Colloidal and Biological Characterization of Dual Drug-Loaded Smart Micellar Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Free and Drug-Loaded Micellar Solutions
2.2. Physicochemical Characterization
2.3. Drug Encapsulation and Loading Efficiency (DEE and DLE)
2.4. Cell Culture
2.5. In Vitro Cytotoxicity Assays
3. Results
3.1. Colloidal Characterization
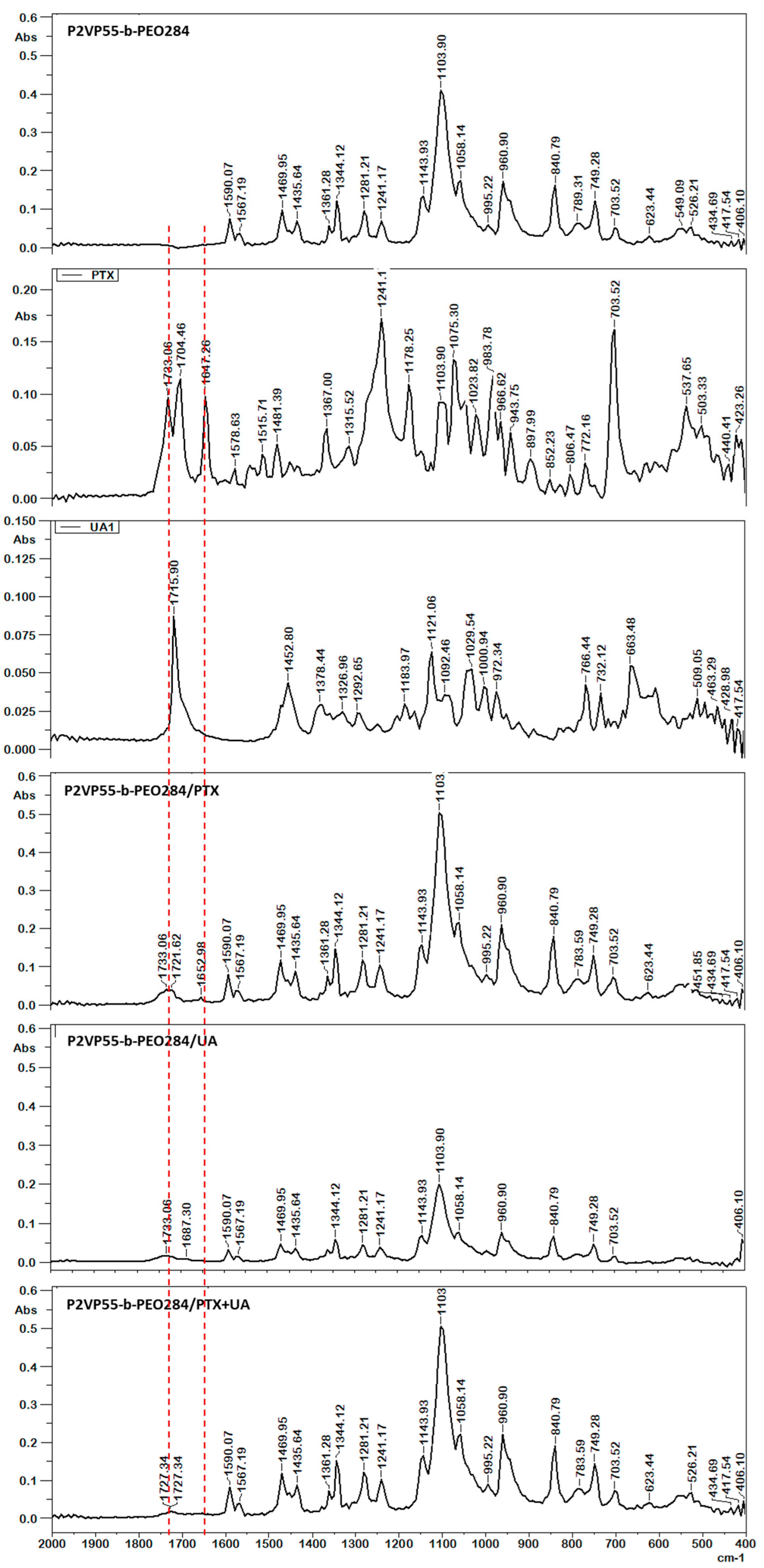
3.2. FT-IR Spectroscopy
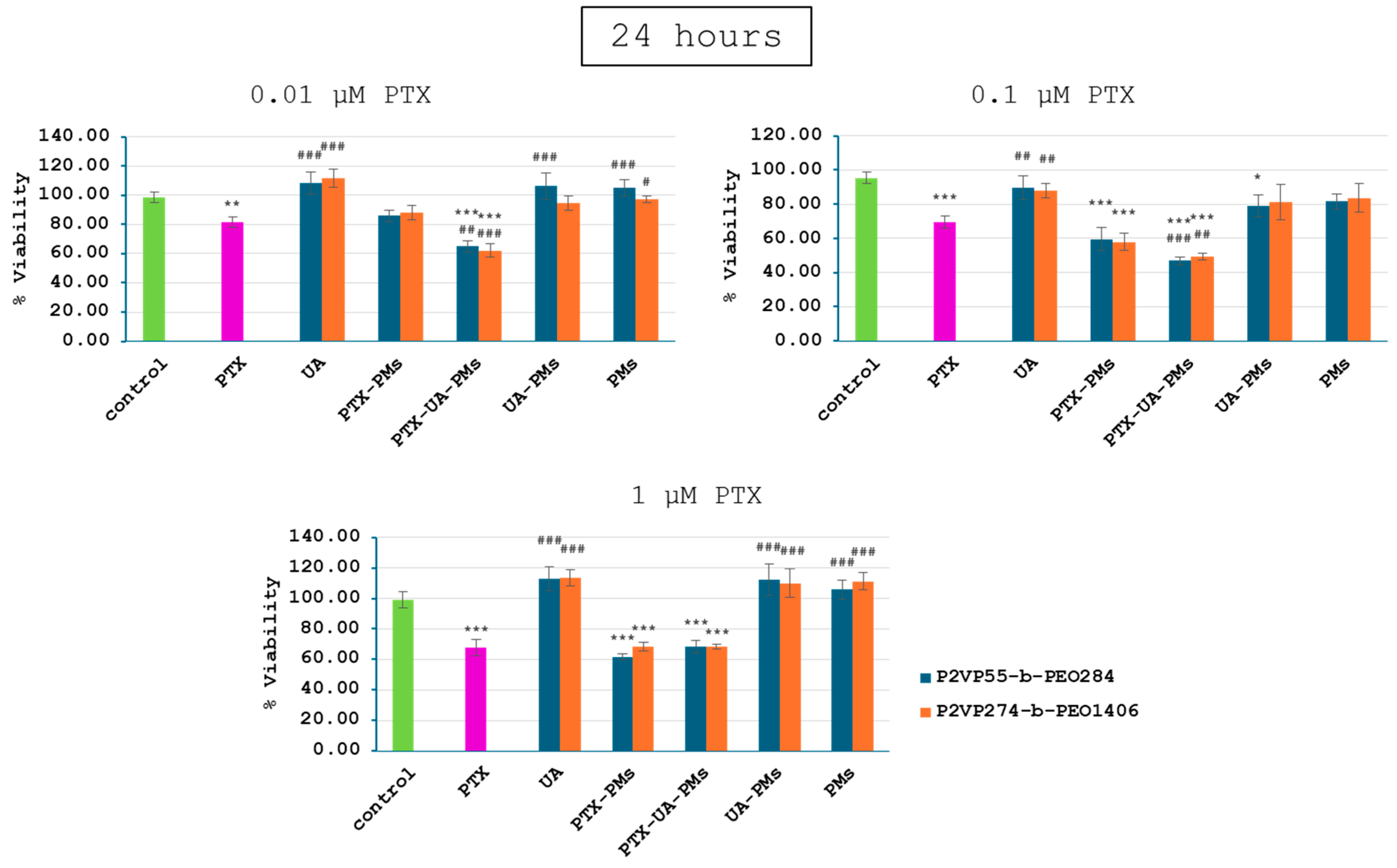
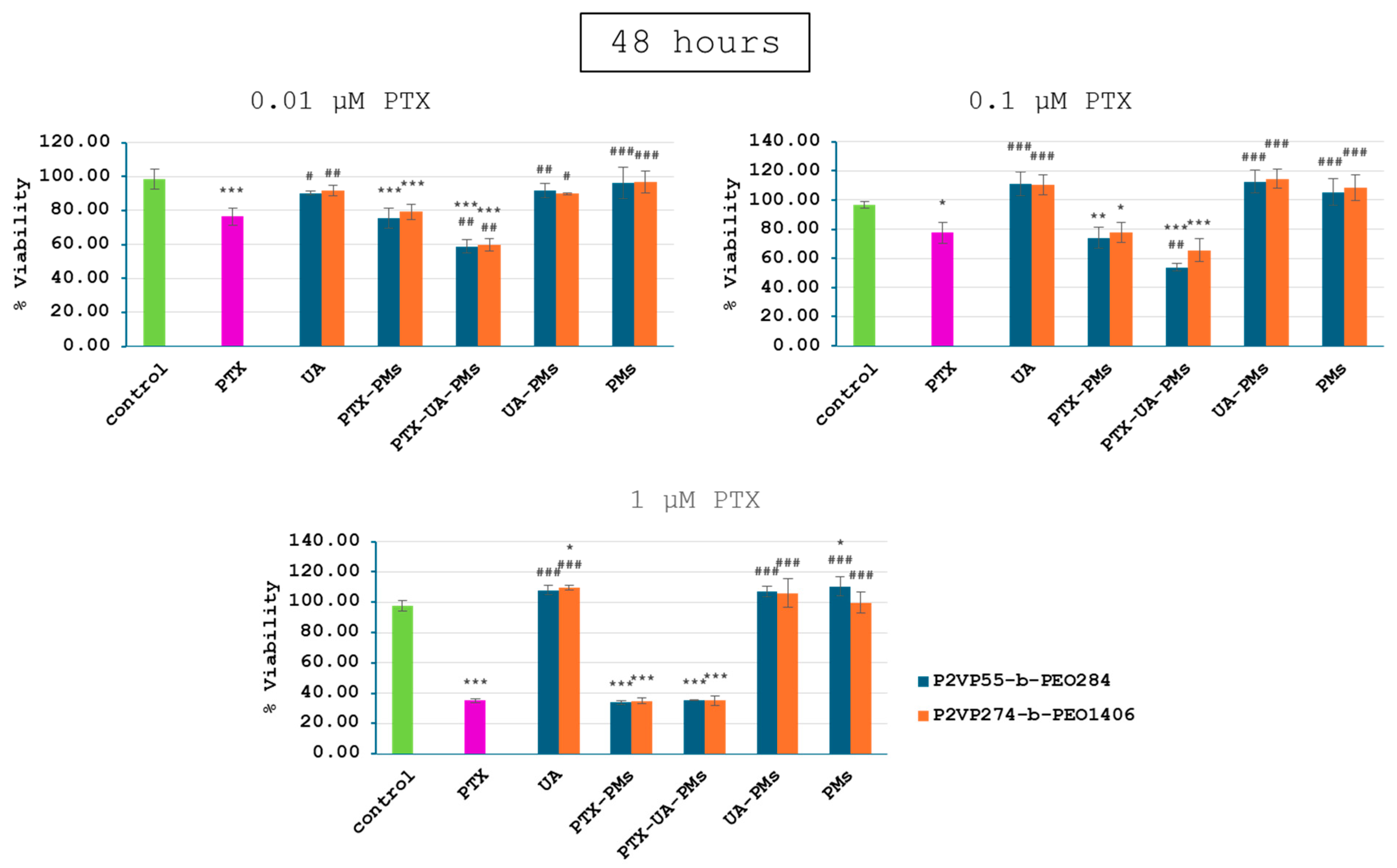
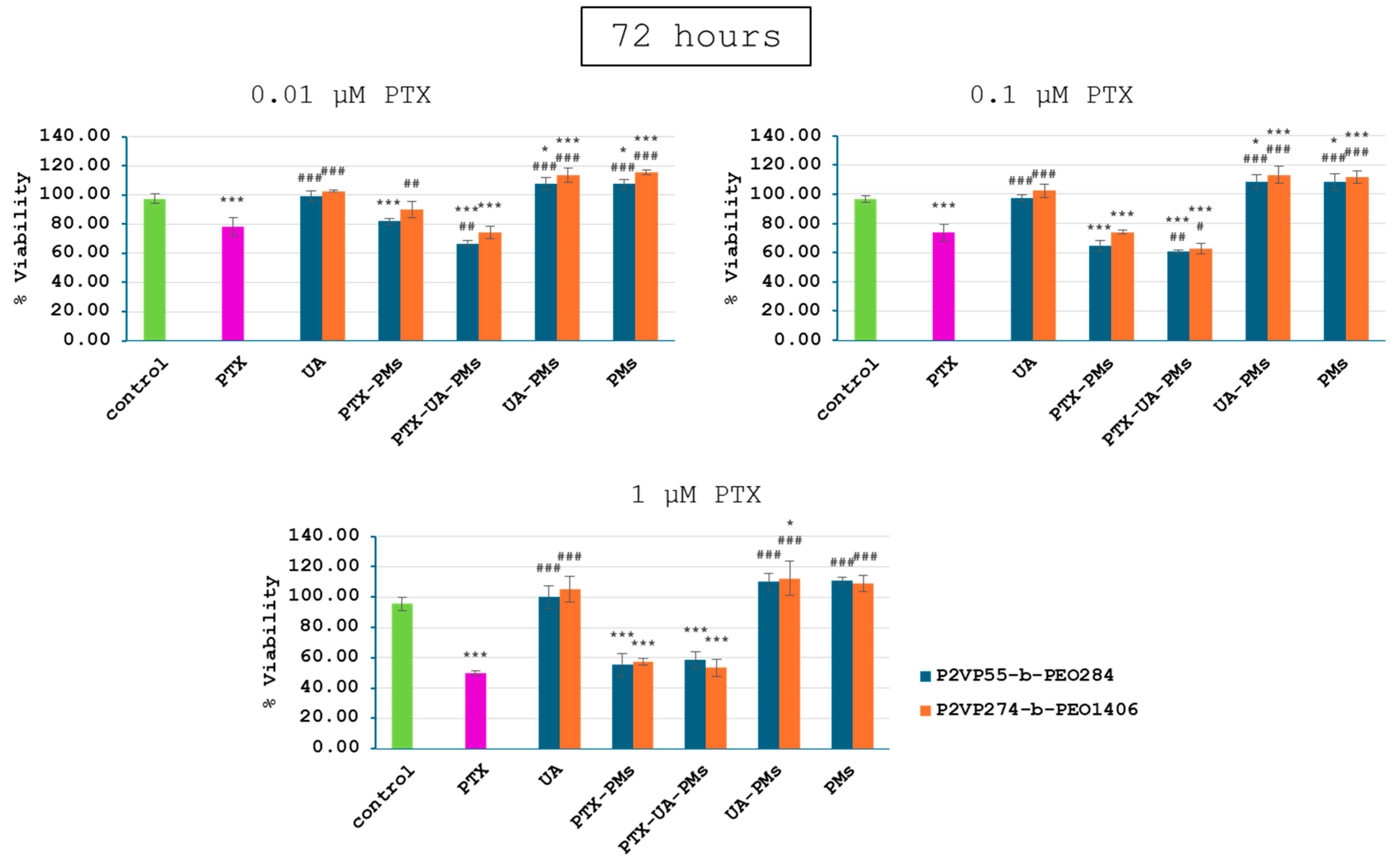
3.3. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Tobechukwu, C.E.; Ugochukwu, S.O.; Ufedo, L.O.; Chinenye, P.N.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Shadrach, C.E.; Onyinyechi, L.K.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Parveen, F.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P. Approaches to Improve EPR-Based Drug Delivery for Cancer Therapy and Diagnosis. J. Pers. Med. 2023, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Zheng, Y.; Oz, Y.; Gu, Y.; Ahamad, N.; Shariati, K.; Chevalier, J.; Kapur, D.; Annabi, N. Rational design of polymeric micelles for targeted therapeutic delivery. Nano Today 2024, 55, 102147. [Google Scholar] [CrossRef]
- Abhilash, R.; Meheli, A.; Kumar, S.P.; Bhudev, C.D.; Seema, B. “Smart” drug delivery: A window to future of translational medicine. Front. Chem. 2023, 10, 1095598. [Google Scholar] [CrossRef] [PubMed]
