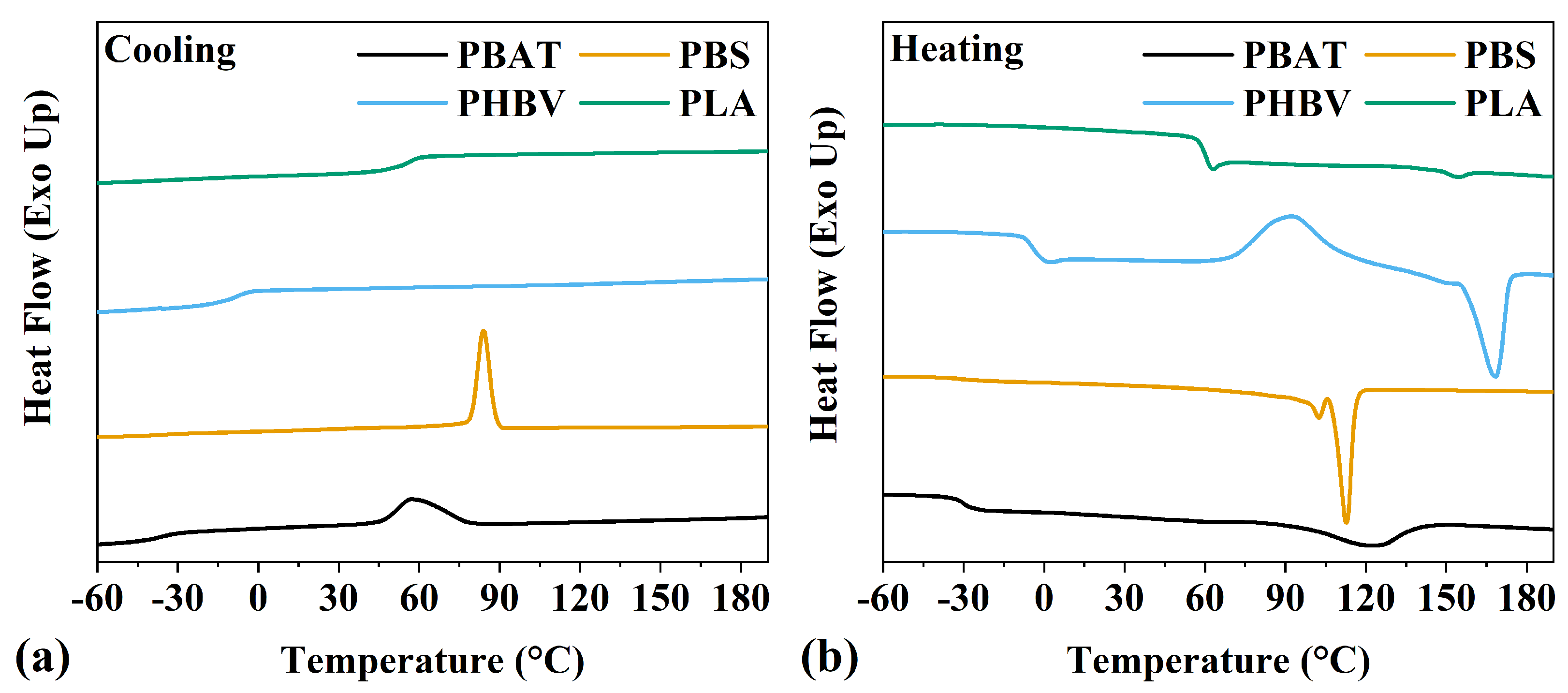
3.2. Thermal Properties
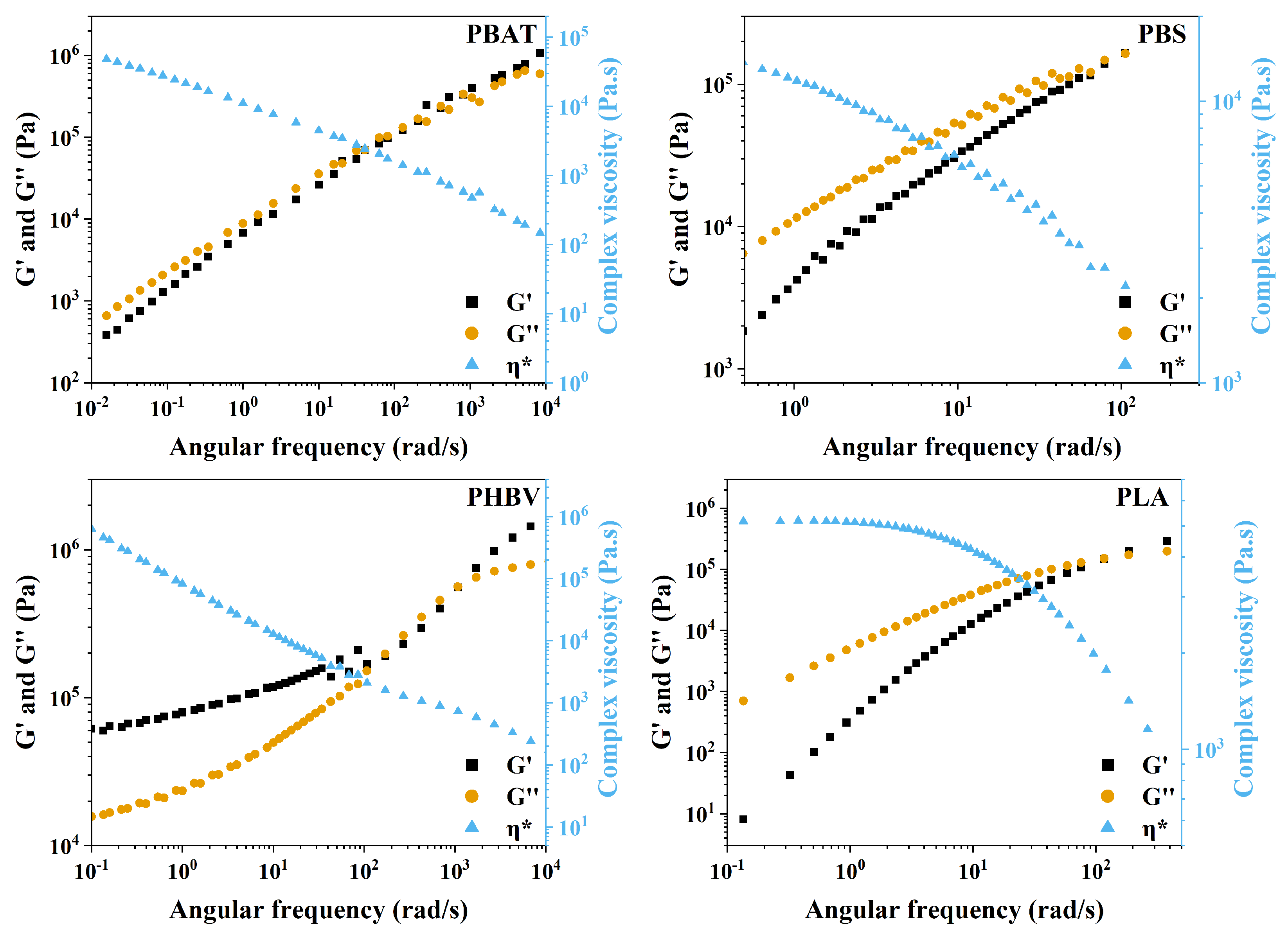
3.3. Mechanical Properties
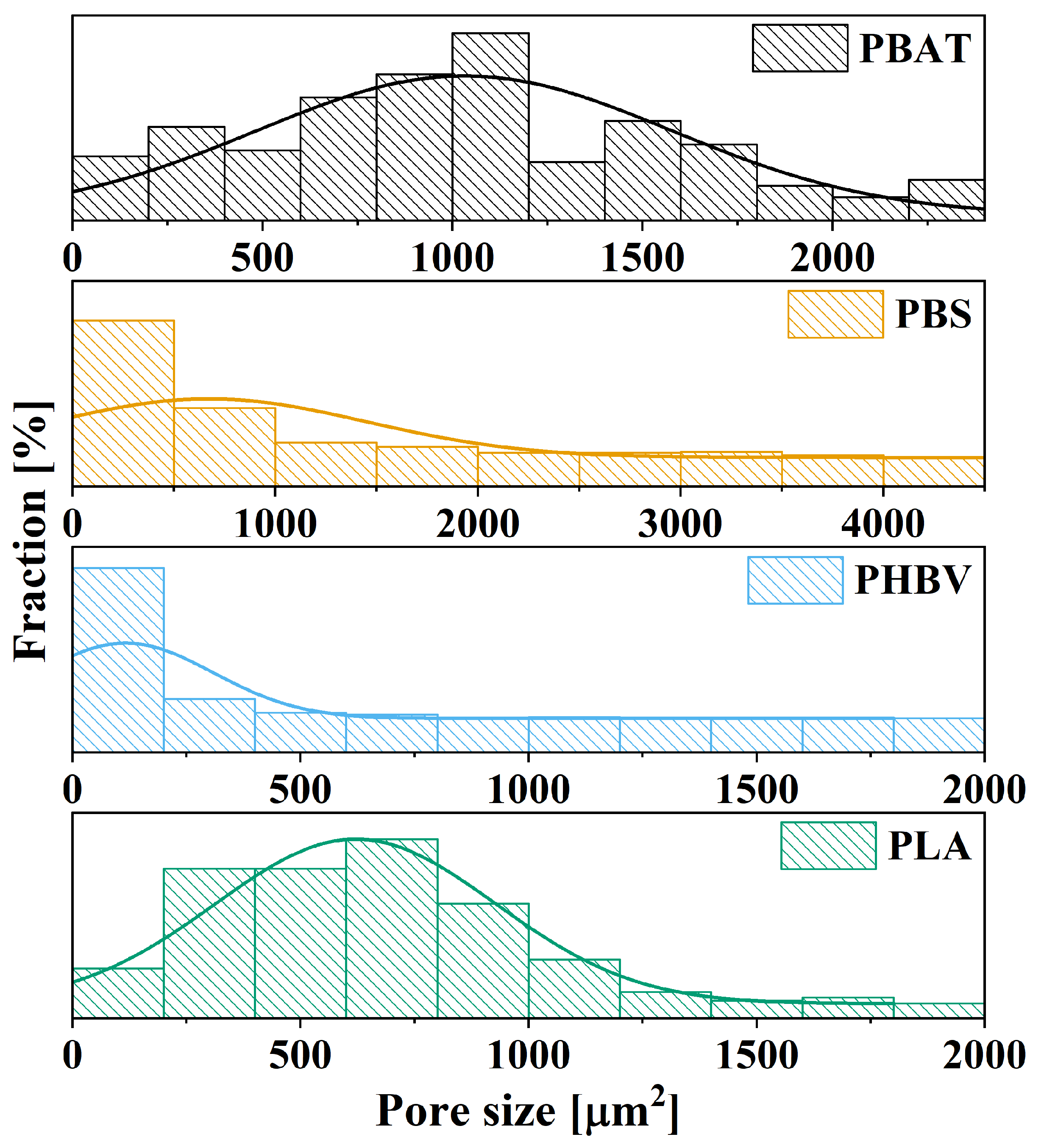
3.4. Porous Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Villarrubia-Gómez, P.; Cornell, S.E.; Fabres, J. Marine plastic pollution as a planetary boundary threat—The drifting piece in the sustainability puzzle. Mar. Policy 2018, 96, 213–220. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Agathokleous, E.; Iavicoli, I.; Barceló, D.; Calabrese, E.J. Ecological risks in a ‘plastic’ world: A threat to biological diversity? J. Hazard. Mater. 2021, 417, 126035. [Google Scholar] [CrossRef]
- Evans, D.M.; Parsons, R.; Jackson, P.; Greenwood, S.; Ryan, A. Understanding plastic packaging: The co-evolution of materials and society. Glob. Environ. Chang. 2020, 65, 102166. [Google Scholar] [CrossRef]
- Ju, J.; Gu, Z.; Liu, X.; Zhang, S.; Peng, X.; Kuang, T. Fabrication of bimodal open-porous poly (butylene succinate)/cellulose nanocrystals composite scaffolds for tissue engineering application. Int. J. Biol. Macromol. 2020, 147, 1164–1173. [Google Scholar] [CrossRef]
- Huang, P.; Wu, F.; Shen, B.; Ma, X.; Zhao, Y.; Wu, M.; Wang, J.; Liu, Z.; Luo, H.; Zheng, W. Bio-inspired lightweight polypropylene foams with tunable hierarchical tubular porous structure and its application for oil-water separation. Chem. Eng. J. 2019, 370, 1322–1330. [Google Scholar] [CrossRef]
- Martín-de León, J.; Pura, J.L.; Bernardo, V.; Ángel Rodríguez-Pérez, M. Transparent nanocellular PMMA: Characterization and modeling of the optical properties. Polymer 2019, 170, 16–23. [Google Scholar] [CrossRef]
- Guo, F.; Liao, X.; Li, S.; Yan, Z.; Tang, W.; Li, G. Heat insulating PLA/HNTs foams with enhanced compression performance fabricated by supercritical carbon dioxide. J. Supercrit. Fluids 2021, 177, 105344. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Wen, B.; Chen, Y.; Wang, X. A Facile and Efficient Method for Preparing Chain Extended Poly(lactic acid) Foams with High Volume Expansion Ratio. J. Polym. Environ. 2020, 28, 17–31. [Google Scholar] [CrossRef]
- Zhi, X.; Liu, J.; Zhang, H.B.; Hong, S.; Yu, Z.Z. Simultaneous enhancements in electrical conductivity and toughness of selectively foamed polycarbonate/polystyrene/carbon nanotube microcellular foams. Compos. Part B Eng. 2018, 143, 161–167. [Google Scholar] [CrossRef]
