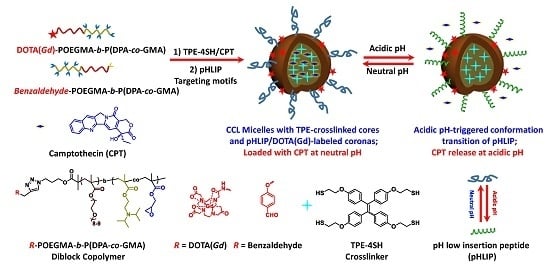
pH-Responsive Tumor-Targetable Theranostic Nanovectors Based on Core Crosslinked (CCL) Micelles with Fluorescence and Magnetic Resonance (MR) Dual Imaging Modalities and Drug Delivery Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Synthesis of N3-POEGMA-Br Macroinitiator (Scheme 2)
2.2.2. Synthesis of N3-POEGMA-b-P(DPA-co-GMA) Block Copolymers (Scheme 2)
2.2.3. Synthesis of DOTA(Gd)-POEGMA-b-P(DPA-co-GMA) and Benzaldehyde-POEGMA-b-P(DPA-co-GMA) Block Copolymers (Scheme 2)
2.2.4. Fabrication of Core Crosslinked (CCL) Mixed Micelles of BP2 and BP3 Using Tetrakis[4-(2-mercaptoethoxy)phenyl]ethylene (TPE-4SH) as the Crosslinker
2.2.5. Functionalization of Mixed Micelles with Targeting Peptide
2.2.6. In Vitro Cytotoxicity Assay
2.2.7. In Vitro MRI (Magnetic Resonance Imaging) Relaxivity Measurement
2.2.8. In Vivo MRI Measurement
2.3. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2014, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Crawley, N.; Thompson, M.; Romaschin, A. Theranostics in the growing field of personalized medicine: An analytical chemistry perspective. Anal. Chem. 2013, 86, 130–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Y.; He, B.; Luo, K.; Gu, Z. Biodegradable polymeric nanoparticles based on amphiphilic principle: Construction and application in drug delivery. Sci. China Chem. 2014, 57, 461–475. [Google Scholar] [CrossRef]
- Tsuchida, K.; Murakami, T. Recent advances in inorganic nanoparticle-based drug delivery systems. Mini Rev. Med. Chem. 2008, 8, 175–183. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Ikkala, O.; ten Brinke, G. Functional materials based on self-assembly of polymeric supramolecules. Science 2002, 295, 2407–2409. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, N.-P.; Li, B.-Y. Preparation of nanosilica-immobilized antioxidant and the antioxidation effects in polypropylene. Chin. J. Polym. Sci. 2014, 32, 1602–1609. [Google Scholar] [CrossRef]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3, 705. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Feng, G.; Kwok, R.T.K.; Ding, D.; Tang, B.; Liu, B. Aiegen based light-up probes for live cell imaging. Sci. China Chem. 2016, 59, 53–61. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xia, T.; Gong, Y.; Wang, X.; Liu, R.-Q.; Zhang, Q.-Y.; Yi, C.-F. Emulsifier-free emulsion polymerized poly(MMA-HEMA-Eu(AA)3Phen)/Fe3O4 magnetic fluorescent bifunctional nanospheres for magnetic resonance and optical imaging. Chin. J. Polym. Sci. 2016, 34, 135–146. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S. Bioapplications of raft polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Stenzel, M.H.; Davis, T.P. Building nanostructures using raft polymerization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 551–595. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Varghese, R.; Wagenknecht, H.A. Non-covalent versus covalent control of self-assembly and chirality of nile red-modified nucleoside and DNA. Chem. Eur. J. 2010, 16, 9040–9046. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Zhang, X.; Leach, A.G.; Houk, K. Beyond picomolar affinities: Quantitative aspects of noncovalent and covalent binding of drugs to proteins. J. Med. Chem. 2008, 52, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Wang, X.; Zhang, Z.-Y.; Jing, X.-B. Prevention of local liver cancer recurrence after surgery using multilayered cisplatin-loaded polylactide electrospun nanofibers. Chin. J. Polym. Sci. 2014, 32, 1111–1118. [Google Scholar] [CrossRef]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Kompella, U.B. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine 2010, 5, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Kataoka, K. Nanostructured devices based on block copolymer assemblies for drug delivery: Designing structures for enhanced drug function. In Polymer Therapeutics II; Springer: Berlin, Germany, 2006; pp. 67–101. [Google Scholar]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer 2004, 90, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Savić, R.; Eisenberg, A.; Maysinger, D. Block copolymer micelles as delivery vehicles of hydrophobic drugs: Micelle–cell interactions. J. Drug Target 2006, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, K.B.; Kowalewski, T.; Wooley, K.L. Water-soluble knedel-like structures: The preparation of shell-cross-linked small particles. J. Am. Chem. Soc. 1996, 118, 7239–7240. [Google Scholar] [CrossRef]
- O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Cross-linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006, 35, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.L.; Pan, D.; Plummer, R.; Chen, Z.; Whittaker, A.K.; Wooley, K.L. Synthesis of gadolinium-labeled shell-crosslinked nanoparticles for magnetic resonance imaging applications. Adv. Funct. Mater. 2005, 15, 1248–1254. [Google Scholar] [CrossRef]
- Ding, J.; Liu, G. Polystyrene-block-poly(2-cinnamoylethyl methacrylate) nanospheres with cross-linked shells. Macromolecules 1998, 31, 6554–6558. [Google Scholar] [CrossRef]
- Li, Y.; Lokitz, B.S.; McCormick, C.L. Thermally responsive vesicles and their structural “locking” through polyelectrolyte complex formation. Angew. Chem. Int. Ed. 2006, 45, 5792–5795. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Liu, G.; Tao, J. Star polymers and nanospheres from cross-linkable diblock copolymers. Macromolecules 1996, 29, 2487–2493. [Google Scholar] [CrossRef]
- Erhardt, R.; Böker, A.; Zettl, H.; Kaya, H.; Pyckhout-Hintzen, W.; Krausch, G.; Abetz, V.; Müller, A.H. Janus micelles. Macromolecules 2001, 34, 1069–1075. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Quinn, J.F.; Caruso, F. Disulfide cross-linked polymer capsules: En route to biodeconstructible systems. Biomacromolecules 2006, 7, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Weaver, J.V.; Save, M.; Armes, S.P. Synthesis of pH-responsive shell cross-linked micelles and their use as nanoreactors for the preparation of gold nanoparticles. Langmuir 2002, 18, 8350–8357. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, S.; Armes, S.P.; Shi, W.; Liu, S. UV irradiation-induced shell cross-linked micelles with pH-responsive cores using ABC triblock copolymers. Macromolecules 2006, 39, 5987–5994. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, S. Facile fabrication of multistimuli-responsive metallo-supramolecular core cross-linked block copolymer micelles. Macromol. Rapid Commun. 2013, 34, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, H.; Luo, S.; Liu, T.; Jiang, Y.; Liu, S. Thiol and pH dual-responsive dynamic covalent shell cross-linked micelles for triggered release of chemotherapeutic drugs. Polym. Chem. 2013, 4, 695–706. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.; Zhang, Y.; Li, Y.; Liu, S. Facile fabrication of reversible core cross-linked micelles possessing thermosensitive swellability. Macromolecules 2007, 40, 9125–9132. [Google Scholar] [CrossRef]
- Wan, X.; Liu, T.; Liu, S. Thermoresponsive core cross-linked micelles for selective ratiometric fluorescent detection of Hg2+ ions. Langmuir 2011, 27, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Liu, J.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission of silole molecules and polymers: Fundamental and applications. J. Inorg. Organomet. Polym. Mater. 2009, 19, 249–285. [Google Scholar] [CrossRef]
