A Highly Hydrophilic and Biodegradable Novel Poly(amide-imide) for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Techniques
2.3. Synthesis of the l-Glycine Based Novel PAI
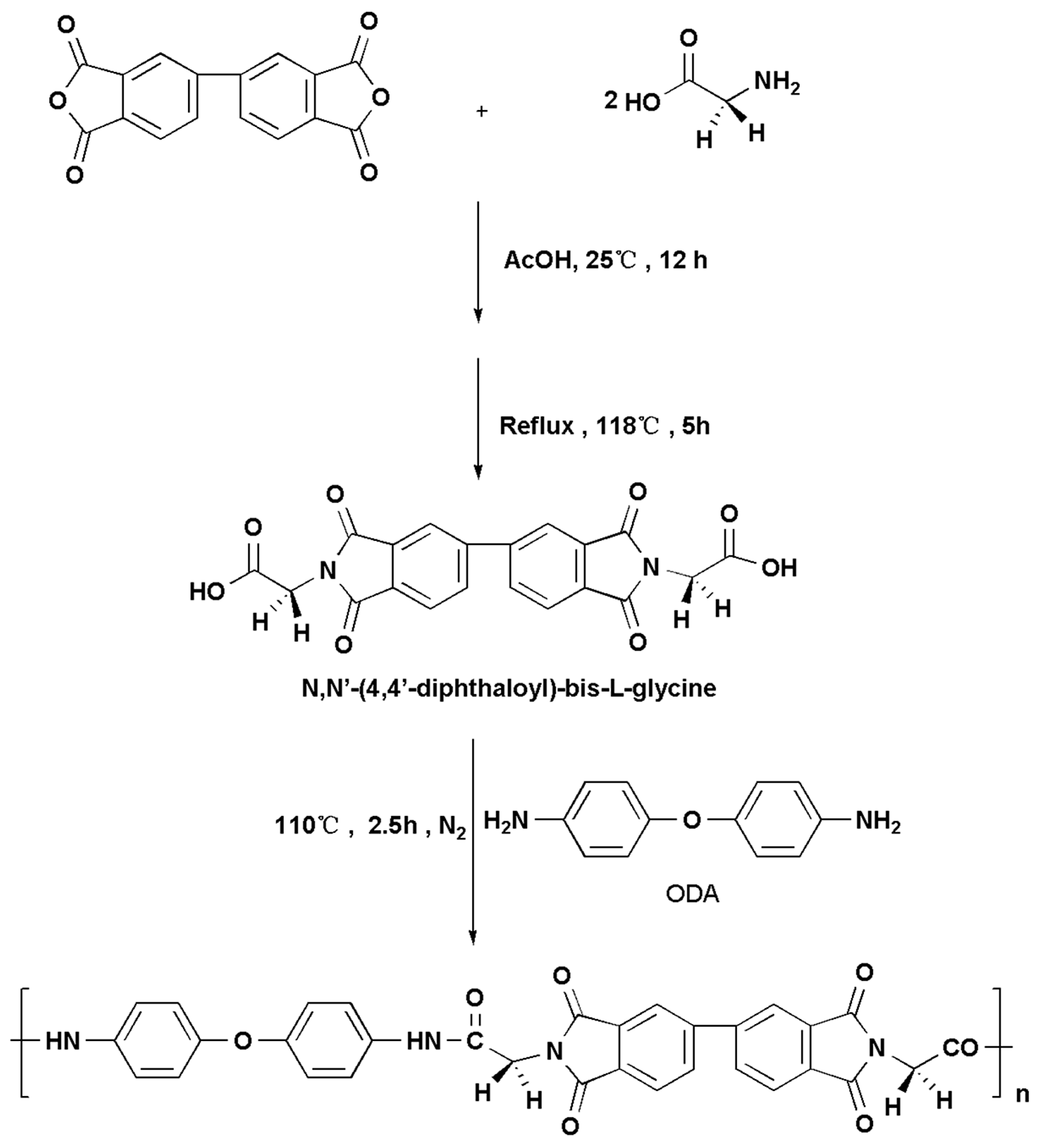
2.3.1. Synthesis of N,N′-(4,4′-Diphthaloyl)-bis-l-glycine (Scheme 1)
2.3.2. Synthesis of the Novel PAI
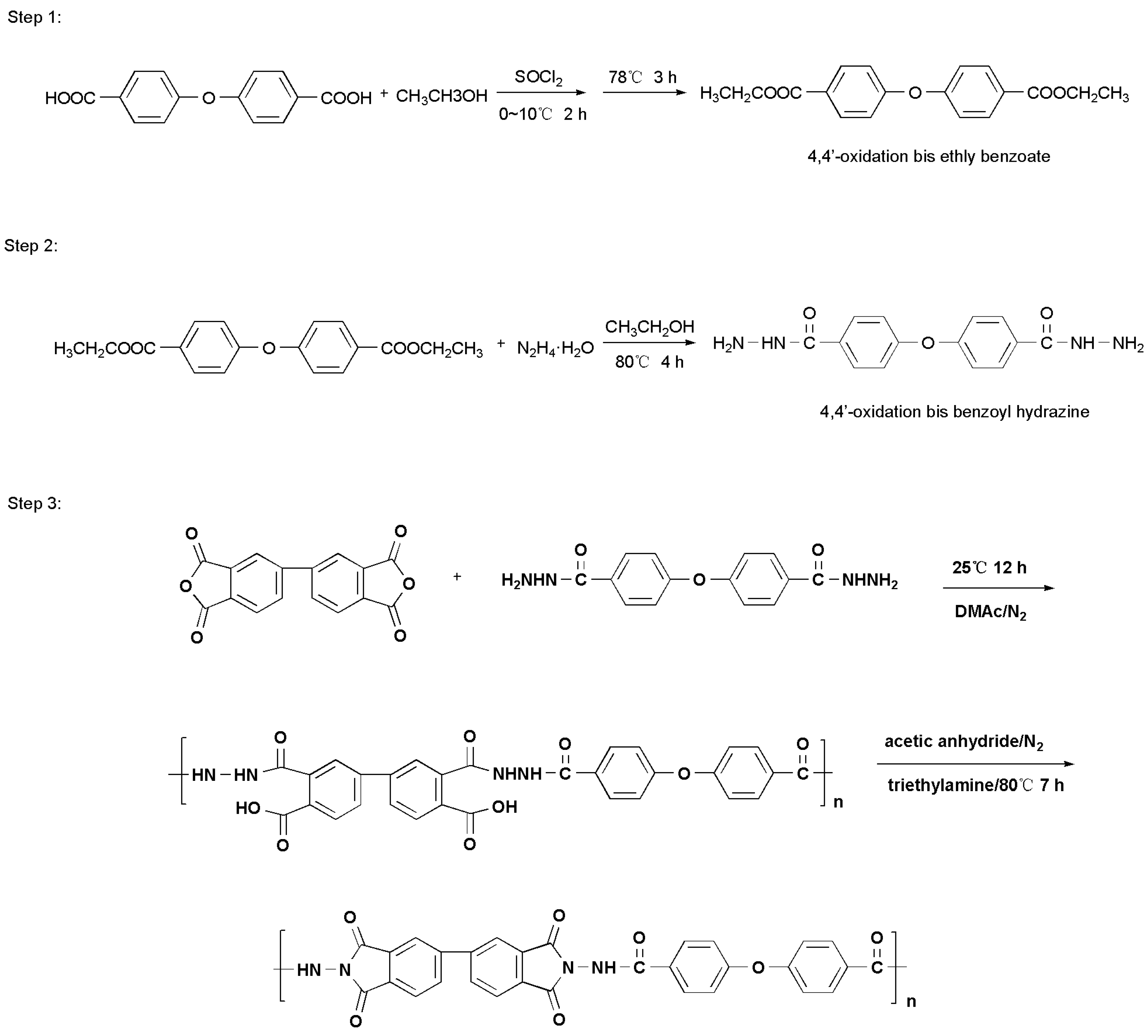
2.4. Synthesis of the Pure PAI
2.4.1. Preparation of 4,4′-Oxidation bis Ethly Benzoate (See Step 1 in Scheme 2)
2.4.2. Synthesis of 4,4′-Oxidation bis Benzoyl Hydrazine (See Step 2 in Scheme 2)
2.4.3. Synthesis of the Pure PAI (See Step 3 in Scheme 2)
2.5. Hydrophilicity
2.6. In Vitro Degradation Assay
2.7. In Vitro Biocompatibility Assay
3. Results and Discussion
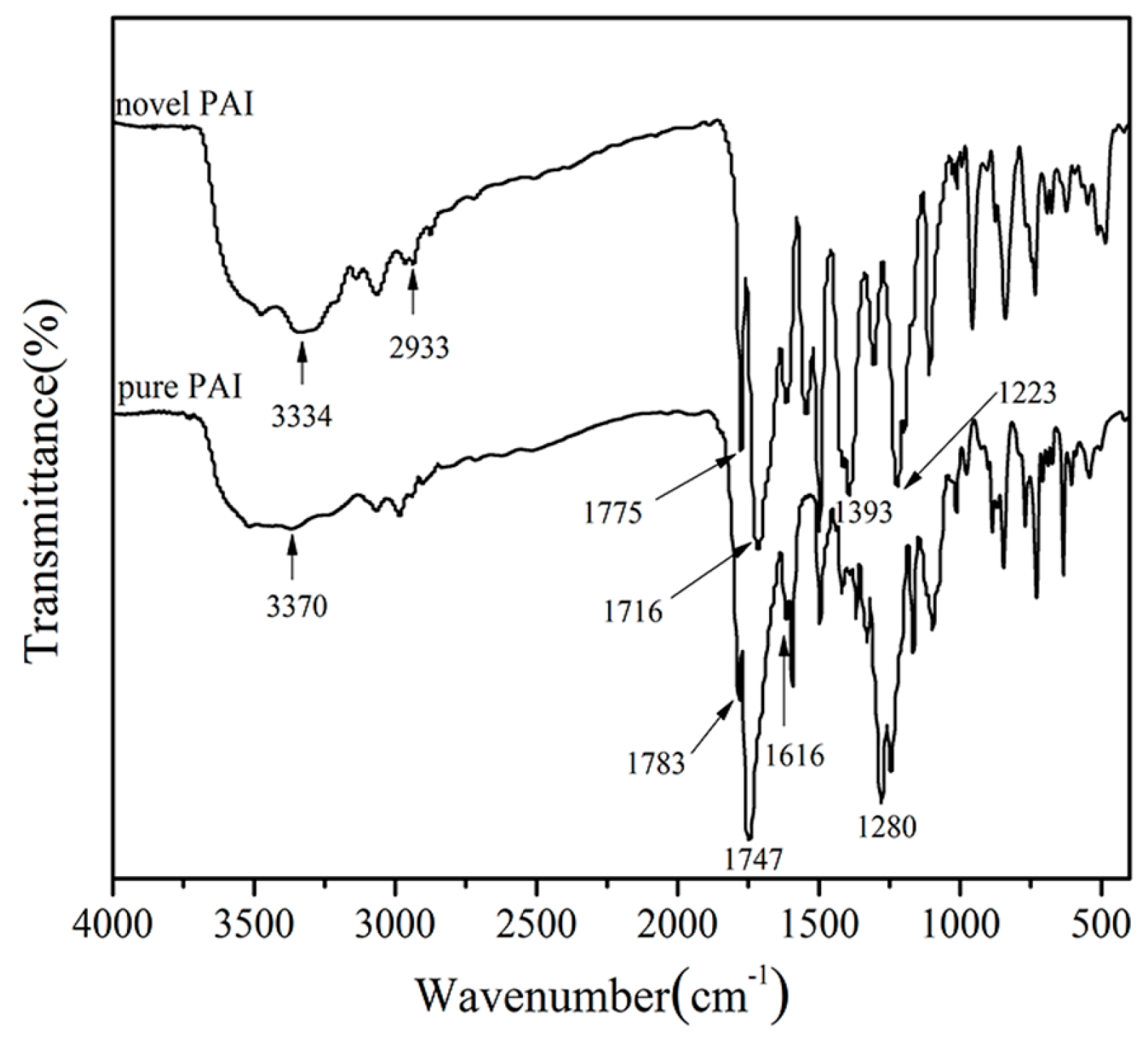
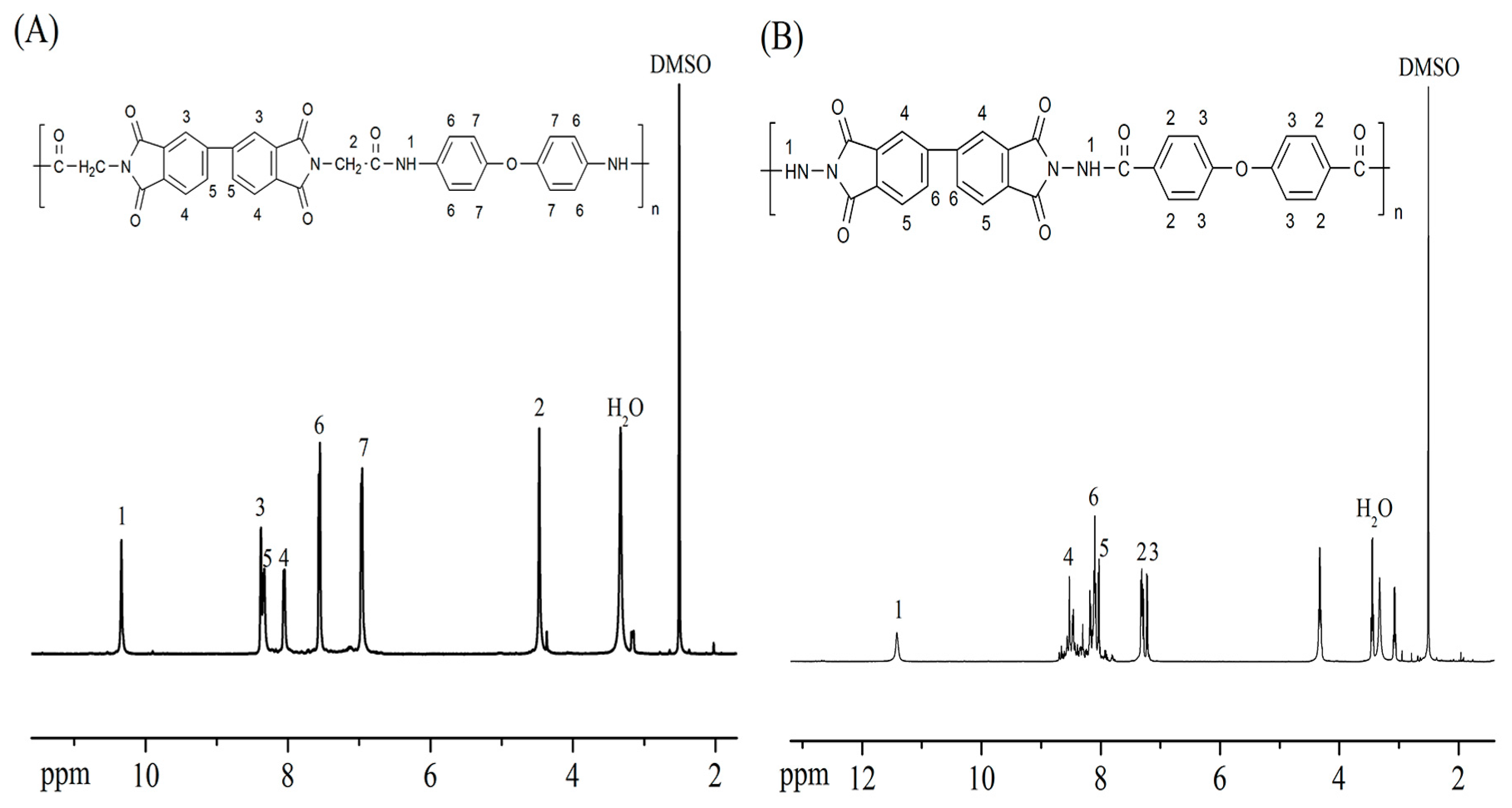
3.1. Polymer Synthesis
3.2. Solubility Property
3.3. Molecular Weight and Thermal Stability
3.4. Hydrophilicity Performance
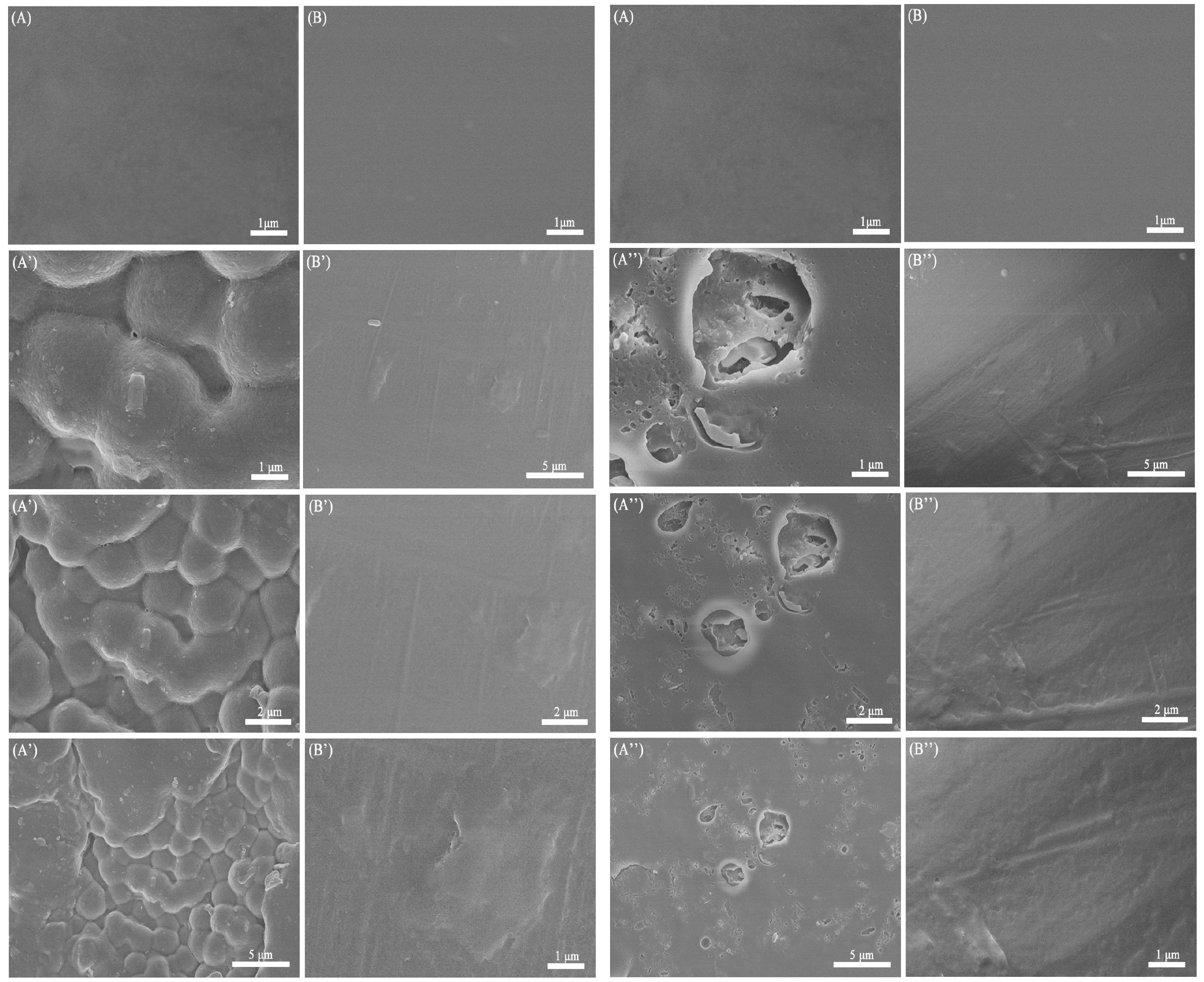
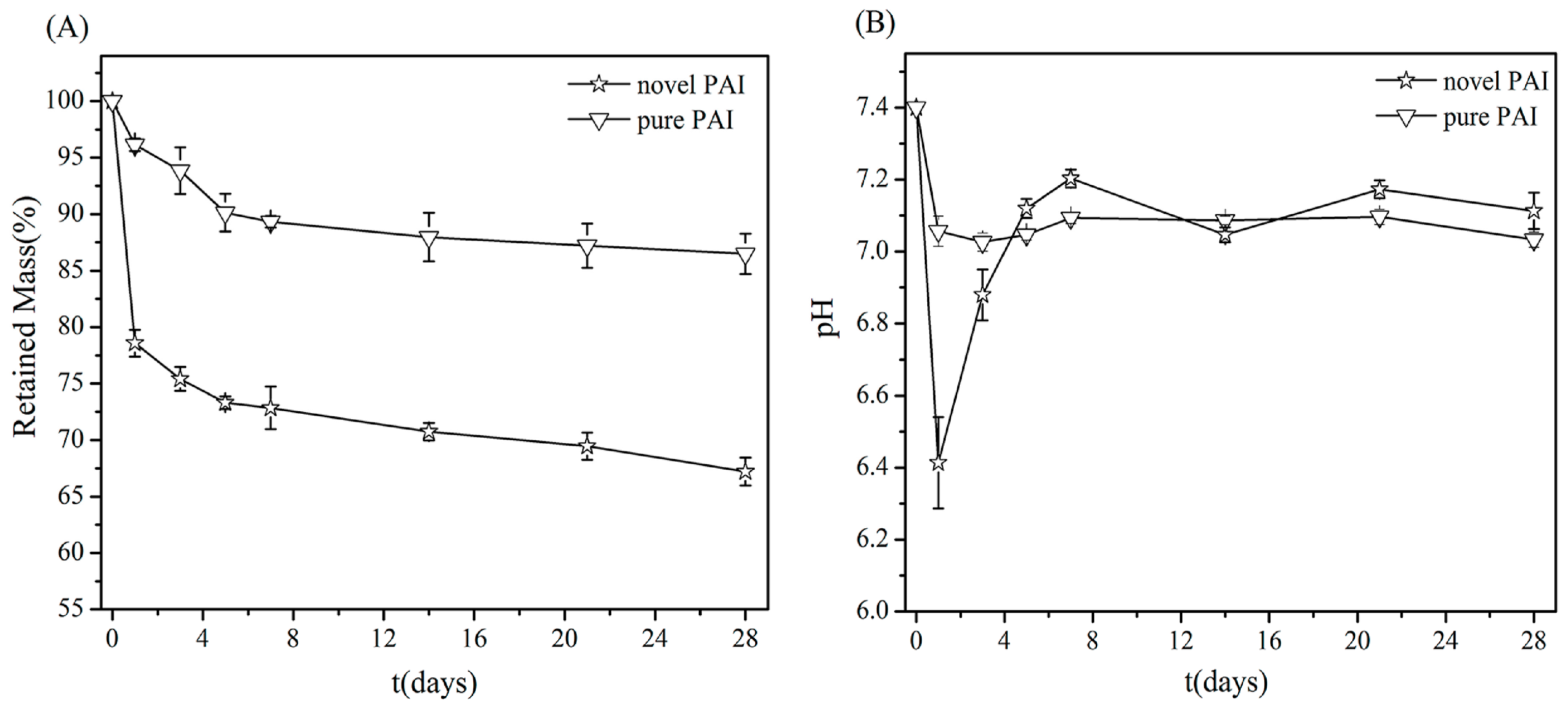
3.5. In Vitro Degradation Assay
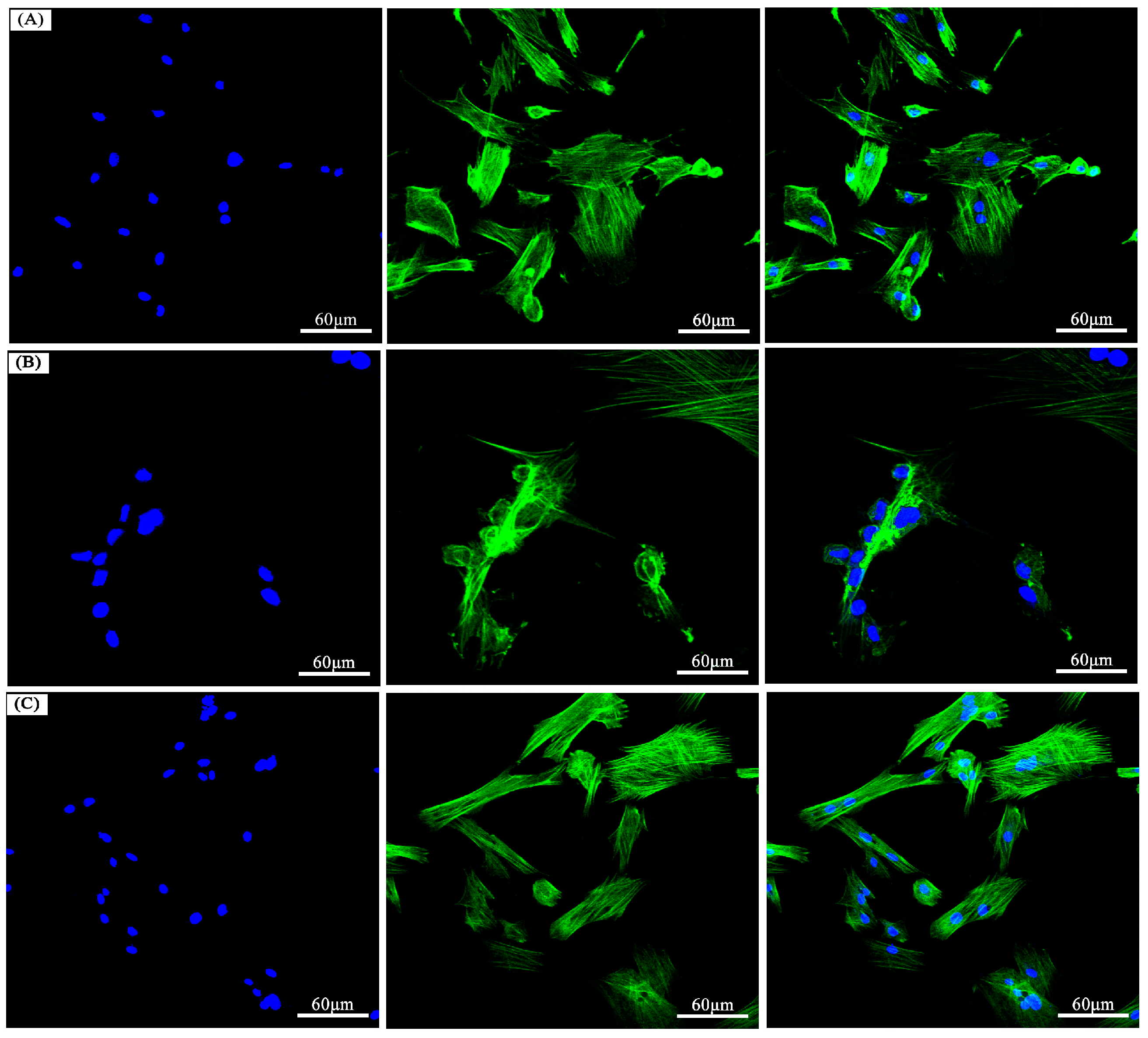
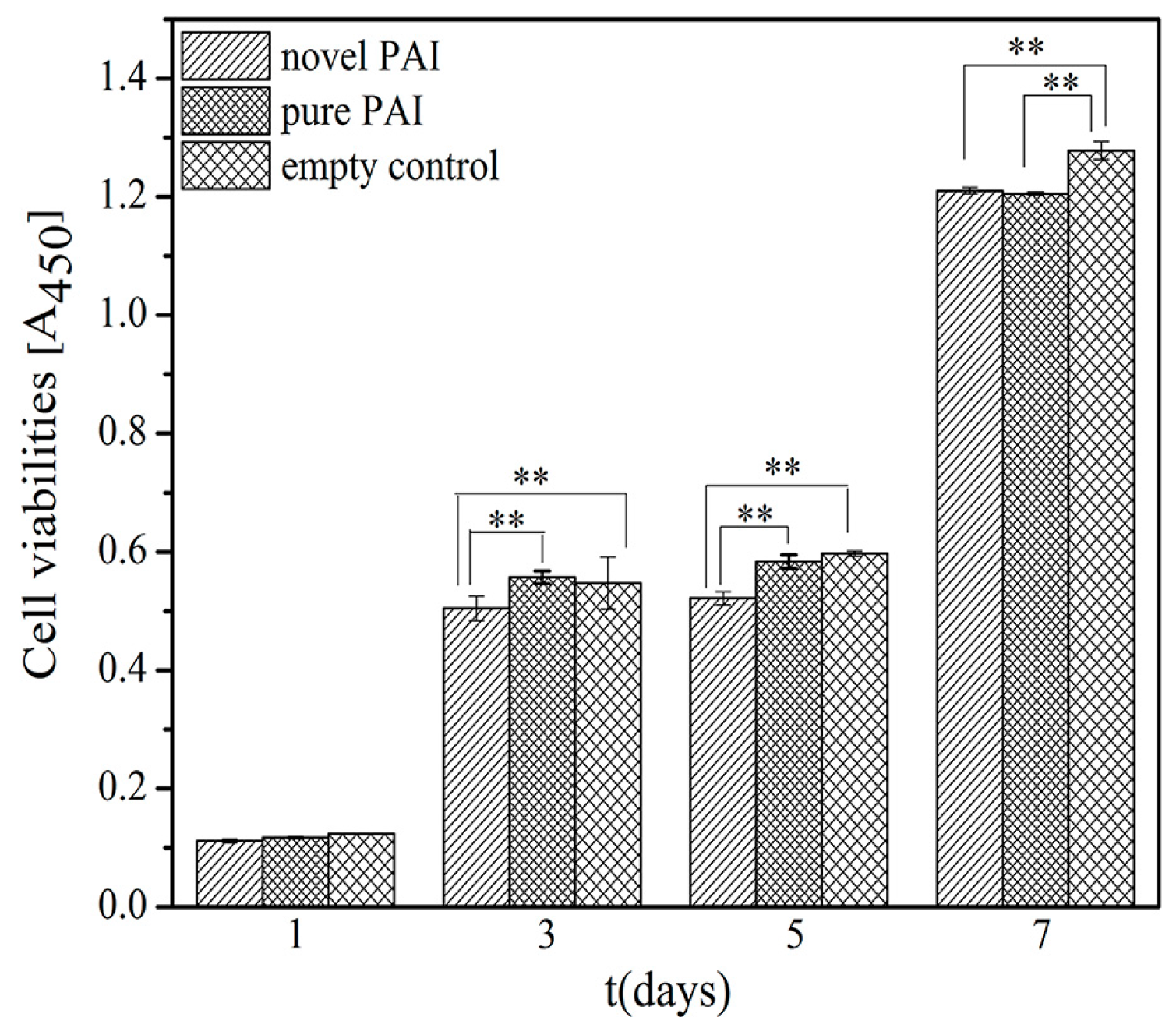
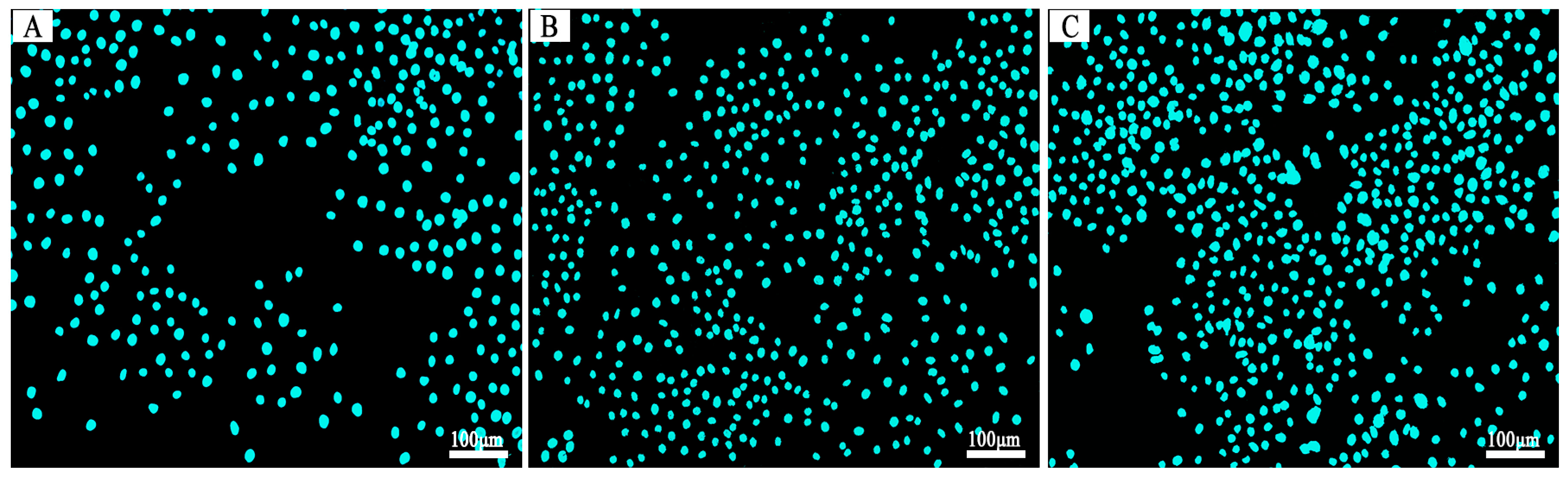