- Zhao, B.; Bai, P.; Yuan, M.; Yan, Z.; Fan, B.; Zhang, R.; Che, R. Recyclable magnetic carbon foams possessing voltage-controllable electromagnetic shielding and oil/water separation. Carbon 2022, 197, 570–578. [Google Scholar] [CrossRef]
- Raepsaet, C.; Alves, P.; Cullen, B.; Gefen, A.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Najafi, B.; Santamaria, N.; Sharpe, A.; Swanson, T.; et al. Clinical research on the use of bordered foam dressings in the treatment of complex wounds: A systematic review of reported outcomes and applied measurement instruments. J. Tissue Viability 2022, 31, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Faba, S.; Aguero, A.; Arrieta, M.P.; Martínez, S.; Romero, J.; Torres, A.; Galotto, M.J. Foaming of 3D-Printed PLA/CaCO3 Composites by Supercritical CO2 Process for Sustainable Food Contact Materials. Polymers 2024, 16, 798. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M. Recent Trends in Polymeric Foams and Porous Structures for Electromagnetic Interference Shielding Applications. Polymers 2024, 16, 195. [Google Scholar] [CrossRef]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable Poly(butylene succinate) and Poly(butylene adipate-co-terephthalate) Blends: Reactive Extrusion and Performance Evaluation. J. Polym. Environ. 2014, 22, 336–349. [Google Scholar] [CrossRef]
- Javadi, A.; Srithep, Y.; Clemons, C.C.; Turng, L.S.; Gong, S. Processing of poly(hydroxybutyrate-co-hydroxyvalerate)-based bionanocomposite foams using supercritical fluids. J. Mater. Res. 2012, 27, 1506–1517. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend in Freshwater with Sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef]
- Georgousopoulou, I.N.; Vouyiouka, S.; Dole, P.; Papaspyrides, C.D. Thermo-mechanical degradation and stabilization of poly(butylene succinate). Polym. Degrad. Stab. 2016, 128, 182–192. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, X.; Chen, H.; Fu, X.; Sun, Y.; Chen, Q.; Liu, W.; Shen, X. Mechanical, Crystallization, Rheological, and Supercritical CO2 Foaming Properties of Polybutylene Succinate Nanocomposites: Impact of Carbon Nanofiber Content. Polymers 2024, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xue, K.; Liu, Z.; Xu, Z.; Zhao, L. The essential role of PBS on PBAT foaming under supercritical CO2 toward green engineering. J. CO2 Util. 2022, 60, 101965. [Google Scholar] [CrossRef]
