Effect of Nano Spinel Ferrites Co0.9Cu0.1Fe2O4 on Non-Isothermal Cold Crystallization Behaviours and Kinetics of Its Composites with Polylactic Acid
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
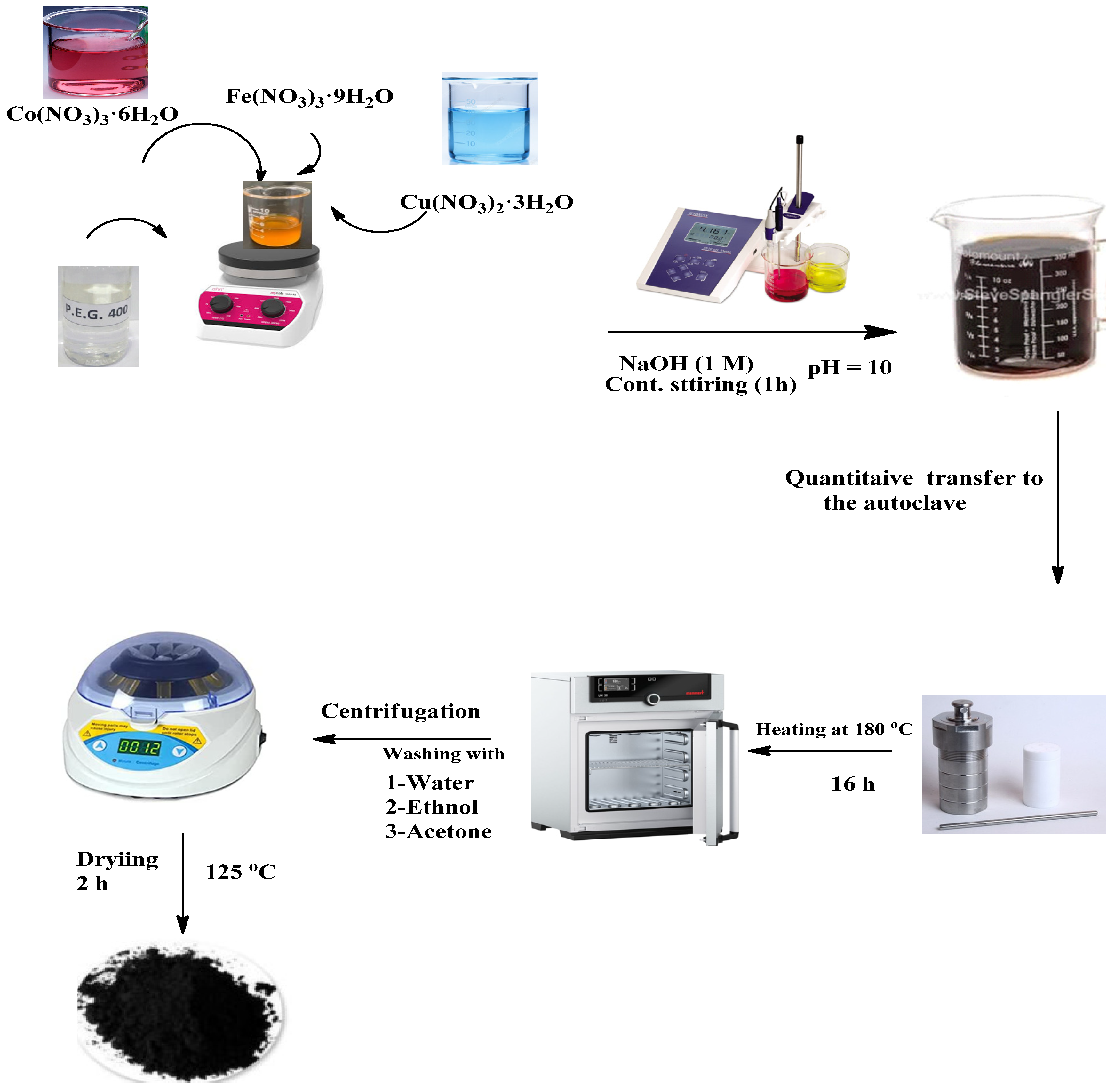
2.2. Preparation of the Investigated Nano Oxides
2.3. Characterizations of the Investigated Nano Oxides
2.4. Preparation of the PLA Nanocomposites
2.5. Characterization of the PLA Nanocomposites
3. Findings and Discussion
3.1. X-ray Diffraction (XRD) Analysis for the Prepared Co0.9Cu0.1Fe2O4 Nano Spinel Ferrite
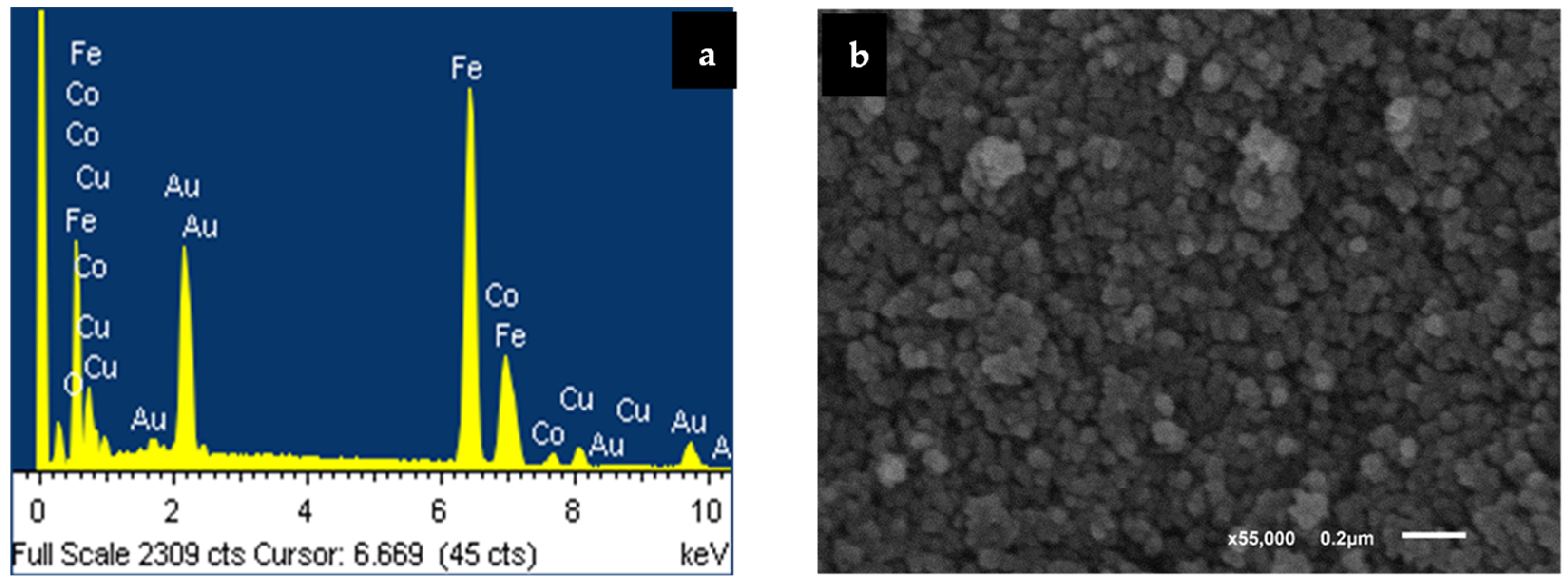
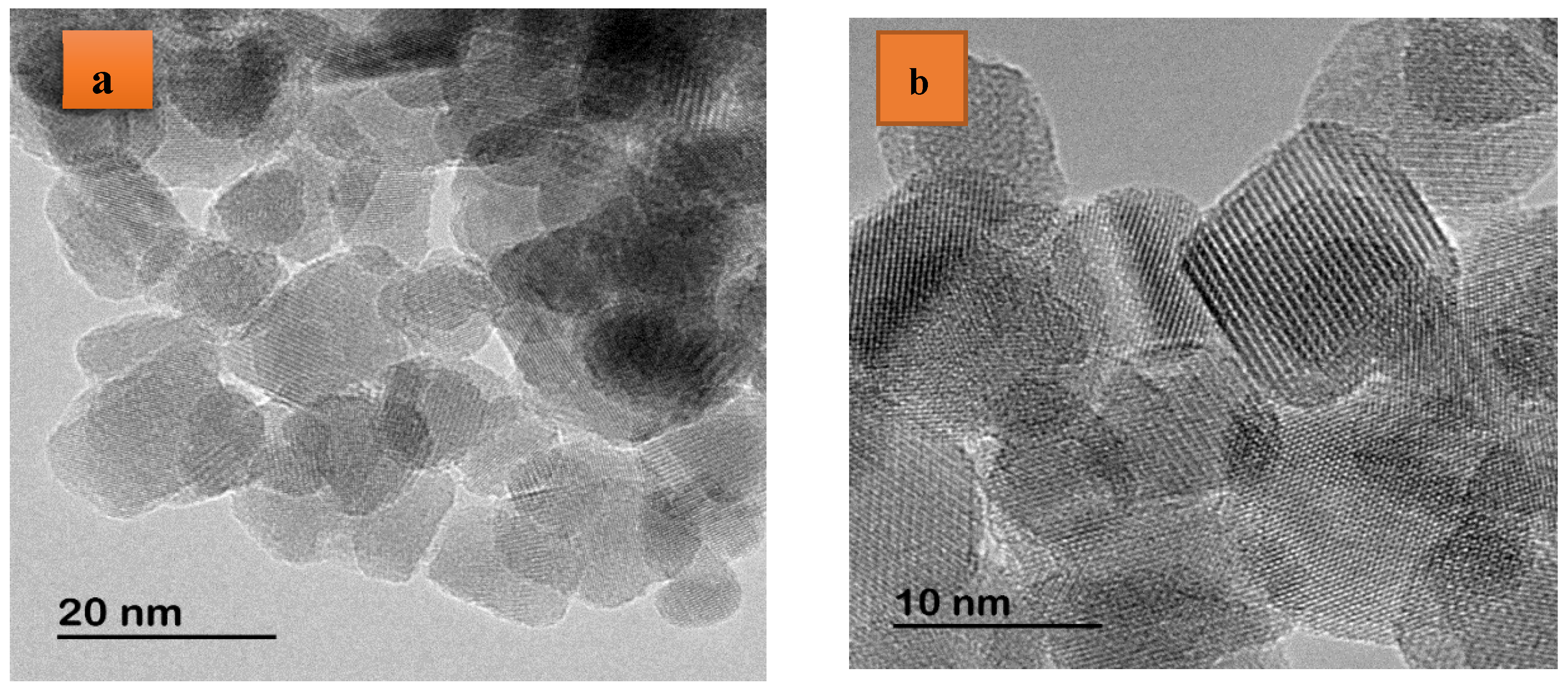
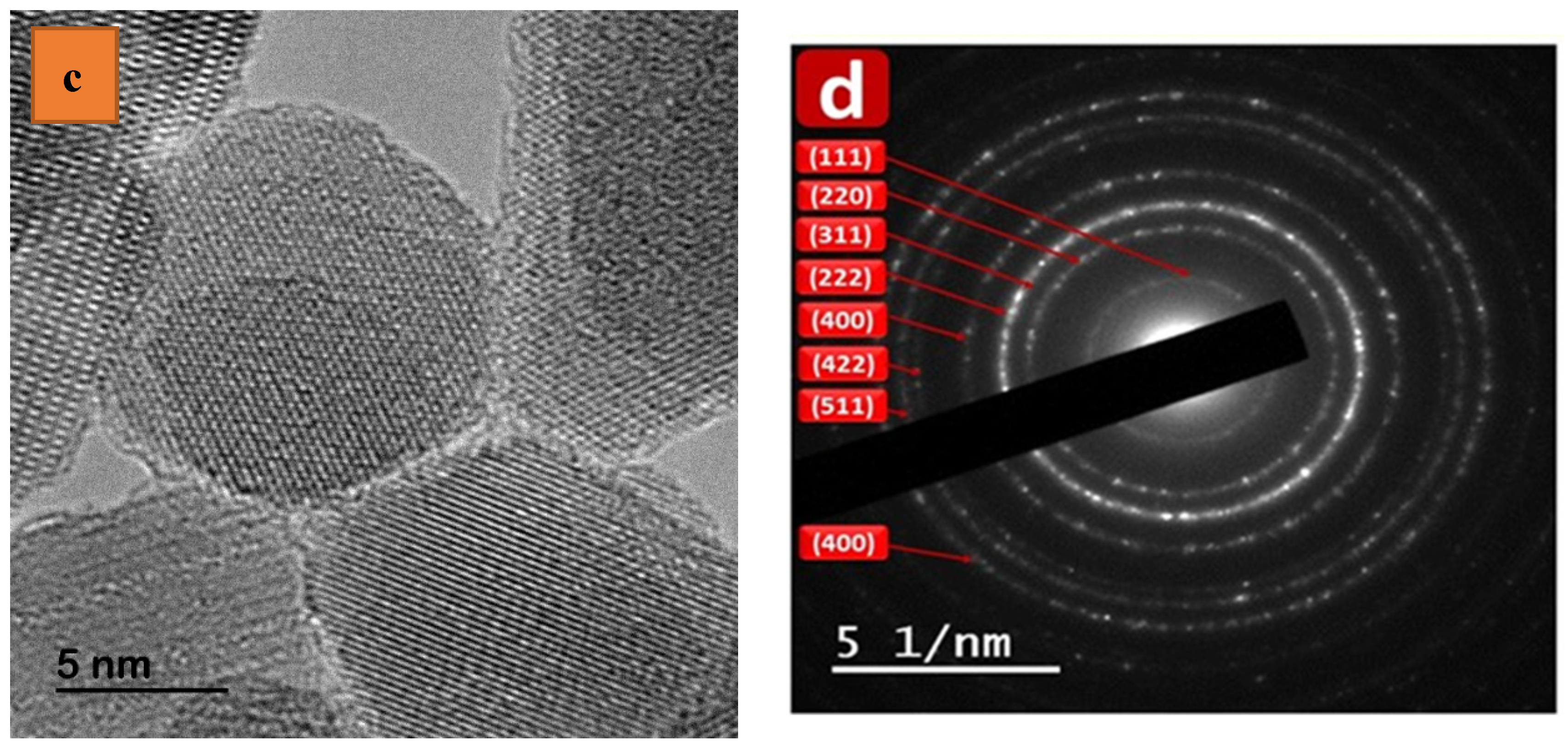
3.2. Electron Microscopy Analyses Using EDXS, SEM, and TEM
3.3. Analysis of Infrared Spectroscopy
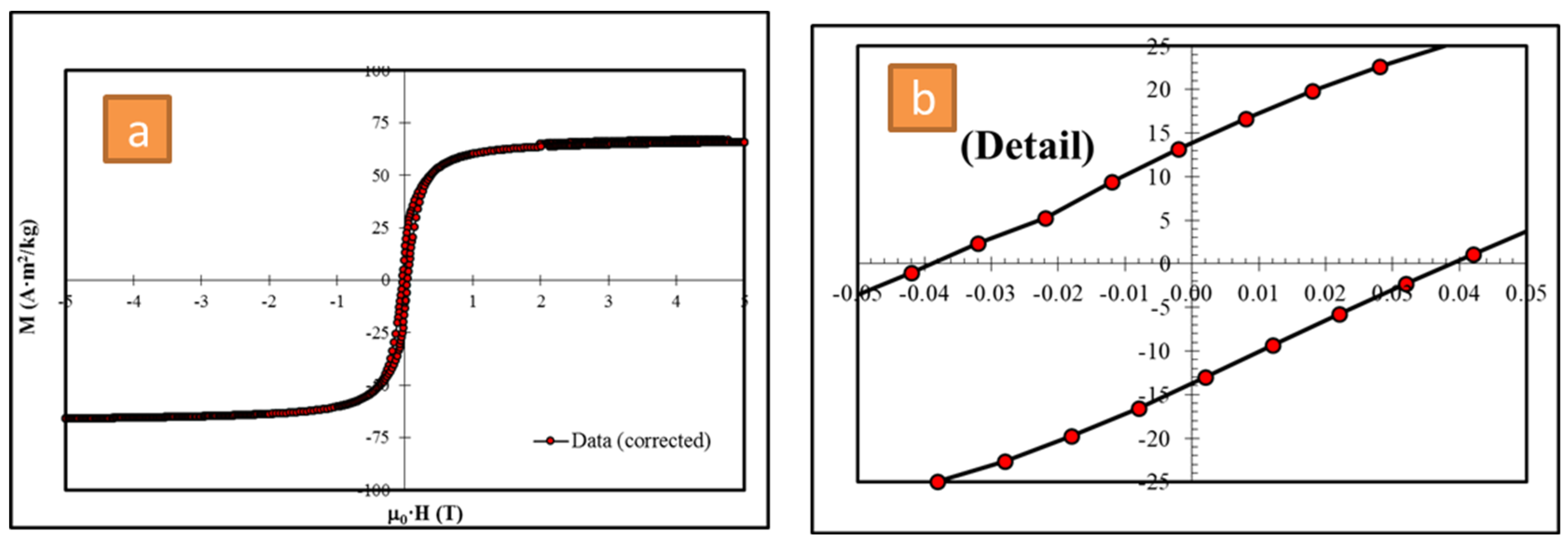
3.4. Magnetic Properties
3.5. Polarized Light Optical Microscopy (POM)
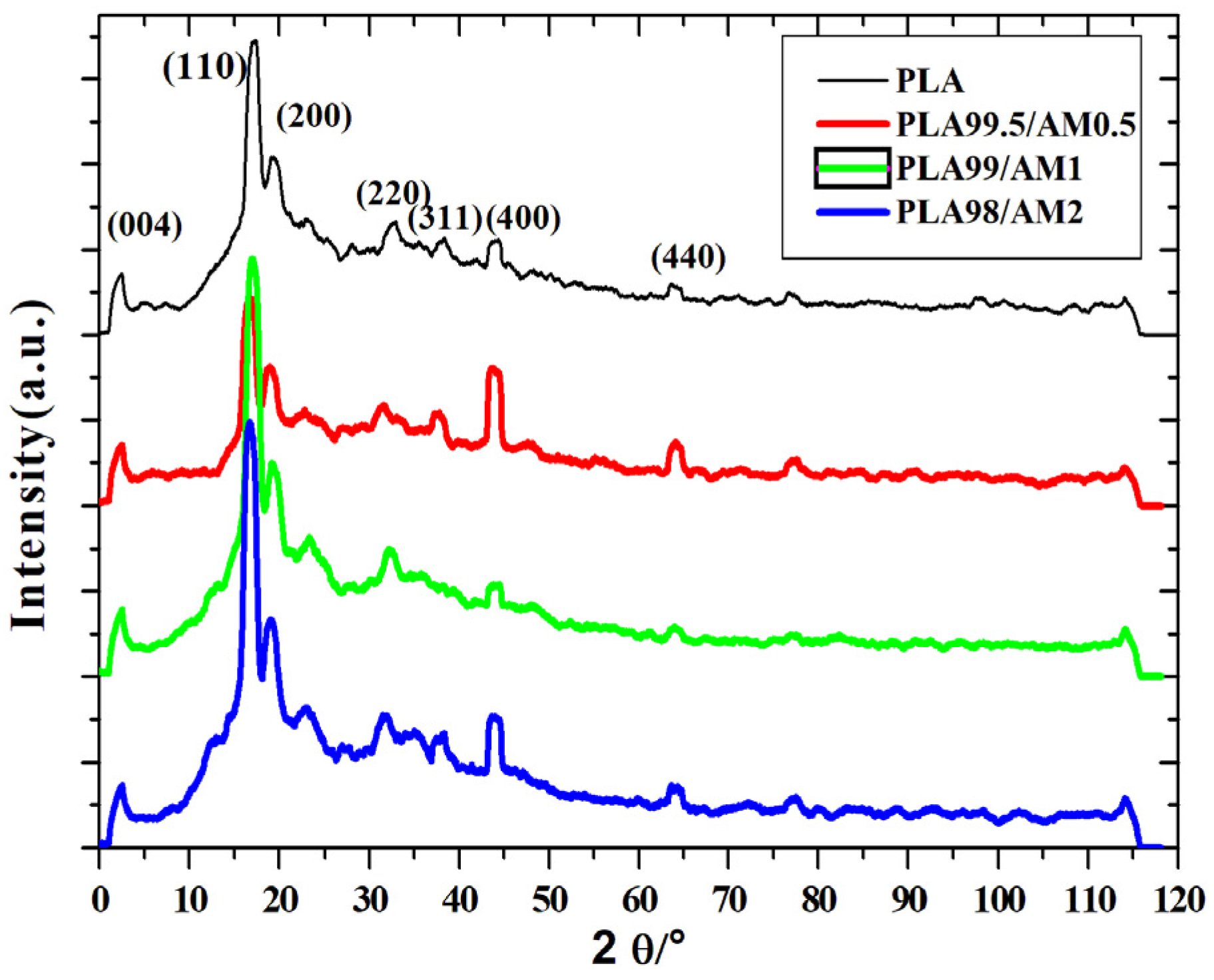
3.6. X-ray Diffraction (XRD) Analysis
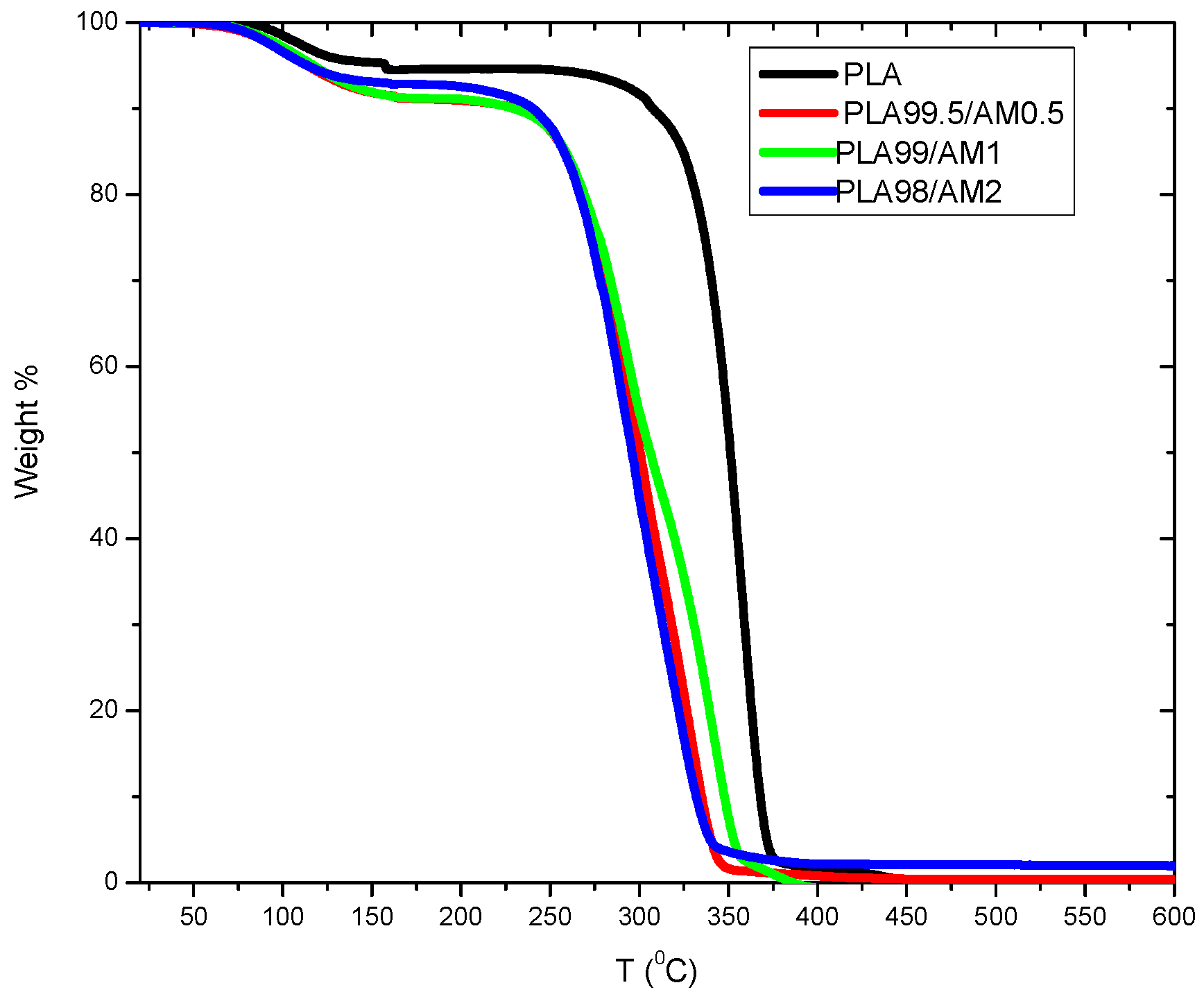
3.7. Thermal Gravimetric Analysis (TGA)
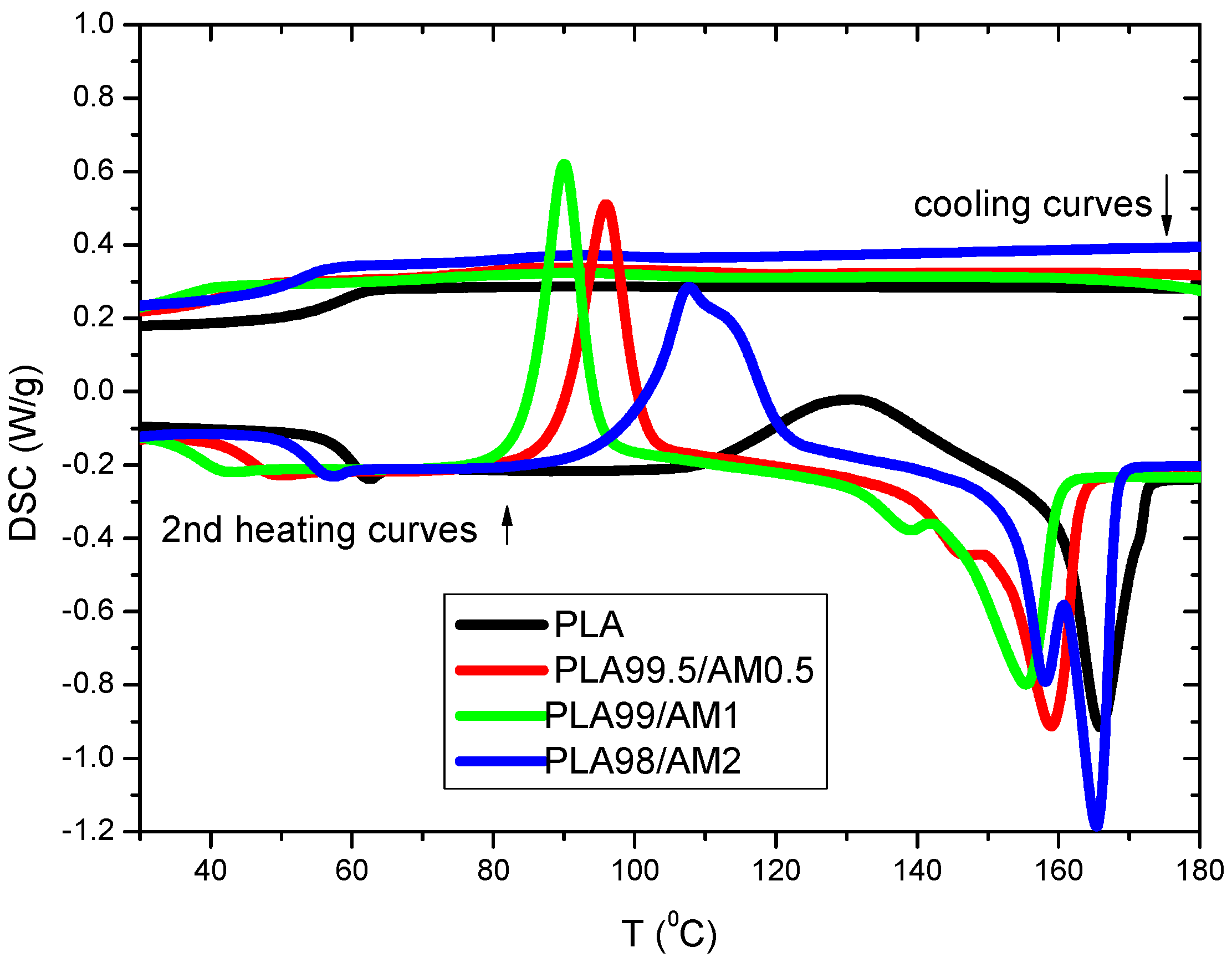
3.8. Differential Scanning Calorimetry (DSC)
3.8.1. Non-Isothermal Cold Crystallization Behaviours of PLA/AM Composites
3.8.2. Non-Isothermal Cold Crystallization Kinetics of PLA/AM Composites
3.8.3. Relative Crystallinity
Half-Time of Crystallization
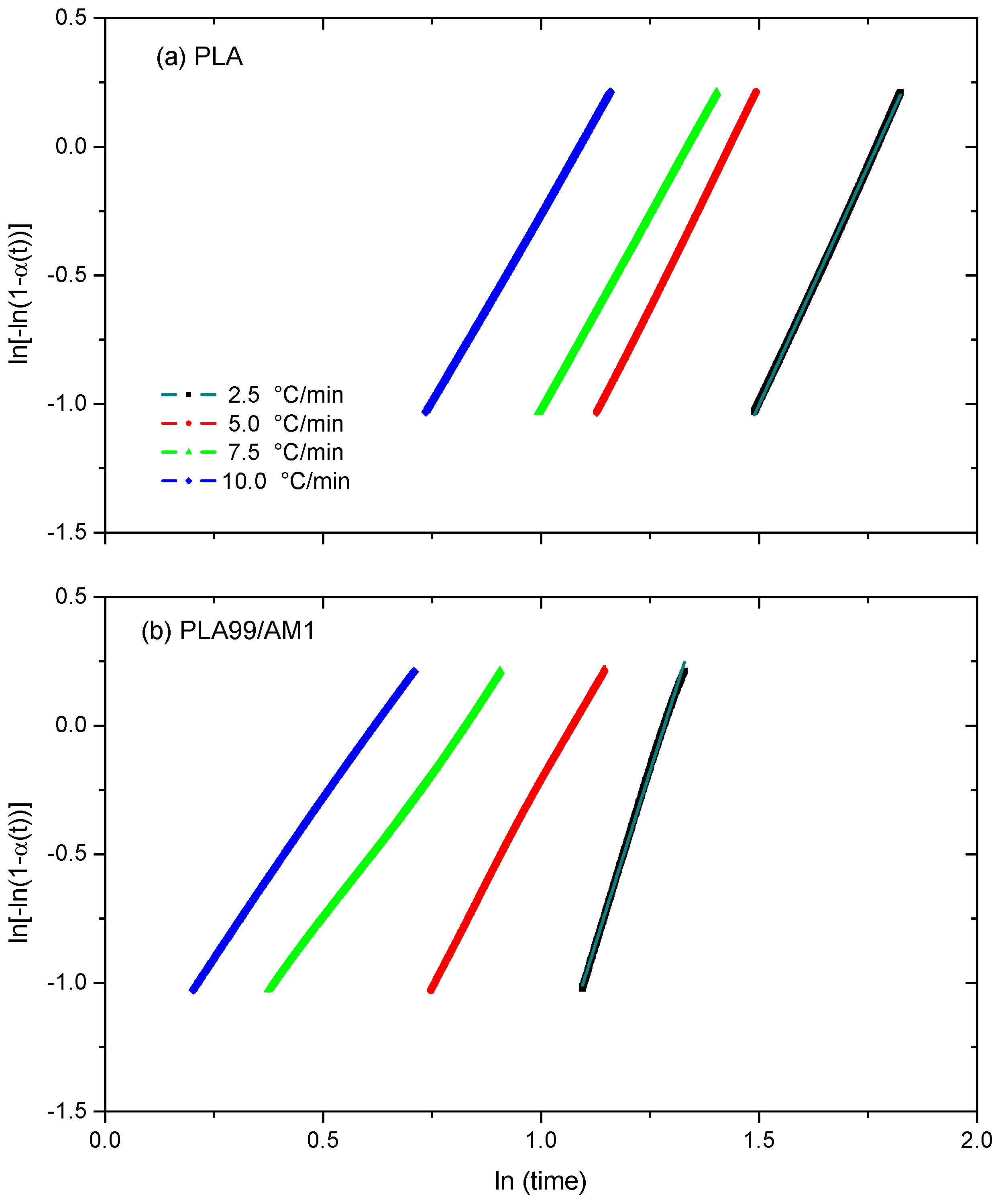
Avrami Model
4. Conclusions
- (i)
- Shift of glass transition temperature (Tg) to a lower value.
- (ii)
- Shift of crystallization temperature to a lower value.
- (iii)
- Shift of melting temperature to a lower value.
- (iv)
- A rise in the rate of crystallization.
- (v)
- Increase in the percent of crystallinity.
- (vi)
- Decrease in thermal stability of PLA samples.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, W.S.; Abu-Dief, A.M. Impact of rare earth europium (RE-Eu3+) ions substitution on microstructural, optical and magnetic properties of CoFe2−xEuxO4 nanosystems. Ceram. Int. 2020, 46, 16196–16209. [Google Scholar] [CrossRef]