3.6. The In Vitro Biocompatibility Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of peek. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B.; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. Peek biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Lu, X.; Yang, L.; Yang, H.; Yuan, W.; Chen, D. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: A prospective, randomized, control study with over 7-year follow-up. Eur. Spine J. 2013, 22, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Judith, M.R.; Kumar, A.; Mishra, V.; Robert, M.C. Analysis of stress distribution in lumbar interbody fusion. Spine 2005, 30, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Tai, C.L.; Lin, C.Y.; Hsieh, P.H.; Chen, W.P. Biomechanical comparison of a new stand-alone anterior lumbar interbody fusion cage with established fixation techniques—A three-dimensional finite element analysis. BMC Musculoskelet. Disord. 2008, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiong, G.; Ren, K.; Raman, S.R.; Liu, Z.; Li, Q.; Ma, C.; Li, D.; Wan, Y. Air DBD plasma treatment on three-dimensional braided carbon fiber-reinforced PEEK composites for enhancement of in vitro bioactivity. Surf. Coat. Technol. 2014, 242, 1–7. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Mirzadeh, H.; Irani, S. Plasma surface modification of poly(l-lactic acid) and poly(lactic-co-glycolic acid) films for improvement of nerve cells adhesion. Radiat. Phys. Chem. 2008, 77, 280–287. [Google Scholar] [CrossRef]
- Chunguang, Z.; Yueming, S.; Chongqi, T.; Hong, D.; Fuxing, P.; Yonggang, Y.; Hong, L. Evaluation of bioabsorbable multiamino acid copolymer/α-tri-calcium phosphate interbody fusion cages in a goat model. Spine 2011, 36, E1615–E1622. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.B.; Ames, C.P.; Crawford, N.R.; Chamberlain, R.H.; Rubino, A.M.; Seim, H.B., III; Turner, A.S. In vivo evaluation of bioresorbable polylactide implants for cervical graft containment in an ovine spinal fusion model. Neurosurg. Focus 2004, 16, 1–6. [Google Scholar] [CrossRef]
- Guo, K.; Chu, C. Synthesis, characterization, and biodegradation of novel poly(ether ester amide) s based on l-phenylalanine and oligoethylene glycol. Biomacromolecules 2007, 8, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pan, D.; Luo, K.; Li, L.; Gu, Z. Biodegradable and amphiphilic block copolymer-doxorubicin conjugate as polymeric nanoscale drug delivery vehicle for breast cancer therapy. Biomaterials 2013, 34, 8430–8443. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Guo, Z.; Cheng, J.; Fang, Z. Biodegradable aliphatic/aromatic copoly(ester-ether)s: The effect of poly(ethylene glycol) on physical properties and degradation behavior. J. Polym. Res. 2011, 18, 187–196. [Google Scholar] [CrossRef]
- Baillargeon, A.L.; Mequanint, K. Biodegradable polyphosphazene biomaterials for tissue engineering and delivery of therapeutics. BioMed Res. Int. 2014, 2014, 761373. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Higashi, M.; Kaneko, T.; Kida, T.; Akashi, M. Hydrolytic and enzymatic degradation of nanoparticles based on amphiphilic poly(γ-glutamic acid)-graft-l-phenylalanine copolymers. Biomacromolecules 2006, 7, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Dinari, M. Progress in synthetic polymers based on natural amino acids. J. Macromol. Sci. Part A 2011, 48, 644–679. [Google Scholar] [CrossRef]
- Horwitz, J.A.; Shum, K.M.; Bodle, J.C.; Deng, M.; Chu, C.C.; Reinhart King, C.A. Biological performance of biodegradable amino acid-based poly(ester amide)s: Endothelial cell adhesion and inflammation in vitro. J. Biomed. Mater. Res. Part A 2010, 95, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Kobayashi, M.; Kise, H. Synthesis and