- Pilla, S.; Kim, S.G.; Auer, G.K.; Gong, S.; Park, C.B. Microcellular extrusion foaming of poly(lactide)/poly(butylene adipate-co-terephthalate) blends. Mater. Sci. Eng. C 2010, 30, 255–262. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, L.; Gao, X.; Xu, Z.; Liu, Z.; Hu, D. Engineering of polybutylene succinate with long-chain branching toward high foamability and degradation. Polym. Degrad. Stab. 2021, 194, 109745. [Google Scholar] [CrossRef]
- Wang, Y.; Huan, L.; Liang, H.; Ding, X.; Mi, J. Foaming biocompatible and biodegradable PBAT/PLGA as fallopian tube stent using supercritical carbon dioxide. Chin. J. Chem. Eng. 2022, 47, 245–253. [Google Scholar] [CrossRef]
- Liparoti, S.; Franco, P.; Pantani, R.; De Marco, I. Supercritical CO2 impregnation of caffeine in biopolymer films to produce anti-cellulite devices. J. Supercrit. Fluids 2022, 179, 105411. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wang, W.; Gong, P.; Yang, Q.; Park, C.B.; Li, G. Ultra-fast degradable PBAT/PBS foams of high performance in compression and thermal insulation made from environment-friendly supercritical foaming. J. Supercrit. Fluids 2022, 181, 105512. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J.; Shi, Z. Biodegradable PLA/PBS open-cell foam fabricated by supercritical CO2 foaming for selective oil-adsorption. Sep. Purif. Technol. 2021, 257, 117949. [Google Scholar] [CrossRef]
- Vorawongsagul, S.; Pratumpong, P.; Pechyen, C. Preparation and foaming behavior of poly (lactic acid)/poly (butylene succinate)/cellulose fiber composite for hot cups packaging application. Food Packag. Shelf Life 2021, 27, 100608. [Google Scholar] [CrossRef]
- Yin, D.; Mi, J.; Zhou, H.; Wang, X.; Tian, H. Fabrication of branching poly (butylene succinate)/cellulose nanocrystal foams with exceptional thermal insulation. Carbohydr. Polym. 2020, 247, 116708. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, G.; Gray, K.; Wilson, S.P.; Muniyasamy, S.; Vincent, S.G.T.; Bush, R.; Palanisami, T. Benchmarking Bioplastics: A Natural Step Towards a Sustainable Future. J. Polym. Environ. 2020, 28, 3055–3075. [Google Scholar] [CrossRef]