- Marzouk, A.A.; Abu-Dief, A.M.; Abdelhamid, A.A. Hydrothermal preparation and characterization of ZnFe2O4 magnetic nanoparticles as an efficient heterogeneous catalyst for the synthesis of multi-substituted imidazoles and study of their anti-inflammatory activity. Appl. Organomet. Chem. 2018, 32, e3794. [Google Scholar] [CrossRef]
- Balaji, A.B.; Pakalapati, H.; Khalid, M.; Walvekar, R.; Siddiqui, H. Natural and Synthetic Biocompatible and Biodegradable Polymers; Elsevier: Amsterdam, The Netherlands, 2017; Volume 25, pp. 3–32. [Google Scholar]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2019, 3, 27–35. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable polymers—An overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Marsilla, K.I.K.; Verbeek, C.J.R. Crystallization of itaconic anhydride grafted poly(lactic acid) during annealing. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Nanthananon, P.; Seadan, M.; Pivsa-Art, S.; Suttiruengwong, S. Enhanced crystallization of poly(lactic acid) through reactive aliphatic bisamide. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012067. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Frache, A. Crystallization kinetics of poly(lactic acid)-talc composites. Express Polym. Lett. 2011, 5, 849–858. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly(lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Tham, W.L.; Poh, B.T.; Mohd Ishak, Z.A.; Chow, W.S. Thermal behaviors and mechanical properties of halloysite nanotube-reinforced poly(lactic acid) nanocomposites. J. Therm. Anal. Calorim. 2014, 118, 1639–1647. [Google Scholar] [CrossRef]
