Copolymers Based on 1,3-Bis(carbazol-9-yl)benzene and Three 3,4-Ethylenedioxythiophene Derivatives as Potential Anodically Coloring Copolymers in High-Contrast Electrochromic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Polymerization of PBCz, P(BCz-co-EDOT), P(BCz-co-ProDOT), and P(BCz-co-EDTT) Films
2.3. Construction of Electrochromic Devices
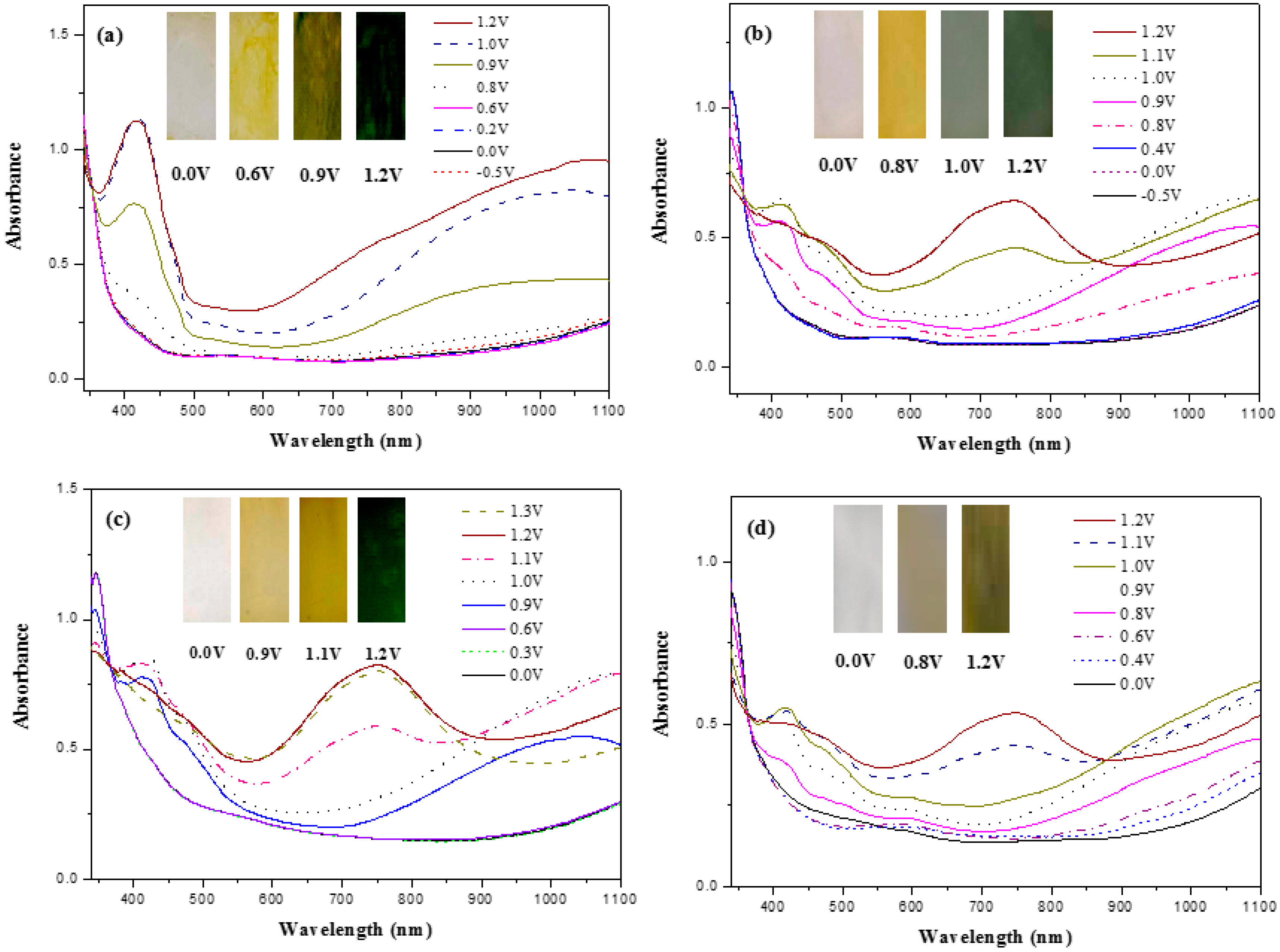
2.4. Electrochemical and Spectroelectrochemical Characterization
3. Results and Discussion
3.1. Electrochemical Polymerization
3.2. Electrochromic Characterizations of Polymer Films
3.3. Spectroelectrochemistry of Electrochromic Devices (ECDs)
3.4. Open Circuit Memory of ECDs
3.5. Stability of Electrochromic Device (ECD)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, E.L.; Ang, T.S.; Ooi, Z.; Sonar, P.; Lin, T.T.; Neo, W.T.; Song, J.; Hobley, J. Optical characterization of the hole polaron in a series of diketopyrrolopyrrole polymers used for organic photovoltaics. Polymers 2015, 7, 69–90. [Google Scholar] [CrossRef]
- Jang, Y.; Seo, J.-W.; Seok, J.; Lee, J.-Y.; Kim, K. Roughening conjugated polymer surface for enhancing the charge collection efficiency of sequentially deposited polymer/fullerene photovoltaics. Polymers 2015, 7, 1497–1509. [Google Scholar] [CrossRef]
- Wu, T.Y.; Lee, N.C.; Chen, Y. Synthesis and characterization of new poly(p-phenylenevinylene) derivative containing 5,5′-diphenyl-2,2-p-(2,5-bis-hexyloxyphenylene)-bis-1,3,4-oxadiazole and distyrylbenzene moieties. Synth. Metals 2003, 139, 263–269. [Google Scholar] [CrossRef]
- Shih, H.-K.; Chen, Y.-H.; Chu, Y.-L.; Cheng, C.-C.; Chang, F.-C.; Zhu, C.-Y.; Kuo, S.-W. Photo-crosslinking of pendent uracil units provides supramolecular hole injection/transport conducting polymers for highly efficient light-emitting diodes. Polymers 2015, 7, 804–818. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chen, S.J.; Chen, P.R.; Wu, T.Y.; Tsai, W.T.; Tseng, C.G. Doping process effect of polyaniline doped with poly(styrenesulfonic acid) supported platinum for methanol oxidation. J. Taiwan Inst. Chem. Eng. 2013, 44, 497–504. [Google Scholar] [CrossRef]
- Kuo, C.W.; Kuo, Z.Y.; Jow, J.J.; Wu, T.Y.; Chen, J.Y.; Zhu, X.X. Enhanced electrocatalytic performance for methanol oxidation via insertion of ruthenium oxide particles into Pt and polyaniline-poly(acrylic acid-co-maleic acid) composite electrode. Int. J. Electrochem. Sci. 2012, 7, 4974–4987. [Google Scholar] [CrossRef]