- Tong, H.; Hong, Y.; Dong, Y.; Häußler, M.; Lam, J.W.; Li, Z.; Guo, Z.; Guo, Z.; Tang, B.Z. Fluorescent “light-up” bioprobes based on tetraphenylethylene derivatives with aggregation-induced emission characteristics. Chem. Commun. 2006, 3705–3707. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Liu, G.; Wang, X.; Wu, T.; Yang, J.; Ye, X.; Zhang, G.; Hu, J.; Liu, S. pH-regulated reversible transition between polyion complexes (PIC) and hydrogen-bonding complexes (HBC) with tunable aggregation-induced emission. ACS Appl. Mater. Interfaces 2016, 8, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, P.; Yang, X.; Tang, R.; Wang, L.; Qin, J.; Li, Z. Construction of deep-blue AIE luminogens with TPE and oxadiazole units. Sci. China Chem. 2013, 56, 1213–1220. [Google Scholar] [CrossRef]
- He, B.; Ye, S.; Guo, Y.; Chen, B.; Xu, X.; Qiu, H.; Zhao, Z. Aggregation-enhanced emission and efficient electroluminescence of conjugated polymers containing tetraphenylethene units. Sci. China Chem. 2013, 56, 1221–1227. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Sun, J.; Qin, A.; Tang, B.Z. New tetraphenylpyridinium-based luminogens with aggregation-induced emission characteristics. Sci. China Chem. 2013, 56, 1187–1190. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, T.; Zhang, G.; Jin, F.; Liu, S. Synergistically enhance magnetic resonance/fluorescence imaging performance of responsive polymeric nanoparticles under mildly acidic biological milieu. Macromol. Rapid Commun. 2013, 34, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Bütün, V.; Armes, S.; Billingham, N. Synthesis and aqueous solution properties of near-monodisperse tertiary amine methacrylate homopolymers and diblock copolymers. Polymer 2001, 42, 5993–6008. [Google Scholar] [CrossRef]
- Amalvy, J.; Wanless, E.; Li, Y.; Michailidou, V.; Armes, S.; Duccini, Y. Synthesis and characterization of novel pH-responsive microgels based on tertiary amine methacrylates. Langmuir 2004, 20, 8992–8999. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Billingham, N.C.; Armes, S.P. A schizophrenic water-soluble diblock copolymer. Angew. Chem. Int. Ed. 2001, 40, 2328–2331. [Google Scholar] [CrossRef]
- Liu, S.; Weaver, J.V.; Tang, Y.; Billingham, N.C.; Armes, S.P.; Tribe, K. Synthesis of shell cross-linked micelles with pH-responsive cores using ABC triblock copolymers. Macromolecules 2002, 35, 6121–6131. [Google Scholar] [CrossRef]
- Jiang, X.; Ge, Z.; Xu, J.; Liu, H.; Liu, S. Fabrication of multiresponsive shell cross-linked micelles possessing pH-controllable core swellability and thermo-tunable corona permeability. Biomacromolecules 2007, 8, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Liu, T.; Zhang, G.; Liu, S. Highly sensitive and selective fluorometric off–on K+ probe constructed via host–guest molecular recognition and aggregation-induced emission. J. Mater. Chem. 2012, 22, 8622–8628. [Google Scholar] [CrossRef]
- De, S.; Khan, A. Efficient synthesis of multifunctional polymers via thiol–epoxy “click” chemistry. Chem. Commun. 2012, 48, 3130–3132. [Google Scholar] [CrossRef] [PubMed]
- Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Evaluation and control of thiol–ene/thiol–epoxy hybrid networks. Polymer 2007, 48, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, X.; Fan, J.; Wang, G.; Liu, S. Aldehyde surface-functionalized shell cross-linked micelles with pH-tunable core swellability and their bioconjugation with lysozyme. Macromolecules 2007, 40, 9074–9083. [Google Scholar] [CrossRef]
- Hunt, J.F.; Rath, P.; Rothschild, K.J.; Engelman, D.M. Spontaneous, pH-dependent membrane insertion of a transbilayer α-helix. Biochemistry 1997, 36, 15177–15192. [Google Scholar] [CrossRef] [PubMed]