- Mohammed, B.H.; Sherwani, A.F.H.; Faraj, R.H.; Qadir, H.H.; Younis, K.H. Mechanical properties and ductility behavior of ultra-high performance fiber reinforced concretes: Effect of low water-to-binder ratios and micro glass fibers. Ain Shams Eng. J. 2021, 12, 1557–1567. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Li, B.; Wan, G.; Zhao, G.; Zhang, A. Strong and thermal-resistance glass fiber-reinforced polylactic acid (PLA) composites enabled by heat treatment. Int. J. Biol. Macromol. 2019, 129, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, W.; Wang, Y.; Yu, K.; Zhou, H.; Wang, X.; Mi, J. Crystallization-induced microcellular foaming of poly(lactic acid) with high volume expansion ratio. Polym. Degrad. Stab. 2017, 144, 231–240. [Google Scholar] [CrossRef]
- Wu, N.; Wang, H. Effect of zinc phenylphosphonate on the crystallization behavior of poly(l-lactide). J. Appl. Polym. Sci. 2013, 130, 2744–2752. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Xiao, H.W.; Li, P.; Ren, X.; Jiang, T.; Yeh, J. Isothermal crystallization kinetics and crystal structure of poly(lactic acid): Effect of triphenyl phosphate and talc. J. Appl. Polym. Sci. 2010, 118, 3558–3569. [Google Scholar] [CrossRef]
- Mohamed, W.S.; Alzaid, M.; SMAbdelbaky, M.; Amghouz, Z.; García-Granda, S.; MAbu-Dief, A. Impact of Co2+ substitution on microstructure and magnetic properties of CoxZn1−xFe2O4 nanoparticles. Nanomaterials 2019, 9, 1602. [Google Scholar] [CrossRef]
- Fariñas, J.C.; Moreno, R.; Pérez, A.; García, M.A.; García-Hernández, M.; Salvador, M.D.; Borrell, A. Microwave-assisted solution synthesis, microwave sintering and magnetic properties of cobalt ferrite. J. Eur. Ceram. Soc. 2018, 38, 2360–2368. [Google Scholar] [CrossRef]
- Karbasi, M.; Maghazeii, F.; Ghanbari, D. Magnetic investigation of microwave synthesized and thermal stable poly vinyl alcohol-cobalt ferrite nanocomposites. J. Nanostruct. 2019, 9, 365–375. [Google Scholar]