- Singhvi, M.; Zinjarde, S.; Gokhale, D. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, W.; Li, X.; Hu, Z.; Wang, B.; Li, M.; Dong, W. Preparation of high-expansion open-cell polylactic acid foam with superior oil-water separation performance. Int. J. Biol. Macromol. 2021, 193, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Hwang, K.S.; Kwon, H.J.; Lee, C.; Kim, C.H.; Kim, T.H.; Heo, S.W.; Kim, J.H.; Lee, J.Y. Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater. Sci. Eng. C 2020, 110, 110693. [Google Scholar] [CrossRef] [PubMed]
- Jack, K.S.; Velayudhan, S.; Luckman, P.; Trau, M.; Grøndahl, L.; Cooper-White, J. The fabrication and characterization of biodegradable HA/PHBV nanoparticle–polymer composite scaffolds. Acta Biomater. 2009, 5, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- Le Moigne, N.; Sauceau, M.; Benyakhlef, M.; Jemai, R.; Benezet, J.C.; Rodier, E.; Lopez-Cuesta, J.M.; Fages, J. Foaming of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/organo-clays nano-biocomposites by a continuous supercritical CO2 assisted extrusion process. Eur. Polym. J. 2014, 61, 157–171. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Trusca, R.; Gabor, A.R.; Nicolae, C.A.; Casarica, A. Biocomposite foams based on polyhydroxyalkanoate and nanocellulose: Morphological and thermo-mechanical characterization. Int. J. Biol. Macromol. 2020, 164, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Köse, G.; Korkusuz, F.; Korkusuz, P.; Purali, N.; Özkul, A.; Hasırcı, V. Bone generation on PHBV matrices: An in vitro study. Biomaterials 2003, 24, 4999–5007. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, M.; Wang, L.; Zhou, H.; Wen, B.; Wang, X.; Zhang, Y. CO2-based fabrication of biobased and biodegradable poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/graphene nanoplates nanocomposite foams: Toward EMI shielding application. Polymer 2022, 253, 125034. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Cui, C.; Mu, Y.; Niu, K. Low-Density and High-Performance Fiber-Reinforced PP/POE Composite Foam via Irradiation Crosslinking. Polymers 2024, 16, 745. [Google Scholar] [CrossRef] [PubMed]
- Worch, J.C.; Prydderch, H.; Jimaja, S.; Bexis, P.; Becker, M.L.; Dove, A.P. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 3, 514–535. [Google Scholar] [CrossRef]
- Stampfer, L.; Bouilhac, C.; Mérian, T.; Chabert, F.; Djilali, T.; Nassiet, V.; Habas, J.P. Investigation of the Reaction between a Homemade PEEK Oligomer and an Epoxy Prepolymer: Optimisation of Critical Parameters Using Physico–Chemical Methods. Polymers 2024, 16, 764. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, Y.; Zhang, L.; Yue, D. Bioinspired Sustainable Polymer with Stereochemistry-Controllable Thermomechanical Properties. Macromolecules 2023, 56, 416–425. [Google Scholar] [CrossRef]
