Synthesis and Characterization of [60]Fullerene-Glycidyl Azide Polymer and Its Thermal Decomposition
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Malonic Acid Glycidyl Azide Polymer Ester
2.3. Synthesis of Bromomalonic Acid Glycidyl Azide Polymer Ester
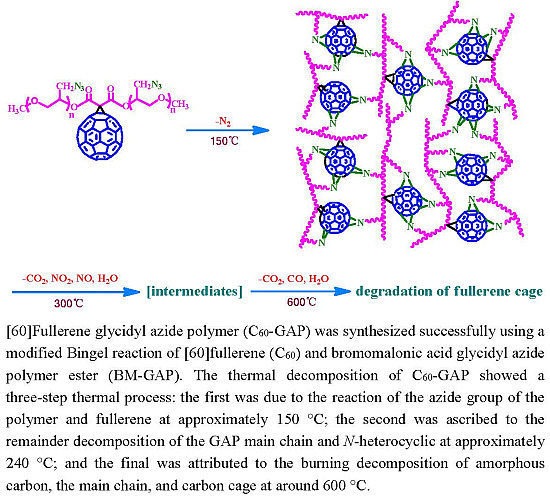
2.4. Synthesis of [60]Fullerene Glycidyl Azide Polymer
2.5. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of C60-GAP





3.2. Thermal Analysis




4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hirsch, A. The Chemistry of the Fullerenes; Thieme Medical Publishers: Stuttgart, Germany, 1994. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes: Their Properties and Applications; Academic Press: Waltham, MA, USA, 1996. [Google Scholar]
- Astefanei, A.; Nunez, O.; Galceran, M.T. Analysis of C60-fullerene derivatives and pristine fullerenes in environmental samples by ultrahigh performance liquid chromatography-atmospheric pressure photoionization-mass spectrometry. J. Chromatogr. A 2014, 1365, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.P.; Jazdzyk, M. The preparation and structures of non-hydrocarbon functionalized fullerene-diamine adducts. Chem. Commun. 2003, 13, 1530–1531. [Google Scholar] [CrossRef]
- Chaves, C.W.; Moraes, D.E. Single or functionalized fullerenes interacting with heme group. AIP Adv. 2014, 9, 097119. [Google Scholar]
- Tzirakis, M.D.; Orfanopoulos, M. Radical reactions of fullerenes: from synthetic organic chemistry to materials science and biology. Chem. Rev. 2013, 7, 5262–5321. [Google Scholar] [CrossRef]
- Han, X.; Wang, T.F.; Lin, Z.K.; Han, D.L.; Li, S.F.; Zhao, F.Q. RDX/AP-CMDB propellants containing fullerenes and carbon black additives. Defence Sci. J. 2009, 3, 284–293. [Google Scholar] [CrossRef]
- Jin, B.; Peng, R.F.; Zhao, F.Q.; Yi, J.H.; Xu, S.Y.; Wang, S.B.; Chu, S.J. Combustion effects of nitrofulleropyrrolidine on RDX-CMDB propellants. Propellants Explos. Pyrotech. 2014, 39, 874–880. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Bhonsle, J.B.; Wang, L.; Shu, S.F.; Chang, T.M.; Hwu, J.R. Efficient one-flask synthesis of water-soluble [60]fullerenols. Tetrahedron 1996, 14, 4936–4972. [Google Scholar]
- Hamwi, A.; Marchand, V. Oxidized fullerene derivatives containing hydroxyl, nitro and fluorine groups. Fullerene Sci. Technol. 1996, 5, 835–851. [Google Scholar]
- Franco, J.U.; Ell, J.R.; Hilton, A.K.; Hammons, J.C.; Olmstead, M.M. C60Cl6, C60Br8 and C60(NO2)6 as selective tools in organic synthesis. Fuller. Nanotub. Carbon Nanostruct. 2009, 4, 349–360. [Google Scholar] [CrossRef]
- Cataldo, F. Preparation of polynitrofullerene by the action of dinitrogen tetroxide on C60. Fullerene Sci. Technol. 1997, 1, 257–265. [Google Scholar] [CrossRef]
- Franco, C.; Ornella, U.; Giancarlo, A. Synthesis and explosive decomposition of polynitro[60]fullerene. Carbon 2013, 62, 413–421. [Google Scholar] [CrossRef]