- PAsogekar, P.A.; Verenkar, V. Structural and magnetic properties of nanosized CoxZn(1−x)Fe2O4 (x = 0.0, 0.5, 1.0) synthesized via autocatalytic thermal decomposition of hydrazinated cobalt zinc ferrous succinate. Ceram. Int. 2019, 45, 21793–21803. [Google Scholar] [CrossRef]
- Sartori, K.; Choueikani, F.; Gloter, A.; Begin-Colin, S.; Taverna, D.; Pichon, B.P. Room temperature blocked magnetic nanoparticles based on ferrite promoted by a threestep thermal decomposition process. J. Am. Chem. Soc. 2019, 141, 9783–9787. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, G.; Alzamora, M.; Contreras, C.; Burlandy, G.; Litterst, F.J.; Baggio-Saitovitch, E. Internal structure and magnetic properties in cobalt ferrite nanoparticles: Influence of the synthesis method. Part. Part. Syst. Charact. 2019, 36, 1900061. [Google Scholar] [CrossRef]
- Sagadevan, S.; Podder, J.; Das, I. Synthesis and characterization of cobalt ferrite (CoFe2O4) nanoparticles prepared by hydrothermal method. In Recent Trends in Materials Science and Applications; Ebenezar, J., Ed.; Springer Proceedings in Physics; Springer: Cham, Switzerland, 2017; Volume 189. [Google Scholar]
- Gmelin, E.; Sarge, M.S. Calibration of differential scanning calorimeters. Pure Appl. Chem. 1995, 67, 1789–1800. [Google Scholar] [CrossRef]
- Migliaresi, C.; Cohn, D.; De Lollis, A.; Fambri, L. Dynamic mechanical and calorimetric analysis of compression-molded PLLA of different molecular weights: Effect of thermal treatments. J. Appl. Polym. Sci. 1991, 43, 83–95. [Google Scholar] [CrossRef]
- Routray, K.L.; Saha, S.; Behera, D. Green synthesis approach for nano sized CoFe2O4through aloe vera mediated sol-gel auto combustion method for high frequency devices. Mater. Chem. Phys. 2018, 224, 29–35. [Google Scholar] [CrossRef]
- Abd El Aleem, M.; El-Remaily, A.A.; Abu-Dief, A.M. CuFe2O4 nanoparticles: An efficient heterogeneous magnetically separable catalyst for synthesis of some novel propynyl-1H-imidazoles derivatives. Tetrahedron 2015, 71, 2579–2584. [Google Scholar]