- Maboudi, A.H.; Lotfipour, M.H.; Rasouli, M.; Azhdari, M.H.; MacLoughlin, R.; Bekeschus, S.; Doroudian, M. Micelle-based nanoparticles with stimuli-responsive properties for drug delivery. Nanotechnol. Rev. 2024, 13, 20230218. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Zhang, K.; Wang, Q.; Chen, Y.; Luo, X. pH-responsive reversibly cross-linked micelles by phenol-yne click via curcumin as a drug delivery system in cancer chemotherapy. J. Mater. Chem. B 2019, 7, 3884–3893. [Google Scholar] [CrossRef]
- Zhuo, S.; Zhang, F.; Yu, J.; Zhang, X.; Yang, G.; Liu, X. pH-Sensitive Biomaterials for Drug Delivery. Molecules 2020, 25, 5649. [Google Scholar] [CrossRef]
- Adeli, F.; Abbasi, F.; Babazadeh, M.; Davaran, S. Thermo/pH dual-responsive micelles based on the host–guest interaction between benzimidazole-terminated graft copolymer and β-cyclodextrin-functionalized star block copolymer for smart drug delivery. J. Nanobiotechnol. 2022, 20, 91. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P. pH-responsive polymers for drug delivery: Trends and opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Hlavatovičová, E.; Fernandez-Alvarez, R.; Byś, K.; Kereïche, S.; Mandal, T.K.; Atanase, L.I.; Štěpánek, M.; Uchman, M. Stimuli-Responsive Triblock Terpolymer Conversion into Multi-Stimuli-Responsive Micelles with Dynamic Covalent Bonds for Drug Delivery through a Quick and Controllable Post-Polymerization Reaction. Pharmaceutics 2023, 15, 288. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Kamburova, K.; Maeda, H.; Radeva, T. Investigation of pH dependence of poly(acrylic acid) conformation by means of electric birefringence. Colloids Surf. A 2010, 354, 61–64. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behavior of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 2016, 12, 2542–2549. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Riess, G. Micellization of pH-stimulable poly(2-vinylpyridine)-b-poly(ethylene oxide) copolymers and their complexation with anionic surfactants. J. Colloid Interface Sci. 2013, 395, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Lerch, J.P.; Caprarescu, S.; Iurciuc, C.E.; Riess, G. Micellization of pH-sensitive poly(butadiene)-block-poly(2 vinylpyridine)-block-poly(ethylene oxide) triblock copolymers: Complex formation with anionic surfactants. J. Appl. Polym. Sci. 2017, 134, 45313–45321. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Ying, N.; Liu, S.; Zhang, M.; Cheng, J.; Luo, L.; Jiang, J.; Shi, G.; Wu, S.; Ji, J.; Su, H.; et al. Nano delivery system for paclitaxel: Recent advances in cancer theranostics. Colloids Surf. B. Biointerfaces 2023, 228, 113419. [Google Scholar] [CrossRef]
- Mustafa, G.; Hassan, D.; Ruiz-Pulido, G.; Pourmadadi, M.; Eshaghi, M.M.; Behzadmehr, R.; Tehrani, F.S.; Rahdar, A.; Medina, D.I.; Pandey, S. Nanoscale drug delivery systems for cancer therapy using paclitaxel—A review of challenges and latest progressions. J. Drug Deliv. Sci. Technol. 2023, 84, 104494. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Khan, K.; Hafeez, A.; Irfan, M.; Armaghan, M.; ur Rahman, A.; Gurer, E.S.; Sharifi-rad, J.; Butnariu, M.; Bagiu, I.C.; et al. Ursolic acid: A natural modulator of signaling networks in different cancers. Cancer Cell Int. 2022, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.Y.; Jin, H.; Nguyen, T.V.; Chai, O.-H.; Park, B.-H.; Kim, S.M. Ursolic Acid Accelerates Paclitaxel-Induced Cell Death in Esophageal Cancer Cells by Suppressing Akt/FOXM1 Signaling Cascade. Int. J. Mol. Sci. 2021, 22, 11486. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Fan, Y.; Ni, Z.; Liu, Q.; Zhu, Z.; Chen, Z.; Hao, W.; Yue, H.; Wu, R.; Kang, X. Ursolic Acid Reverses the Chemoresistance of Breast Cancer Cells to Paclitaxel by Targeting MiRNA-149-5p/MyD88. Front. Oncol. 2019, 9, 501. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hou, Y.; Yu, J.; Lu, Y.; Chang, L.; Jiang, M.; Wu, X. Synergism of ursolic acid and cisplatin promotes apoptosis and enhances growth inhibition of cervical cancer cells via suppressing NF-κB p65. Oncotarget 2017, 8, 97416–97427. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, G.Q.; Zhang, K.; Zhou, Y.C.; Li, X.L.; Xu, W.; Zhang, H.; Shao, Y.; Zhang, Z.Y.; Sun, W.H. Cyclooxygenase-2 mediated synergistic effect of ursolic acid in combination with paclitaxel against human gastric carcinoma. Oncotarget 2017, 8, 92770–92777. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.; Lingelser, J.P.; Gallot, Y. Synthèse et caractérisation de copolymères séquencés poly(vinyl-2 pyridine):poly(oxyéthylène). Makromol. Chem. 1982, 183, 2961–2969. [Google Scholar] [CrossRef]
- Matsushita, Y.; Shimizu, K.; Nakao, Y.; Choshi, H.; Noda, I.; Nagasawa, M. Preparation and Characterization of Poly(2-vinylpyridine) with Narrow Molecular Weight Distributions. Polym. J. 1986, 18, 361–366. [Google Scholar] [CrossRef]
- Zhou, Z.; Tong, H.H.Y.; Li, L.; Shek, F.L.Y.; Lv, Y.; Zheng, Y. Synthesis, characterization and thermal analysis of ursolic acid solid forms. Cryst. Res. Technol. 2015, 50, 538–548. [Google Scholar] [CrossRef]
- Huaman, M.A.L.; Quispe, A.L.T.; Quispe, R.I.H.; Flores, C.A.S.; Caycho, J.R. A simple method to obtain ursolic acid. Results Chem. 2021, 3, 100144. [Google Scholar] [CrossRef]
- Mirina, S.; Abad, K.; Zafar, I.; Ismail, K.; Abida, R.; Asmat, U.; Fazli, N.; Ahmad, K.S. Design and Characterization of Paclitaxel-Loaded Polymeric Nanoparticles Decorated with Trastuzumab for the Effective Treatment of Breast Cancer. Front. Pharmacol. 2022, 13, 855294. [Google Scholar] [CrossRef] [PubMed]