- Huang, F.; Wu, L.; Li, B.G. Sulfonated biodegradable PBAT copolyesters with improved gas barrier properties and excellent water dispersibility: From synthesis to structure-property. Polym. Degrad. Stab. 2020, 182, 109391. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Bagrov, V.V.; Komarov, P.D.; Ilyin, S.O.; Ivchenko, P.V. The Use of Branching Agents in the Synthesis of PBAT. Polymers 2022, 14, 1720. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, T. Synthesis, characterization of phosphorus-containing copolyester and its application as flame retardants for poly(butylene succinate) (PBS). Chemosphere 2019, 235, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, J.; Wu, S.; Qi, Z.; Xu, J.; Guo, B. Synthesis, physical properties and photodegradation of functional poly(butylene succinate) covalently linking UV stabilizing moieties in molecular chains. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 524, 160–168. [Google Scholar] [CrossRef]
- Moorkoth, D.; Nampoothiri, K.M. Production and characterization of poly(3-hydroxy butyrate-co-3 hydroxyvalerate) (PHBV) by a novel halotolerant mangrove isolate. Bioresour. Technol. 2016, 201, 253–260. [Google Scholar] [CrossRef]
- Bakare, R.A.; Bhan, C.; Raghavan, D. Synthesis and Characterization of Collagen Grafted Poly(hydroxybutyrate–valerate) (PHBV) Scaffold for Loading of Bovine Serum Albumin Capped Silver (Ag/BSA) Nanoparticles in the Potential Use of Tissue Engineering Application. Biomacromolecules 2014, 15, 423–435. [Google Scholar] [CrossRef]
- Pérez, J.M.; Ruiz, C.; Fernández, I. Synthesis of a Biodegradable PLA: NMR Signal Deconvolution and End-Group Analysis. J. Chem. Educ. 2022, 99, 1000–1007. [Google Scholar] [CrossRef]
- Song, J.; Mi, J.; Zhou, H.; Wang, X.; Zhang, Y. Chain extension of poly (butylene adipate-co-terephthalate) and its microcellular foaming behaviors. Polym. Degrad. Stab. 2018, 157, 143–152. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, F.; Zhang, S.; Huang, H. Structure and improved properties of PPC/PBAT blends via controlling phase morphology based on melt viscosity. J. Appl. Polym. Sci. 2020, 137, 48924. [Google Scholar] [CrossRef]