- Abu-Dief, A.M.; Abdelbaky, M.S.; Martínez-Blanco, D.; Amghouz, Z.; García-Granda, S. Effect of chromium substitution on the structural and magnetic properties of nanocrystalline zinc ferrite. Mater. Chem. Phys. 2016, 174, 164–171. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, W.S. α-Bi2O3 nanorods: Synthesis, characterization and UV-photocatalytic activity. Mater. Res. Express 2017, 4, 035039. [Google Scholar] [CrossRef]
- Alahmadi, M.; Alsaedi, W.H.; Mohamed, W.S.; Hassan, H.M.; Ezzeldien, M.; Abu-Dief, A.M. Development of Bi2O3/MoSe2 mixed nanostructures for photocatalytic degradation of methylene blue dye. J. Taibah Univ. Sci. 2023, 17, 2161333. [Google Scholar] [CrossRef]
- Tahir, M.B.; Iqbal, T.; Hassan, A.; Ghazal, S. Wet chemical Co-precipitation synthesis of nickel ferrite nanoparticles and their characterization. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1430–1438. [Google Scholar] [CrossRef]
- Mehran, E.; Shayesteh, S.F.; Sheykhan, M. Structural and magnetic properties of turmeric functionalized CoFe2O4 nanocomposite powder. Chin. Phys. B 2016, 25, 107504. [Google Scholar] [CrossRef]
- Thakur, P.; Sharma, R.; Sharma, V.; Barman, P.B.; Kumar, M.; Barman, D.; Katyal, S.C.; Sharma, P. Gd doped Mn-Zn soft ferrite nanoparticles: Superparamagnetism and its correlation with other physical properties. J. Magn. Magn. Mater. 2017, 432, 208–217. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared Spectra of Ferrites. Phys. Rev. 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Bakhshi, H.; Shokuhfar, A.; Afghahi, S.S.S. Structural, magnetic and Raman study of CoFe2O4@C core–shell nanoparticles. Ceram. Int. 2015, 41, 10736–10744. [Google Scholar] [CrossRef]
- Sharma, R.; Thakur, P.; Kumar, M.; Thakur, N.; Negi, N.S.; Sharma, P.; Sharma, V. Improvement in magnetic behaviour of cobalt doped magnesium zinc nano-ferrites via co-precipitation route. J. Alloys Compd. 2016, 684, 569–581. [Google Scholar] [CrossRef]