biodegradation of poly(ester amide)s containing amino acid residues: The effect of the stereoisomeric composition of l- and d-phenylalanines on the enzymatic degradation of the polymers. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 385–392. [Google Scholar] [CrossRef]
- Yodoya, S.; Takagi, T.; Kurotani, M.; Hayashi, T.; Nagata, M.; Oka, M.; Hayashi, T. Preparation and properties of A–B–A tri-block copolymer membranes consisting of N-hydroxypropyl-l-glutamine as the A component and l-alanine as the B component. Eur. Polym. J. 2003, 39, 2059–2067. [Google Scholar] [CrossRef]
- Okamoto, Y. Chiral polymers. Prog. Polym. Sci. 2000, 25, 159–162. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Birchall, A.; Bush, S.; North, M. Copolymerization of peptide derived monomers and methyl methacrylate. Polymer 2001, 42, 375–389. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y. Biodegradable copoly(amino acid)s based on 6-aminocaproic acid and l-leucine. J. Polym. Environ. 2011, 19, 177–181. [Google Scholar] [CrossRef]
- Azari, S.; Zou, L. Using zwitterionic amino acid l-dopa to modify the surface of thin film composite polyamide reverse osmosis membranes to increase their fouling resistance. J. Membr. Sci. 2012, 401–402, 68–75. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Liaw, D.J.; Hsu, P.N.; Liaw, B.Y. Synthesis and characterization of novel polyamide-imides containing noncoplanar 2,2′-dimethyl-4,4′-biphenylene unit. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 63–70. [Google Scholar] [CrossRef]
- Liaw, D.J.; Liaw, B.Y. Synthesis and characterization of new polyamide-imides containing pendent adamantyl groups. Polymer 2001, 42, 839–845. [Google Scholar] [CrossRef]
- Ma, X.; Lee, N.H.; Oh, H.J.; Hwang, J.S.; Kim, S.J. Preparation and characterization of silica/polyamide-imide nanocomposite thin films. Nanoscale Res. Lett. 2010, 5, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Liaw, D.J.; Chen, W.H. High glass transitions of novel organosoluble polyamide-imides based on noncoplanar and rigid diimide-dicarboxylic acid. Polym. Degrad. Stab. 2006, 91, 1731–1739. [Google Scholar] [CrossRef]
- Babooram, K.; Francis, B.; Bissessur, R.; Narain, R. Synthesis and characterization of novel (amide-imide)-silica composites by the sol–gel process. Compos. Sci. Technol. 2008, 68, 617–624. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khani, M.; Sabzalian, M.R. Synthesis and biodegradability assessment of poly(amide-imide)s containing N-trimellitylimido-l-amino acid and 5-(2-benzimidazole)-1,3-phenylenediamine. Polym. Bull. 2014, 71, 2159–2172. [Google Scholar] [CrossRef]
