Non-Isothermal Cold-Crystallization Behavior and Kinetics of Poly(l-Lactic Acid)/WS2 Inorganic Nanotube Nanocomposites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Processing
2.2. Characterization Techniques
3. Results and Discussion
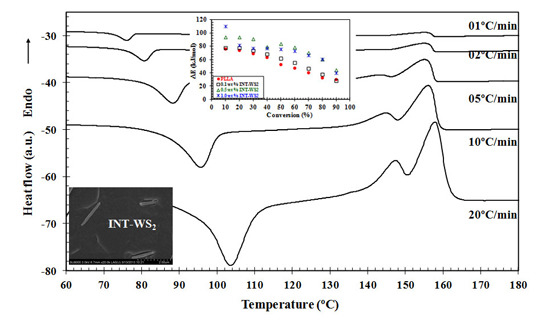
3.1. Non-Isothermal Cold-Crystallization Behavior

| INT-WS2 content (wt %) | ϕh (°C/min) | Tcc (°C) | (1-λ)cc (%) | Tm1 (°C) | Tm2 (°C) | (1-λ)m (%) |
|---|---|---|---|---|---|---|
| 0.0 | 1 | 78.5 | 33.7 | 133.8 | 152.0 | 38.9 |
| 2 | 83.7 | 37.7 | 135.3 | 151.6 | 41.1 | |
| 5 | 91.5 | 44.5 | 137.1 | 151.3 | 45.3 | |
| 10 | 99.9 | 45.7 | 139.1 | 152.1 | 46.8 | |
| 20 | 109.8 | 45.4 | 143.4 | 153.9 | 46.1 | |
| 0.1 | 1 | 77.6 | 32.2 | 134.9 | 152.9 | 39.7 |
| 2 | 83.0 | 34.4 | 136.0 | 152.3 | 40.8 | |
| 5 | 91.1 | 42.9 | 137.8 | 151.6 | 46.2 | |
| 10 | 98.6 | 44.8 | 140.0 | 152.7 | 47.1 | |
| 20 | 108.4 | 44.6 | 143.6 | 154.8 | 46.4 | |
| 0.5 | 1 | 75.9 | 28.1 | 138.5 | 154.2 | 40.8 |
| 2 | 80.5 | 32.4 | 139.8 | 154.2 | 42.7 | |
| 5 | 88.3 | 40.6 | 135.8 | 151.0 | 47.0 | |
| 10 | 94.7 | 42.6 | 137.8 | 151.3 | 47.9 | |
| 20 | 103.5 | 44.4 | 140.9 | 153.0 | 47.5 | |
| 1.0 | 1 | 76.0 | 25.0 | 140.4 | 155.1 | 43.0 |
| 2 | 80.7 | 30.5 | 141.6 | 155.0 | 46.1 | |
| 5 | 88.2 | 33.1 | 143.2 | 155.1 | 48.2 | |
| 10 | 95.6 | 40.5 | 145.0 | 156.0 | 49.7 | |
| 20 | 103.6 | 43.0 | 147.4 | 157.8 | 45.5 |




3.2. Lui Model

| INT-WS2 content (wt %) | x (%) | α | F (T) | ΔEa (kJ/mol) |
|---|---|---|---|---|
| 0.0 | 10 | 1.24 | 83.58 | 100.8 |
| 30 | 1.25 | 90.89 | ||
| 50 | 1.26 | 95.76 | ||
| 70 | 1.27 | 101.73 | ||
| 90 | 1.29 | 114.42 | ||
| 0.1 | 10 | 1.24 | 80.29 | 102.7 |
| 30 | 1.24 | 87.02 | ||
| 50 | 1.26 | 93.30 | ||
| 70 | 1.27 | 99.31 | ||
| 90 | 1.30 | 111.33 | ||
| 0.5 | 10 | 1.23 | 73.09 | 112.8 |
| 30 | 1.24 | 79.65 | ||
| 50 | 1.24 | 83.31 | ||
| 70 | 1.25 | 87.24 | ||
| 90 | 1.26 | 95.24 | ||
| 1.0 | 10 | 1.29 | 62.41 | 111.9 |
| 30 | 1.22 | 74.47 | ||
| 50 | 1.24 | 81.18 | ||
| 70 | 1.24 | 85.88 | ||
| 90 | 1.26 | 95.10 |
3.3. Effective Energy Barrier