- Yao, S.F.; Chen, X.T.; Ye, H.M. Investigation of Structure and Crystallization Behavior of Poly(butylene succinate) by Fourier Transform Infrared Spectroscopy. J. Phys. Chem. B 2017, 121, 9476–9485. [Google Scholar] [CrossRef] [PubMed]
- Montanheiro, T.L.d.A.; Passador, F.R.; Oliveira, M.P.d.; Durán, N.; Lemes, A.P. Preparation and characterization of maleic anhydride grafted poly (hydroxybutirate-CO-hydroxyvalerate)–PHBV-g-MA. Mater. Res. 2016, 19, 229–235. [Google Scholar] [CrossRef]
- Fei, B.; Chen, C.; Wu, H.; Peng, S.; Wang, X.; Dong, L. Quantitative FTIR study of PHBV/bisphenol A blends. Eur. Polym. J. 2003, 39, 1939–1946. [Google Scholar] [CrossRef]
- Cuiffo, M.A.; Snyder, J.; Elliott, A.M.; Romero, N.; Kannan, S.; Halada, G.P. Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure. Appl. Sci. 2017, 7, 579. [Google Scholar] [CrossRef]
- Herrera-Kao, W.A.; Loría-Bastarrachea, M.I.; Pérez-Padilla, Y.; Cauich-Rodríguez, J.V.; Vázquez-Torres, H.; Cervantes-Uc, J.M. Thermal degradation of poly(caprolactone), poly(lactic acid), and poly(hydroxybutyrate) studied by TGA/FTIR and other analytical techniques. Polym. Bull. 2018, 75, 4191–4205. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Mark, J.E. Physical Properties of Polymer Handbook; Springer: New York, NY, USA, 2006. [Google Scholar]
- Federico, C.; Bouvard, J.; Combeaud, C.; Billon, N. Large strain/time dependent mechanical behaviour of PMMAs of different chain architectures. Application of time-temperature superposition principle. Polymer 2018, 139, 177–187. [Google Scholar] [CrossRef]
- Zhang, K.; Mohanty, A.K.; Misra, M. Fully Biodegradable and Biorenewable Ternary Blends from Polylactide, Poly(3-hydroxybutyrate-co-hydroxyvalerate) and Poly(butylene succinate) with Balanced Properties. ACS Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, L.; Grochowicz, M. Efficiency of Twin-Screw Extrusion of Biodegradable Poly (Butylene Succinate)-Wheat Bran Blend. Materials 2021, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Benini, K.C.C.d.; Ornaghi, H.L.; de Medeiros, N.M.; Pereira, P.H.F.; Cioffi, M.O.H. Thermal characterization and lifetime prediction of the PHBV/nanocellulose biocomposites using different kinetic approaches. Cellulose 2020, 27, 7503–7522. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N.R. Dynamic Mechanical Analysis; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Yang, M.; Li, W.; Dong, P.; Ma, Y.; He, Y.; Zhao, Z.; Chen, L. Temperature and strain rate sensitivity of yield strength of amorphous polymers: Characterization and modeling. Polymer 2022, 251, 124936. [Google Scholar] [CrossRef]