- Zhai, R.S.; Arindam, D.; Chien, K.H.; Chau, C.H.; Taizoon, C.; Long, Y.C. Polymeric fullerene oxide films produced by decomposition of hexanitro[60]fullerene. Carbon 2004, 2, 395–403. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, H.; Patil, M.; Thiel, W.; Pant, C.S.; Banerjee, S. Synthesis, characterization, thermal and computational studies of novel tetra-azido esters as energetic plasticizers. Thermochim. Acta 2013, 562, 96–104. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, K.T.; Jung, H.; Kim, S.H.; Park, S.; Jeon, H.B.; Paik, H.; Min, B.S.; Kin, W. Characterization of 1,2,3-triazole crosslinked polymers based on azide chain-ends prepolymers and a dipolarophile curing agent as propellant binders: The effect of a plasticizer. J. Taiwan Inst. Chem. Eng. 2014, 6, 3110–3116. [Google Scholar] [CrossRef]
- Min, B.S.; Kim, C.K.; Ryoo, M.S.; Kim, S.Y. Azide-bearing polymer-based solid composite propellant prepared by a dual curing system consisting of a urethane-forming reaction and a dipolar addition reaction. Fuel 2014, 15, 165–171. [Google Scholar] [CrossRef]
- Ding, Y.Z.; Hu, C.; Guo, X.; Che, Y.Y.; Huang, J. Structure and mechanical properties of novel composites based on glycidyl azide polymer and propargyl-terminated polybutadiene as potential binder of solid propellant. J. Appl. Polym. Sci. 2014, 7, 40007. [Google Scholar]
- Huang, T.; Jin, B.; Peng, R.F.; Chu, S.J. Synthesis and characterization of a new energetic plasticizer: Acyl-terminated GAP. Int. J. Polym. Anal. Charact. 2014, 6, 522–531. [Google Scholar] [CrossRef]
- Jin, B.; Dong, H.S.; Peng, R.F.; Shen, J.; Tan, B.S.; Chu, S.J. Synthesis and characterization of poly(vinyl 2,4,6-trinitrophenylacetal) as a new energetic binder. J. Appl. Polym. Sci. 2011, 3, 1643–1648. [Google Scholar] [CrossRef]
- Frankel, M.B.; Grant, L.R.; Flanagan, J.E. Historical development of glycidyl azide polymer. J. Propuls. Power 1992, 3, 560–563. [Google Scholar] [CrossRef]
- Kim, E.S.; Yang, V.; Lian, Y.C. Modeling of HMX/GAP pseudo-propellant combustion. Combust. Flame 2002, 3, 227–245. [Google Scholar] [CrossRef]
- Hu, C.; Guo, X.; Jing, Y.; Chen, J.; Zhang, C.; Huang, J. Structure and mechanical properties of crosslinked glycidyl azide polymers via click chemistry as potential binder of solid propellant. J. Appl. Polym. Sci. 2014, 16, 40636. [Google Scholar]
- Li, N.; Xiong, X.F.; Mo, H.C.; Lu, X.M.; Liu, Y.J. Synthesis and properties of azide terminated and acetate terminated glycidyl azide polymer. J. Solid Rocket Technol. 2013, 2, 234–236. [Google Scholar]
- Jin, B.; Shen, J.; Peng, R.F.; Zheng, R.Z.; Chu, S.J. Efficient cyclopropanation of [60]fullerene starting from bromo-substituted active methylene compounds without using a basic catalyst. Tetrahedron Lett. 2014, 36, 5007–5010. [Google Scholar] [CrossRef]
- Guerra, S.; Trinh, T.M.N.; Schillinger, F.; Muhlberger, L.; Sigwalt, D.; Holler, M. The di-t-butylsilylene protecting group as a bridging unit in linear and macrocyclic bis-malonates for the regioselective multifunctionalization of C60. Tetrahedron Lett. 2013, 46, 6251–6257. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Luo, Y.J.; Li, X.M. Synthesis and characterization of BAMO-r-GAP copolymer. Polym. Mater. Sci. Eng. 2012, 9, 1–4. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Raju, K.M. Synthesis and characterization of low molecular weight azido polymers as high energetic plasticizers. Int. J. Polym. Anal. Charact. 2004, 5, 289–304. [Google Scholar] [CrossRef]
- Sigwalt, D.; Schillinger, F.; Guerra, S.; Holler, M.; Berville, M.; Nierengarten, J.F. An expeditious regioselective synthesis of [60]fullerene e,e,e tris-adduct building blocks. Tetrahedron Lett. 2013, 32, 4241–4244. [Google Scholar] [CrossRef]