| Samples | Mn (P2VP) (g/mol) a | Mn (PEO) (g/mol) a | Mn (Copolymer) (g/mol) | Mw/Mn (Copolymer) a |
|---|---|---|---|---|
| P2VP55-b-PEO284 | 5800 | 12,500 | 18,300 | 1.11 |
| P2VP274-b-PEO1406 | 28,800 | 61,900 | 90,700 | 1.29 |
| Sample | Drug | Cop/Drug Ratio | Quantity (mg) | ||
|---|---|---|---|---|---|
| m (Copolymer) | m (PTX) | m (UA) | |||
| P2VP55-b-PEO284 | PTX | 20/1 | 100 | 5 | |
| 10/1 | 100 | 10 | |||
| 5/1 | 100 | 20 | |||
| 2/1 | 100 | 50 | |||
| PTX + UA | 10/1 | 100 | 5 | 5 | |
| 5/1 | 100 | 10 | 10 | ||
| 2/1 | 100 | 25 | 25 | ||
| UA | 10/1 | 100 | 10 | ||
| Free PMs | 100 | ||||
| P2VP274-b-PEO1406 | PTX | 10/1 | 100 | 10 | |
| PTX + UA | 10/1 | 100 | 5 | 5 | |
| UA | 10/1 | 100 | 10 | ||
| Free PMs | 100 | ||||
| Sample | Copolymer/ Drug Ratio (mg/mg) | DEE (%) | DLE (%) | Z-Average (nm) | Dv (nm) | PDI | ZP (mV) | |
|---|---|---|---|---|---|---|---|---|
| P2VP55-b-PEO284 | Free PMs | - | - | - | 43.1 | 34.5 | 0.261 | −15.5 |
| PTX | 20 | 94.0 | 4.5 | 45.0 | 38.1 | 0.143 | −17.2 | |
| 10 | 91.6 | 8.3 | 51.8 | 45.7 | 0.107 | −18.2 | ||
| 5 | 64.2 | 10.7 | 71.1 | 59.8 | 0.122 | −17.6 | ||
| 2 | 56.0 | 18.7 | n.a | 43.1–85% 312–15% | n.a | −3.3 | ||
| UA | 10 | 92.1 | 8.3 | 348.9 | 477.3 | 0.228 | −1.5 | |
| PTX + UA | 10 | 64.7 | 5.9 | 223.2 | 584–27% 41–73% | 0.624 | −4.3 | |