- Wu, T.Y.; Kuo, C.W.; Chen, Y.L.; Chang, J.K. Copolymers based on indole-6-carboxylic acid and 3,4-ethylenedioxythiophene as platinum catalyst support for methanol oxidation. Catalysts 2015, 5, 1657–1672. [Google Scholar] [CrossRef]
- Wu, T.Y.; Sheu, R.B.; Chen, Y. Synthesis, optically acid-sensory and electrochemical properties of novel polyoxadiazole derivatives. Macromolecules 2004, 37, 725–733. [Google Scholar] [CrossRef]
- Cho, J.; Cheon, K.H.; Park, K.H.; Kwon, S.K.; Kim, Y.H.; Chung, D.S. Colloids of semiconducting polymers for high-performance, environment-friendly polymer field effect transistors. Org. Electron. 2015, 24, 160–164. [Google Scholar] [CrossRef]
- Chang, K.H.; Wang, H.P.; Wu, T.Y.; Sun, I.W. Optical and electrochromic characterizations of four 2,5-dithienylpyrrole-based conducting polymer films. Electrochim. Acta 2014, 119, 225–235. [Google Scholar] [CrossRef]
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic systems and the prospects for devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Tavoli, F.; Alizadeh, N. Enhancement effect of transition metal cations on the electrochromic properties of nanostructure tiron doped polypyrrole film. J. Electroanal. Chem. 2015, 746, 39–44. [Google Scholar] [CrossRef]
- Ming, S.; Zhang, S.; Liu, H.; Zhao, Y.; Mo, D.; Xu, J. Methacrylate modified polythiophene: Electrochemistry and electrochromics. Int. J. Electrochem. Sci. 2015, 10, 6598–6609. [Google Scholar]
- Wu, T.Y.; Li, W.B.; Kuo, C.W.; Chou, C.F.; Liao, J.W.; Chen, H.R.; Tseng, C.G. Study of poly(methyl methacrylate)-based gel electrolyte for electrochromic device. Int. J. Electrochem. Sci. 2013, 8, 10720–10732. [Google Scholar]
- Zhen, S.; Xu, J.; Lu, B.; Zhang, S.; Zhao, L.; Li, J. Tuning the optoelectronic properties of polyfuran by design of furan-EDOT monomers and free-standing films with enhanced redox stability and electrochromic performances. Electrochim. Acta 2014, 146, 666–678. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Hsueh, J.C. Electrochemical synthesis and electrochromic properties of new conjugated polycarbazoles from di(carbazol-9-yl)-substituted triphenylamine and N-phenylcarbazole derivatives. J. Electroanal. Chem. 2015, 758, 100–110. [Google Scholar] [CrossRef]