- Shirosaki, T.; Harisaki, R.; Horikawa, M.; Sakurai, H.; Nagaoka, S.; Ihara, H. Synthesis of a series of malonic diester-introduced fullerene derivatives. Synth. Commun. 2014, 2, 275–279. [Google Scholar] [CrossRef]
- Wang, G.W.; Li, J.X.; Xu, Y. Synthesis of C60-fused tetrahydrothiophene derivatives via nucleophilic cycloaddition of thiocyanates. Org. Biomol. Chem. 2008, 16, 2995–2999. [Google Scholar] [CrossRef]
- Li, F.B.; You, X.; Wang, G.W. Synthesis of disubstituted [60]fullerene-fused lactones: Ferric perchlorate promoted reaction of [60]fullerene with malonate esters. Org. Lett. 2010, 21, 4896–4899. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Liu, Z.R.; Wang, X.H.; Heng, S.Y.; Pan, Q.; Zhao, F.Q. Investigation on thermal decomposition of GAP by FTIR spectroanalysis. J. Solid Rocket Technol. 2010, 5, 549–553. [Google Scholar]
- Korobeinichev, O.P.; Kuibida, L.V.; Volkov, E.N.; Shmakov, A.G. Mass spectrometric study of combustion and thermal decomposition of GAP. Combust. Flame 2002, 1, 136–150. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S. The effect of nitrate esters on the thermal decomposition mechanism of GAP. J. Hhazard. Mater. 2008, 1, 112–117. [Google Scholar] [CrossRef]
- Kay, K.Y.; Han, K.J.; Yu, Y.J.; Park, Y.D. Dendritic fullerenes (C60) with photoresponsive azobenzene groups. Tetrahedron Lett. 2002, 29, 5053–5056. [Google Scholar] [CrossRef]
- González, S.; Martín, N.; Swartz, A.; Guldi, D.M. Addition reaction of azido-exTTFs to C60: Synthesis of fullerotriazoline and azafulleroid electroactive dyads. Org. Lett. 2003, 4, 557–560. [Google Scholar] [CrossRef]
- Fazhoğlu, H.; Hacaloğlu, J. The effect of triethanolamine on thermal decomposition of GAP. J. Macromol. Sci. A 2002, 7, 759–768. [Google Scholar] [CrossRef]
- Fazlıoğlu, H.; Hacaloğlu, J. Thermal decomposition of glycidyl azide polymer by direct insertion probe mass spectrometry. J. Anal. Appl. Pyrolysis 2002, 2, 327–338. [Google Scholar] [CrossRef]
- Jin, B.; Shen, J.; Peng, R.F.; Shu, Y.J.; Tan, B.S.; Chu, S.J.; Dong, H.S. Synthesis, characterization, thermal stability and sensitivity properties of the new energetic polymer through the azidoacetylation of poly(vinyl alcohol). Polym. Degrad. Stab. 2012, 4, 473–480. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Jin, B.; Peng, R.F.; Chen, C.D.; Zheng, R.Z.; He, Y.; Chu, S.J. Synthesis and Characterization of [60]Fullerene-Glycidyl Azide Polymer and Its Thermal Decomposition. Polymers 2015, 7, 896-908. https://doi.org/10.3390/polym7050896
Huang T, Jin B, Peng RF, Chen CD, Zheng RZ, He Y, Chu SJ. Synthesis and Characterization of [60]Fullerene-Glycidyl Azide Polymer and Its Thermal Decomposition. Polymers. 2015; 7(5):896-908. https://doi.org/10.3390/polym7050896
Chicago/Turabian StyleHuang, Ting, Bo Jin, Ru Fang Peng, Cong Di Chen, Rong Zong Zheng, Yi He, and Shi Jin Chu. 2015. "Synthesis and Characterization of [60]Fullerene-Glycidyl Azide Polymer and Its Thermal Decomposition" Polymers 7, no. 5: 896-908. https://doi.org/10.3390/polym7050896
APA StyleHuang, T., Jin, B., Peng, R. F., Chen, C. D., Zheng, R. Z., He, Y., & Chu, S. J. (2015). Synthesis and Characterization of [60]Fullerene-Glycidyl Azide Polymer and Its Thermal Decomposition. Polymers, 7(5), 896-908. https://doi.org/10.3390/polym7050896