- Polychronopoulos, N.D.; Vlachopoulos, J. Polymer Processing and Rheology. In Functional Polymers; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–47. [Google Scholar] [CrossRef]
- Pal, A.K.; Wu, F.; Misra, M.; Mohanty, A.K. Reactive extrusion of sustainable PHBV/PBAT-based nanocomposite films with organically modified nanoclay for packaging applications: Compression moulding vs. cast film extrusion. Compos. Part B Eng. 2020, 198, 108141. [Google Scholar] [CrossRef]
- Ugartemendia, J.M.; Muñoz, M.E.; Sarasua, J.R.; Santamaria, A. Phase behavior and effects of microstructure on viscoelastic properties of a series of polylactides and polylactide/poly(ϵ-caprolactone) copolymers. Rheol. Acta 2014, 53, 857–868. [Google Scholar] [CrossRef]
- Dealy, J.M.; Wissbrun, K.F. Linear Viscoelasticity; Springer: Dordrecht, The Netherlands, 1999; pp. 42–102. [Google Scholar] [CrossRef]
- Yin, D.; Xiang, A.; Li, Y.; Qi, H.; Tian, H.; Fan, G. Effect of Plasticizer on the Morphology and Foaming Properties of Poly(vinyl alcohol) Foams by Supercritical CO2 Foaming Agents. J. Polym. Environ. 2019, 27, 2878–2885. [Google Scholar] [CrossRef]
- Rainglet, B.; Chalamet, Y.; Bounor-Legaré, V.; Delage, K.; Forest, C.; Cassagnau, P. Polypropylene foams under CO2 batch conditions: From formulation and rheological modeling to cell-growth simulation. Polymer 2021, 218, 123496. [Google Scholar] [CrossRef]
- Long, H.; Xu, H.; Shaoyu, J.; Jiang, T.; Zhuang, W.; Li, M.; Jin, J.; Ji, L.; Ying, H.; Zhu, C. High-Strength Bio-Degradable Polymer Foams with Stable High Volume-Expansion Ratio Using Chain Extension and Green Supercritical Mixed-Gas Foaming. Polymers 2023, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Oluwabunmi, K.E.; Zhao, W.; D’Souza, N.A. Carbon Capture Utilization for Biopolymer Foam Manufacture: Thermal, Mechanical and Acoustic Performance of PCL/PHBV CO2 Foams. Polymers 2021, 13, 2559. [Google Scholar] [CrossRef]
- Milovanovic, S.; Lukic, I.; Horvat, G.; Novak, Z.; Frerich, S.; Petermann, M.; García-González, C.A. Green Processing of Neat Poly(lactic acid) Using Carbon Dioxide under Elevated Pressure for Preparation of Advanced Materials: A Review (2012–2022). Polymers 2023, 15, 860. [Google Scholar] [CrossRef]