- Lim, S.Z.H.; Neo, W.T.; Cho, C.M.; Wang, X.B.; Tan, A.Y.X.; Chan, H.S.O.; Xu, J.W. Electrochromic π-conjugated copolymers derived from azulene, fluorene, and dialkyloxybenzothiadiazole. Aust. J. Chem. 2013, 66, 1048–1056. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Fu, Y.; Xu, J.; Nie, G. Electrochromic property of a copolymer based on 5-cyanoindole and 3,4-ethylenedioxythiophene and its application in electrochromic devices. J. Electroanal. Chem. 2013, 700, 17–23. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chen, B.K.; Li, W.B.; Tseng, L.Y.; Wu, T.Y.; Tseng, C.G.; Chen, H.R.; Huang, Y.C. Effects of supporting electrolytes on spectroelectrochemical and electrochromic properties of polyaniline-poly(styrene sulfonic acid) and poly(ethylenedioxythiophene)-poly(styrene sulfonic acid)-based electrochromic device. J. Chin. Chem. Soc. 2014, 61, 563–570. [Google Scholar] [CrossRef]
- Kerszulis, J.A.; Johnson, K.E.; Kuepfert, M.; Khoshabo, D.; Dyerac, A.L.; Reynolds, J.R. Tuning the painter’s palette: Subtle steric effects on spectra and colour in conjugated electrochromic polymers. J. Mater. Chem. C 2015, 3, 3211–3218. [Google Scholar] [CrossRef]
- Atılgan, N.; Cihaner, A.; Önal, A.M. Electrochromic performance and ion sensitivity of a 2,4-terthienyl based fluorescent polymer. React. Funct. Polym. 2010, 70, 244–250. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kumar, A.; Meijer, E.W.; Reynolds, J.R. Enhanced contrast ratio and rapid switching in electrochromics based on poly(3,4-propylenedioxythiophene) derivatives. Adv. Mater. 1999, 11, 1379–1382. [Google Scholar] [CrossRef]
- Sefer, E.; Bilgili, H.; Gultekin, B.; Tonga, M.; Koyuncu, S. A narrow range multielectrochromism from 2,5-di-(2-thienyl)-1H-pyrrole polymer bearing pendant perylenediimide moiety. Dyes Pigments 2015, 113, 121–128. [Google Scholar] [CrossRef]
- Carbas, B.B.; Kivrak, A.; Teke, E.; Zora, M.; Önal, A.M. Electrochemical polymerization of a new low-voltage oxidized thienylenepyrrole derivative and its electrochromic device application. J. Electroanal. Chem. 2014, 729, 15–20. [Google Scholar] [CrossRef]
- Capan, A.; Ozturk, T. Electrochromic properties of 3-arylthieno[3,2-b]thiophenes. Synth. Metals 2014, 188, 100–103. [Google Scholar] [CrossRef]
- Chen, C.C.; Chiang, C.Y.; Wu, T.Y.; Sun, I.W. Improved electrochromic properties of poly(3,4-ethylenedioxythiophene) in 1-butyl-3-methylimidazolium dicyanamide. ECS Electrochem. Lett. 2013, 2, H43–H45. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Liu, R.; Liu, J.; He, Q. Electrosyntheses, characterizations and electrochromic properties of a copolymer based on 4,4′-di(N-carbazoyl)biphenyl and 2,2′-bithiophene. Sol. Energy Mater. Sol. Cells 2011, 95, 1867–1874. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Xiao, J.; Cui, C.; Liu, R. Synthesis and electropolymerization of 9H-carbazol-9-ylpyrene and its electrochromic properties and electrochromic device application. Int. J. Electrochem. Sci. 2012, 7, 2781–2795. [Google Scholar]