- Andreev, O.A.; Dupuy, A.D.; Segala, M.; Sandugu, S.; Serra, D.A.; Chichester, C.O.; Engelman, D.M.; Reshetnyak, Y.K. Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 7893–7898. [Google Scholar] [CrossRef] [PubMed]
- Reshetnyak, Y.K.; Andreev, O.A.; Segala, M.; Markin, V.S.; Engelman, D.M. Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 15340–15345. [Google Scholar] [CrossRef] [PubMed]
- Andreev, O.A.; Karabadzhak, A.G.; Weerakkody, D.; Andreev, G.O.; Engelman, D.M.; Reshetnyak, Y.K. pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc. Natl. Acad. Sci. USA 2010, 107, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Reshetnyak, Y.K.; Andreev, O.A.; Lehnert, U.; Engelman, D.M. Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proc. Natl. Acad. Sci. USA 2006, 103, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Lewis, D.J.; Watson, S.P.; Thomas, S.G.; Pikramenou, Z. pH-controlled delivery of luminescent europium coated nanoparticles into platelets. Proc. Natl. Acad. Sci. USA 2012, 109, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Meng, H.; Wang, N.; Donovan, M.J.; Fu, T.; You, M.; Chen, Z.; Zhang, X.; Tan, W. A controlled-release nanocarrier with extracellular pH value driven tumor targeting and translocation for drug delivery. Angew. Chem. Int. Ed. 2013, 52, 7487–7491. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, Z.; Lv, Y.; Fan, H.; Bai, H.; Meng, H.; Long, Y.; Fu, T.; Zhang, X.; Tan, W. Gold nanorod-photosensitizer conjugate with extracellular pH-driven tumor targeting ability for photothermal/photodynamic therapy. Nano Res. 2014, 7, 1291–1301. [Google Scholar] [CrossRef]
- Yu, M.; Guo, F.; Wang, J.; Tan, F.; Li, N. Photosensitizer-loaded pH-responsive hollow gold nanospheres for single light-induced photothermal/photodynamic therapy. ACS Appl. Mater. Interfaces 2015, 7, 17592–17597. [Google Scholar] [CrossRef] [PubMed]
- Kyrychenko, A. Nanogold decorated by phlip peptide: Comparative force field study. Phys. Chem. Chem. Phys. 2015, 17, 12648–12660. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Daniels, J.; Wijesinghe, D.; Andreev, O.A.; Reshetnyak, Y.K. Phlip®-mediated delivery of PEGylated liposomes to cancer cells. J. Controlled Release 2013, 167, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, D.; Arachchige, M.C.; Lu, A.; Reshetnyak, Y.K.; Andreev, O.A. pH dependent transfer of nano-pores into membrane of cancer cells to induce apoptosis. Sci. Rep. 2013, 3, 3560. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol polymers for pH-responsive, targeted drug delivery to cancer cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef] [PubMed]
- Oana, H.; Kishimura, A.; Yonehara, K.; Yamasaki, Y.; Washizu, M.; Kataoka, K. Spontaneous formation of giant unilamellar vesicles from microdroplets of a polyion complex by thermally induced phase separation. Angew. Chem. Int. Ed. 2009, 48, 4613–4616. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Huh, Y.M.; Yang, J.; Lee, K.; Suh, J.S.; Haam, S. pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by mri. Adv. Mater. 2011, 23, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhang, G.; Liu, S. pH-disintegrable polyelectrolyte multilayer-coated mesoporous silica nanoparticles exhibiting triggered co-release of cisplatin and model drug molecules. Macromol. Rapid Commun. 2011, 32, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Sumerlin, B.S.; Lowe, A.B.; Thomas, D.B.; McCormick, C.L. Aqueous solution properties of pH-responsive ab diblock acrylamido copolymers synthesized via aqueous raft. Macromolecules 2003, 36, 5982–5987. [Google Scholar] [CrossRef]