| 5 | 65.0 | 8.4 | 305.7 | 654–66% 50–34% | 0.440 | −2.1 | ||
| 2 | 53.8 | 17.9 | 612.4 | 1150–96% 168–4% | 0.356 | −1.9 | ||
| P2VP274-b-PEO1406 | Free PMs | - | - | - | 140.5 | 141.7 | 0.044 | −10.1 |
| PTX | 10 | 62.3 | 5.5 | 1761.3 | 1178–95% 135–5% | 0.859 | −1.7 | |
| UA | 61.9 | 5.4 | 309.8 | n.d | 0.453 | −1.4 | ||
| PTX + UA | 71.2 | 3.2 | 1625.3 | 1596–95% 231–5% | 0.657 | −1.5 | ||
| PTX | UA | ||
|---|---|---|---|
| P2VP55-b-PEO284 | P2VP274-b-PEO1406 | ||
| C1 | 0.01 µM PTX | 34.72 nM UA | 20.65 nM UA |
| C2 | 0.1 µM PTX | 347.2 nM UA | 206.5 nM UA |
| C3 | 1 µM PTX | 3472 nM UA | 2065 nM UA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, H.; Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Hermenean, A. Colloidal and Biological Characterization of Dual Drug-Loaded Smart Micellar Systems. Polymers 2024, 16, 1189. https://doi.org/10.3390/polym16091189
Herman H, Rata DM, Cadinoiu AN, Atanase LI, Hermenean A. Colloidal and Biological Characterization of Dual Drug-Loaded Smart Micellar Systems. Polymers. 2024; 16(9):1189. https://doi.org/10.3390/polym16091189
Chicago/Turabian StyleHerman, Hildegard, Delia M. Rata, Anca N. Cadinoiu, Leonard I. Atanase, and Anca Hermenean. 2024. "Colloidal and Biological Characterization of Dual Drug-Loaded Smart Micellar Systems" Polymers 16, no. 9: 1189. https://doi.org/10.3390/polym16091189