- Koyuncu, S.; Gultekin, B.; Zafer, C.; Bilgili, H.; Can, M.; Demic, S.; Kaya, I.; Icli, S. Electrochemical and optical properties of biphenyl bridged-dicarbazole oligomer films: Electropolymerization and electrochromism. Electrochim. Acta 2009, 54, 5694–5702. [Google Scholar] [CrossRef]
- Chen, S.; Gao, Q.; Zhao, J.; Cui, C.; Yang, W.; Zhang, X. Electrosyntheses, characterizations and electrochromic properties of a novel copolymer of 4,4′-di(N-carbazoyl)biphenyl with 4H-cyclopenta[2,1-b:3,4-b′]dithiophene. Int. J. Electrochem. Sci. 2012, 7, 5256–5272. [Google Scholar]
- Seshadri, V.; Padilla, J.; Bircan, H.; Radmard, B.; Draper, R.; Wood, M.; Otero, T.F.; Sotzing, G.A. Optimization, preparation, and electrical short evaluation for 30 cm2 active area dual conjugated polymer electrochromic windows. Org. Electron. 2007, 8, 367–381. [Google Scholar] [CrossRef]
- Udum, Y.A.; Gündoğdu Hızlıateş, C.; Ergün, Y.; Toppare, L. Electrosynthesis and characterization of an electrochromic material containing biscarbazole–oxadiazole units and its application in an electrochromic device. Thin Solid Films 2015, 595, 61–67. [Google Scholar] [CrossRef]
- Kuo, C.W.; Hsieh, T.H.; Hsieh, C.K.; Liao, J.W.; Wu, T.Y. Electrosynthesis and characterization of four electrochromic polymers based on carbazole and indole-6-carboxylic acid and their applications in high-contrast electrochromic devices. J. Electrochem. Soc. 2014, 161, D782–D790. [Google Scholar] [CrossRef]












| Electrodes | Anodic polymer | Feed species of anodic polymer | Feed molar ratio of anodic polymers |
|---|---|---|---|
| (a) | PBCz | 2 mM BCz | Neat BCz |
| (b) | P(BCz-co-EDOT) | 2 mM BCz + 2 mM EDOT | 1:1 |
| (c) | P(BCz-co-ProDOT) | 2 mM BCz + 2 mM ProDOT-Me2 | 1:1 |
| (d) | P(BCz-co-EDTT) | 2 mM BCz + 2 mM EDTT | 1:1 |
| Electrodes | λ (nm) a | Tox | Tred | ΔT | ΔOD | Qd (mC∙cm−2) | η (cm2∙C−1) | τc∙(s) | τb∙(s) |
|---|---|---|---|---|---|---|---|---|---|
| (a) | 1050 | 18.5 | 37.1 | 18.6 | −0.301 | −1.67 | 180.3 | 7.0 | 6.0 |
| (b) | 732 | 38.5 | 74.5 | 36.0 | −0.287 | −3.67 | 78.2 | 6.0 | 2.5 |
| (c) | 748 | 17.0 | 69.5 | 52.5 | −0.612 | −4.00 | 153.5 | 7.0 | 2.4 |
| (d) | 749 | 15.2 | 65.2 | 50.0 | −0.637 | −4.60 | 138.5 | 6.5 | 2.5 |