- Guice, K.B.; Marrou, S.R.; Gondi, S.R.; Sumerlin, B.S.; Loo, Y.-L. pH response of model diblock and triblock copolymer networks containing polystyrene and poly(2-hydroxyethyl methacrylate-co-2-(dimethylamino) ethyl methacrylate). Macromolecules 2008, 41, 4390–4397. [Google Scholar] [CrossRef]
- Sumerlin, B.S.; Lowe, A.B.; Thomas, D.B.; Convertine, A.J.; Donovan, M.S.; McCormick, C.L. Aqueous solution properties of pH-responsive ab diblock acrylamido–styrenic copolymers synthesized via aqueous reversible addition–fragmentation chain transfer. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1724–1734. [Google Scholar] [CrossRef]
- Liu, G.; Hu, J.; Zhang, G.; Liu, S. Rationally engineering phototherapy modules of eosin-conjugated responsive polymeric nanocarriers via intracellular endocytic pH gradients. Bioconjug. Chem. 2015, 26, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, J.; Yin, J.; Liu, S. Click coupling fullerene onto thermoresponsive water-soluble diblock copolymer and homopolymer chains at defined positions. Macromolecules 2009, 42, 5007–5016. [Google Scholar] [CrossRef]
- Prasuhn, D.E., Jr.; Yeh, R.M.; Obenaus, A.; Manchester, M.; Finn, M. Viral MRI contrast agents: Coordination of Gd by native virions and attachment of Gd complexes by azide–alkyne cycloaddition. Chem. Commun. 2007, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kohlmeir, E.K.; Meade, T.J. Synthesis of multimeric MR contrast agents for cellular imaging. J. Am. Chem. Soc. 2008, 130, 6662–6663. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, J.; Tian, J.; Ge, Z.; Zhang, G.; Luo, K.; Liu, S. Polyprodrug amphiphiles: Hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J. Am. Chem. Soc. 2013, 135, 17617–17629. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 2005, 12, 468–474. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Rodriguez, L.M.; Lubag, A.J.M.; Malloy, C.R.; Martinez, G.V.; Gillies, R.J.; Sherry, A.D. Responsive MRI agents for sensing metabolism in vivo. Acc. Chem. Res. 2009, 42, 948–957. [Google Scholar] [CrossRef] [PubMed]






| Entry | Samples a | Mn a (kDa) | Mn b (kDa) | Mw/Mn b |
|---|---|---|---|---|
| P1 | N3-POEGMA32-Br | 17.6 | 17.1 | 1.09 |
| BP1 | N3-POEGMA32-b-P(DPA0.86-co-GMA0.14)42 | 26.1 | 21.0 | 1.14 |
| BP2 | DOTA(Gd)-POEGMA32-b-P(DPA0.86-co-GMA0.14)42 | – | 21.8 | 1.18 |
| BP3 | Benzaldehyde-POEGMA32-b-P(DPA0.86-co-GMA0.14)42 | 26.1 | 22.4 | 1.16 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Liu, G.; Wang, X.; Zhang, G.; Hu, J. pH-Responsive Tumor-Targetable Theranostic Nanovectors Based on Core Crosslinked (CCL) Micelles with Fluorescence and Magnetic Resonance (MR) Dual Imaging Modalities and Drug Delivery Performance. Polymers 2016, 8, 226. https://doi.org/10.3390/polym8060226
Tian S, Liu G, Wang X, Zhang G, Hu J. pH-Responsive Tumor-Targetable Theranostic Nanovectors Based on Core Crosslinked (CCL) Micelles with Fluorescence and Magnetic Resonance (MR) Dual Imaging Modalities and Drug Delivery Performance. Polymers. 2016; 8(6):226. https://doi.org/10.3390/polym8060226
Chicago/Turabian StyleTian, Sidan, Guhuan Liu, Xiaorui Wang, Guoying Zhang, and Jinming Hu. 2016. "pH-Responsive Tumor-Targetable Theranostic Nanovectors Based on Core Crosslinked (CCL) Micelles with Fluorescence and Magnetic Resonance (MR) Dual Imaging Modalities and Drug Delivery Performance" Polymers 8, no. 6: 226. https://doi.org/10.3390/polym8060226
APA StyleTian, S., Liu, G., Wang, X., Zhang, G., & Hu, J. (2016). pH-Responsive Tumor-Targetable Theranostic Nanovectors Based on Core Crosslinked (CCL) Micelles with Fluorescence and Magnetic Resonance (MR) Dual Imaging Modalities and Drug Delivery Performance. Polymers, 8(6), 226. https://doi.org/10.3390/polym8060226