- Vinothkumar, P.; Manoharan, C.; Shanmugapriya, B.; Bououdina, M. Effect of reaction time on structural, morphological, optical and photocatalytic properties of copper oxide (CuO) nanostructures. J. Mater. Sci. Mater. Electron. 2019, 30, 6249–6262. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Nassar, I.F.; Elsayed, W.H. Magnetic NiFe2O4 nanoparticles: Efficient, heterogeneous and reusable catalyst for synthesis of acetylferrocene chalcones and their anti-tumour activity. Appl. Organomet. Chem. 2016, 30, 917–923. [Google Scholar] [CrossRef]
- Aleem Ali El-Remaily, M.A.E.; Abu-Dief, A.M.; El-Khatib, R.M. A robust synthesis and characterization of superparamagnetic CoFe2O4 nanoparticles as an efficient and reusable catalyst for green synthesis of some heterocyclic rings. Appl. Organomet. Chem. 2016, 30, 1022–1029. [Google Scholar] [CrossRef]
- Mohamed, W.S.; Hadia, N.M.A.; Alzaid, M.; Abu-Dief, A.M. Impact of Cu2+ cations substitution on structural, morphological, optical and magnetic properties of Co1-xCuxFe2O4 nanoparticles synthesized by a facile hydrothermal approach. Solid State Sci. 2022, 125, 106841. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, Z. Enhanced nonisothermal and isothermal cold crystallization kinetics of biodegradable poly(l-lactide) by trisilanolisobutyl-polyhedral oligomeric silsesquioxanes in their nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43896. [Google Scholar] [CrossRef]
- Tri, P.N.; Domenek, S.; Guinault, A.; Sollogoub, C. Crystallization behavior of poly(lactide)/poly(β-hydroxybutyrate)/talc composites. J. Appl. Polym. Sci. 2013, 129, 3355–3365. [Google Scholar] [CrossRef]