3.4. Melting Behaviour



4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Sodergard, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janrkor, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–882. [Google Scholar] [CrossRef]
- Othman, N.; Acosta-Ramírez, A.; Mehrkhodavandi, P.; Dorgan, J.R.S.; Hatzikiriakos, G. Solution and melt viscoelastic properties of controlled microstructure poly(lactide). J. Rheol. 2011, 55, 987–1005. [Google Scholar] [CrossRef]
- Othman, N.; Xu, C.; Mehrkhodavandi, P.; Hatzikiriakos, S.G. Thermorheological and mechanical behavior of polylactide and its enantiomeric diblock copolymers and blends. Polymer 2012, 53, 2443–2452. [Google Scholar] [CrossRef]
- Saeidou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. Opportunities and challenges in the use of inorganic fullerene-like nanoparticles to produce advanced polymer nanocomposites. Prog. Polym. Sci. 2013, 38, 1163–1231. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M. Thermoplastic polymer nanocomposites based on inorganic fullerene-like nanoparticles and inorganic nanotubes. Inorganics 2014, 2, 291–312. [Google Scholar] [CrossRef] [Green Version]
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of tungsten disulphide. Nature 1992, 360, 444–445. [Google Scholar] [CrossRef]
- Margulis, L.; Salitra, G.; Tenne, R.; Talianker, M. Nested fullerene-like structures. Nature 1993, 365, 113–114. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Nath, M. Inorganic nanotubes. Dalton Trans. 2003, 1–24. [Google Scholar] [CrossRef]
- Remškar, M. Inorganic nanotubes. Adv. Mater. 2004, 16, 1497–1504. [Google Scholar] [CrossRef]
- Tenne, R. Inorganic nanotubes and fullerene-like nanoparticles. Nat. Nanotechnol. 2006, 1, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Tenne, R.; Redlich, M. Recent progress in the research of inorganic fullerene-like nanoparticles and inorganic nanotubes. Chem. Soc. Rev. 2001, 39, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Zak, A.; Sallacan-Ecker, L.; Margolin, A.; Genut, M.; Tenne, R. Insight into the growth mechanism of WS2 nanotubes in the scaled-up fluidized-bed reactor. Nano 2009, 4, 91–98. [Google Scholar] [CrossRef]
- Zak, A.; Sallacan Ecker, L.; Fleischer, N.; Tenne, R. Large-scale synthesis of WS2 multiwall nanotubes and their dispersion, an update. Sens. Transducers. J. 2011, 12, 1–10. [Google Scholar]
- Zhu, Y.Q.; Sekine, T.; Brigatti, K.S.; Firth, S.; Tenne, R.; Rosentsveig, R.; Kroto, H.W.; Walton, D.R. Shock-wave resistance of WS2 nanotubes. J. Am. Chem. Soc. 2003, 125, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Komarneni, M.; Sand, A.; Nevin, P.; Zak, A.; Burghaus, U. Absorption and reaction kinetics of small organic molecules on WS2 nanotubes: An ultra-high vacuum study. Chem. Phys. Lett. 2009, 479, 109–112. [Google Scholar] [CrossRef]
- Pardo, M.; Shuster-Meiseles, T.; Levin-Zaidman, S.; Rudich, A.; Rudich, Y. Low cytotoxicity of inorganic nanotubes and fullerene-like nanostructures in human bronchial epithelial cells: Relation to inflammatory gene induction and antioxidant response. Environ. Sci. Technol. 2014, 48, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.B.; Zak, A.; Tenne, R.; Kartvelishvily, E.; Levin-Zaidman, S.; Neumann, Y.; Stiubea-Cohen, R.; Palmon, A.; Hovav, A.H.; Aframian, D.J. Biocompatibility of tungsten disulfide inorganic nanotubes and fullerene-like nanoparticles with salivary gland cells. Tissue Eng. Part A 2015, 21, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.S.; Zak, A.; Zussman, E. WS2 nanotubes embedded in PMMA nanofibers as energy absorptive material. J. Mater. Chem. 2001, 21, 16086–16093. [Google Scholar] [CrossRef]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Parmar, P.; Lin, L.; Qin, Y.X.; Kasper, F.K.; Mikos, A.G.; Sitharaman, B. Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering. Acta Biomater. 2013, 9, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Marco, C.; Ellis, G. Inorganic WS2 nanotubes that improve the crystallization behavior of poly(3-hydroxybutyrate). CrystEngComm 2014, 16, 1126–1135. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M. Nanocomposite biomaterials based on poly(etherether-ketone) (PEEK) and WS2 inorganic nanotubes. J. Mater. Chem. B 2014, 2, 4509–4520. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G. Development of novel melt-processable biopolymer nanocomposites based on poly(l-lactic acid) and WS2 inorganic nanotubes. CrystEngComm 2013, 16, 5062–5072. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Miller, R.L. Kinetics of crystallization from the melt and chain folding in polyethylene fractions revisited: theory and experiment. Polymer 1997, 38, 3151–3212. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Property of Polymers, 2nd ed; Wiley: New York, NY, USA, 1970. [Google Scholar]
- Di Lorenzo, M.L.; Silvestre, C. Non-isothermal crystallization of polymers. Prog. Polym. Sci. 1999, 24, 917–950. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase changes. I General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. III Granulation, phase change, and microstructure. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 128, 150–158. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Fridman, H. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C 1964, 6, 183–195. [Google Scholar] [CrossRef]
- He, Y.; Fan, Z.Y.; Wei, J.; Li, S.M. Morphology and melt crystallization of poly(l-lactide) obtained by ring opening polymerization of l-lactide with zinc catalyst. Polym. Eng. Sci. 2006, 46, 1583–1589. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, J.; Shao, L.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y. Comparative study of poly(l-lactide) nanocomposites with organic montmorillonite and carbon nanotubes. J. Polym. Sci. Polym. Phys. 2013, 51, 183–196. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naffakh, M.; Marco, C.; Ellis, G. Non-Isothermal Cold-Crystallization Behavior and Kinetics of Poly(l-Lactic Acid)/WS2 Inorganic Nanotube Nanocomposites. Polymers 2015, 7, 2175-2189. https://doi.org/10.3390/polym7111507
Naffakh M, Marco C, Ellis G. Non-Isothermal Cold-Crystallization Behavior and Kinetics of Poly(l-Lactic Acid)/WS2 Inorganic Nanotube Nanocomposites. Polymers. 2015; 7(11):2175-2189. https://doi.org/10.3390/polym7111507
Chicago/Turabian StyleNaffakh, Mohammed, Carlos Marco, and Gary Ellis. 2015. "Non-Isothermal Cold-Crystallization Behavior and Kinetics of Poly(l-Lactic Acid)/WS2 Inorganic Nanotube Nanocomposites" Polymers 7, no. 11: 2175-2189. https://doi.org/10.3390/polym7111507