- Van Dijk, M.; Tunc, D.; Smit, T.; Higham, P.; Burger, E.; Wuisman, P. In vitro and in vivo degradation of bioabsorbable plla spinal fusion cages. J. Biomed. Mater. Res. 2002, 63, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 2008, 29, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.D.; Park, H.S.; Kang, M.H.; Li, Y.; Kim, H.E.; Koh, Y.H.; Estrin, Y. Reinforcement of polyetheretherketone polymer with titanium for improved mechanical properties and in vitro biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhao, Q.; Gong, P.; Zhang, J.; Liao, D.; Yuan, Q.; Wei, N. Carrier selection for bone marrow mesenchymal stem cells transplantation in periodontal regeneration: Calcium alginate or fibrin gel. Arch. Med. Sci. 2009, 5, 493–499. [Google Scholar]


| Polymer | Formula | C (%) | H (%) | N (%) | |
|---|---|---|---|---|---|
| novel PAI | C32H20N4O7 (572.52)n | Calcd. | 67.13 | 3.50 | 9.79 |
| Found | 66.89 | 3.84 | 9.19 | ||
| pure PAI | C30H16N4O7 (544.47)n | Calcd. | 66.18 | 2.94 | 10.29 |
| Found | 65.60 | 2.91 | 9.81 |