- Li, C.; Dou, Q. Non-isothermal crystallization kinetics and spherulitic morphology of nucleated poly(lactic acid): Effect of dilithium hexahydrophthalate as a novel nucleating agent. Thermochim. Acta 2014, 594, e31–e38. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing kinetics of nonisothermal crystallization of poly(ethylene-terephthalate) determined by DSC. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Li, M.; Hu, D.; Wang, Y.; Shen, C. Nonisothermal Crystallization Kinetics of Poly(lactic acid) Formulations Comprising Talc With Poly(ethylene glycol). Polym. Eng. Sci. 2010, 50, 2298–2305. [Google Scholar] [CrossRef]
- Li, C.; Dou, Q.; Bai, Z.; Lu, Q. Non-isothermal crystallization behaviors and spherulitic morphology of poly(lactic acid) nucleated by a novel nucleating agent. J. Therm. Anal. Calorim. 2015, 122, 407–417. [Google Scholar] [CrossRef]
- Wu, J.; Zou, X.; Jing, B.; Dai, W. Effect of Sepiolite on the Crystallization Behavior of Biodegradable Poly(lactic acid) as an Efficient Nucleating Agent. Society 2015, 55, 1104–1112. [Google Scholar] [CrossRef]
- Shi, N.; Dou, Q. Non-isothermal cold crystallization kinetics of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/treated calcium carbonate composites. J. Therm. Anal. Calorim. 2015, 119, 635–642. [Google Scholar] [CrossRef]
- Xiong, J.; Gong, D.-P.; Sun, Y.-M.; Zhao, X.-P. Effect of sulfur phase transition on polypropylene crystallization. Polym. Technol. Mater. 2019, 58, 1342–1353. [Google Scholar] [CrossRef]





| Sample Name | PLA/Spinel Ferrites Co0.9Cu0.1Fe2O4 (AM) |
|---|---|
| PLA | 100:0 |
| PLA99.5/AM0.5 | 99.5:0.5 |
| PLA99/AM1 | 99:1 |
| PLA98/AM2 | 98:2 |
| Name of PLA Samples | PLA Phase | |
|---|---|---|
| Tonset | Tend | |
| PLA | 334 | 371 |
| PLA99.5/AM0.5 | 269 | 342 |
| PLA99/AM1 | 281 | 356 |
| PLA98/AM2 | 261 | 335 |
| Samples | Tg (°C) | ΔCp (J/g °C) | Tcc (°C) | ΔHcc (J/g °C) | Tm (°C) | ΔHm (J/g °C) | Xc (%) |
|---|---|---|---|---|---|---|---|
| PLA | 59 | 0.55 | 131 | 27.97 | 166 | 33.43 | 29.88 |
| PLA99.5/AM0.5 | 46 | 0.54 | 96 | 40.78 | 147–159 | 46.01 | 43.79 |