| Electrodes | λ (nm) a | Tox | Tred | ΔT | ΔOD | Qd (mC∙cm−2) | η (cm2∙C−1) | τc (s) | τb (s) |
|---|---|---|---|---|---|---|---|---|---|
| ECD (a) | 639 | 14.5 | 41.5 | 27.0 | −0.467 | −1.45 | 322.1 | 3.0 | 3.0 |
| ECD (b) | 630 | 32.5 | 58.0 | 25.5 | −0.252 | −0.80 | 315.0 | 3.5 | 3.1 |
| ECD (c) | 642 | 21.5 | 52.5 | 31.0 | −0.388 | −0.75 | 517.3 | 3.4 | 2.6 |
| ECD (c3) | 642 | 10.0 | 51.0 | 41.0 | −0.708 | −1.70 | 416.5 | 3.0 | 3.0 |
| ECD (c4) | 642 | 6.1 | 41.1 | 35.0 | −0.826 | −2.45 | 337.1 | 3.5 | 2.9 |
| ECD (d) | 631 | 23.0 | 43.0 | 20.0 | −0.272 | −0.75 | 362.7 | 3.0 | 3.0 |
| ECD configuration | ΔTmax (%) | ηmax (cm2∙C−1) | Reference |
|---|---|---|---|
| poly(4,4′-di(N-carbazoyl)biphenyl-co-2,2′-bithiophene)/PEDOT | 28.6 (700 nm) | 234 (700 nm) | [27] |
| poly(9H-carbazol-9-ylpyrene)/PEDOT | 23 (623 nm) | 290 (623 nm) | [28] |
| poly(4,4′-di(N-carbazolyl)biphenyl)/PEDOT | 19 (550 nm) | - | [29] |
| poly(4,4′-di(N-carbazoyl)biphenyl-co-4H-cyclopenta[2,1-b:3,4-b′]dithiophene)/PEDOT | 39.8 (628 nm) | 319.98 (628 nm) | [30] |
| poly(3,6-bis(2-(3,4-ethylenedioxy)thienyl)-N-methylcarbazole)/PEDOT | ca. 30 | - | [31] |
| poly(2,5-bis(9-methyl-9H-carbazol-3-yl)-1,3,4-oxadiazole)/PEDOT | 35 (620 nm) | - | [32] |
| poly(carbazole-co-indole-6-carboxylic acid)/PProDOT-Me2 | 32 (575 nm) | 372.7 | [33] |
| P(BCz-co-ProDOT)/double-layer PEDOT-PSS | 31 (642 nm) | 517 (642 nm) | This work |
| P(BCz-co-ProDOT)/triple-layer PEDOT-PSS | 41 (642 nm) | 417 (642 nm) | This work |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-W.; Wu, T.-L.; Lin, Y.-C.; Chang, J.-K.; Chen, H.-R.; Wu, T.-Y. Copolymers Based on 1,3-Bis(carbazol-9-yl)benzene and Three 3,4-Ethylenedioxythiophene Derivatives as Potential Anodically Coloring Copolymers in High-Contrast Electrochromic Devices. Polymers 2016, 8, 368. https://doi.org/10.3390/polym8100368
Kuo C-W, Wu T-L, Lin Y-C, Chang J-K, Chen H-R, Wu T-Y. Copolymers Based on 1,3-Bis(carbazol-9-yl)benzene and Three 3,4-Ethylenedioxythiophene Derivatives as Potential Anodically Coloring Copolymers in High-Contrast Electrochromic Devices. Polymers. 2016; 8(10):368. https://doi.org/10.3390/polym8100368
Chicago/Turabian StyleKuo, Chung-Wen, Teng-Lu Wu, Yuan-Chung Lin, Jeng-Kuei Chang, Ho-Rei Chen, and Tzi-Yi Wu. 2016. "Copolymers Based on 1,3-Bis(carbazol-9-yl)benzene and Three 3,4-Ethylenedioxythiophene Derivatives as Potential Anodically Coloring Copolymers in High-Contrast Electrochromic Devices" Polymers 8, no. 10: 368. https://doi.org/10.3390/polym8100368