| Solvent | Novel PAI | Pure PAI |
|---|---|---|
| NMP | ++ | ++ |
| DMAc | ++ | ++ |
| DMF | ++ | ++ |
| DMSO | + | ++ |
| Pyridine | − | ++ |
| THF | − | ++ |
| CHCl3 | − | − |
| CH2Cl2 | − | − |
| Acetone | − | − |
| Methylbenzene | − | − |
| Dimethylbenzene | − | − |
| Polymer | Mw a (kDa) | Mn a (kDa) | Mz a (kDa) | PDI | Mz/Mw |
|---|---|---|---|---|---|
| novel PAI | 36.518 | 26.062 | 44.674 | 1.401 | 1.223 |
| pure PAI | 36.045 | 24.631 | 44.622 | 1.463 | 1.238 |
| Polymer | T5 (°C) b | T10 (°C) b |
|---|---|---|
| novel PAI | 312 | 380 |
| pure PAI | 233 | 291 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Q.; Zhou, Q.; Liu, L.; Dai, H. A Highly Hydrophilic and Biodegradable Novel Poly(amide-imide) for Biomedical Applications. Polymers 2016, 8, 441. https://doi.org/10.3390/polym8120441
Zou Q, Zhou Q, Liu L, Dai H. A Highly Hydrophilic and Biodegradable Novel Poly(amide-imide) for Biomedical Applications. Polymers. 2016; 8(12):441. https://doi.org/10.3390/polym8120441
Chicago/Turabian StyleZou, Qiying, Qian Zhou, Langlang Liu, and Honglian Dai. 2016. "A Highly Hydrophilic and Biodegradable Novel Poly(amide-imide) for Biomedical Applications" Polymers 8, no. 12: 441. https://doi.org/10.3390/polym8120441