| PLA99/AM1 | 39 | 0.48 | 90 | 40.80 | 139–155 | 42.38 | 44.03 |
| PLA98/AM2 | 55 | 0.48 | 108 | 46.18 | 158–165 | 54.75 | 50.34 |
| Samples | Heating Rate (°C/min) | Tcc (°C) | ∆Hcc (J/g °C) | Xc (%) |
|---|---|---|---|---|
| PLA | 2.5 | 33.7 | 31.55 | 101 |
| 5 | 36.4 | 34.1 | 111 | |
| 7.5 | 42.3 | 39.51 | 125 | |
| 10 | 35.8 | 33.51 | 127 | |
| PLA99/AM1 | 2.5 | 28.5 | 26.41 | 67 |
| 5 | 41.1 | 38.04 | 77 | |
| 7.5 | 43.7 | 40.46 | 78–85 | |
| 10 | 55.6 | 51.53 | 102 |
| Sample | Φ | t0.5 Min | CRP (1/°C) | n | k | R2 |
|---|---|---|---|---|---|---|
| PLA | 2.5 | 5.33 | 0.024 | 3.71 | 0.064 | 1.00 |
| 5 | 3.77 | 3.42 | 0.375 | 1.00 | ||
| 7.5 | 3.37 | 3.06 | 0.581 | 1.00 | ||
| 10 | 2.63 | 2.94 | 0.726 | 1.00 | ||
| PLA99/AM1 | 2.5 | 3.36 | 0.045 | 3.37 | 0.072 | 1.00 |
| 5 | 2.59 | 3.12 | 0.512 | 1.00 | ||
| 7.5 | 1.96 | 2.29 | 0.777 | 1.00 | ||
| 10 | 1.59 | 2.44 | 0.86 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaedi, W.H.; Abu Al-Ola, K.A.; Alhaddad, O.; Albelwe, Z.; Alawaji, R.; Abu-Dief, A.M. Effect of Nano Spinel Ferrites Co0.9Cu0.1Fe2O4 on Non-Isothermal Cold Crystallization Behaviours and Kinetics of Its Composites with Polylactic Acid. Polymers 2024, 16, 1190. https://doi.org/10.3390/polym16091190
Alsaedi WH, Abu Al-Ola KA, Alhaddad O, Albelwe Z, Alawaji R, Abu-Dief AM. Effect of Nano Spinel Ferrites Co0.9Cu0.1Fe2O4 on Non-Isothermal Cold Crystallization Behaviours and Kinetics of Its Composites with Polylactic Acid. Polymers. 2024; 16(9):1190. https://doi.org/10.3390/polym16091190
Chicago/Turabian StyleAlsaedi, Wael H., Khulood A. Abu Al-Ola, Omaima Alhaddad, Zyzafon Albelwe, Renad Alawaji, and Ahmed M. Abu-Dief. 2024. "Effect of Nano Spinel Ferrites Co0.9Cu0.1Fe2O4 on Non-Isothermal Cold Crystallization Behaviours and Kinetics of Its Composites with Polylactic Acid" Polymers 16, no. 9: 1190. https://doi.org/10.3390/polym16091190