- Amani, H.; Mostafavi, E.; Arzaghi, H.; Davaran, S.; Akbarzadeh, A.; Akhavan, O.; Pazoki-Toroudi, H.; Webster, T.J. Three-Dimensional Graphene Foams: Synthesis, Properties, Biocompatibility, Biodegradability, and Applications in Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, G.; Li, Y.; Edy, R.; Gao, P.; Tang, H.; Bao, Z.; Mei, Y. Three-dimensional carbon/ZnO nanomembrane foam as an anode for lithium-ion battery with long-life and high areal capacity. J. Mater. Chem. A 2018, 6, 7227–7235. [Google Scholar] [CrossRef]
- Qu, Z.; Yin, D.; Zhou, H.; Wang, X.; Zhao, S. Cellular morphology evolution in nanocellular poly (lactic acid)/thermoplastic polyurethane blending foams in the presence of supercritical N2. Eur. Polym. J. 2019, 116, 291–301. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.W.; Wang, Y.Z.; Fu, T.; Zhao, H.B. P-doped PANI/AgMWs nano/micro coating towards high-efficiency flame retardancy and electromagnetic interference shielding. Compos. Part B Eng. 2022, 238, 109944. [Google Scholar] [CrossRef]




| Polymer | Mn (kDa) | Mw (kDa) | PDI |
|---|---|---|---|
| PBAT | 52 | 132 | 2.5 |
| PBS | 100 | 233 | 2.3 |
| PHBV | 207 | 440 | 2.1 |
| PLA | 105 | 189 | 1.8 |
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | T5 (°C) | T10 (°C) | T20 (°C) |
|---|---|---|---|---|---|---|
| PBAT | −30 | 74 | 123 | 367 | 380 | 391 |
| PBS | −31 | 84 | 113 | 323 | 347 | 367 |
| PHBV | −4 | 86 * | 170 | 225 | 275 | 283 |
| PLA | 54 | 112 * | 154 | 335 | 347 | 357 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, G.M.R.; Mukherjee, A.; Picchioni, F.; Bose, R.K. Characterization of Biodegradable Polymers for Porous Structure: Further Steps toward Sustainable Plastics. Polymers 2024, 16, 1147. https://doi.org/10.3390/polym16081147
Lima GMR, Mukherjee A, Picchioni F, Bose RK. Characterization of Biodegradable Polymers for Porous Structure: Further Steps toward Sustainable Plastics. Polymers. 2024; 16(8):1147. https://doi.org/10.3390/polym16081147
Chicago/Turabian StyleLima, Guilherme M. R., Adrivit Mukherjee, Francesco Picchioni, and Ranjita K. Bose. 2024. "Characterization of Biodegradable Polymers for Porous Structure: Further Steps toward Sustainable Plastics" Polymers 16, no. 8: 1147. https://doi.org/10.3390/polym16081147






