Cis-1,4-Polymerization of Isoprene by 1,3-Bis(oxazolinymethylidene)isoindoline-Ligated Rare-Earth Metal Dialkyl Complexes
Abstract
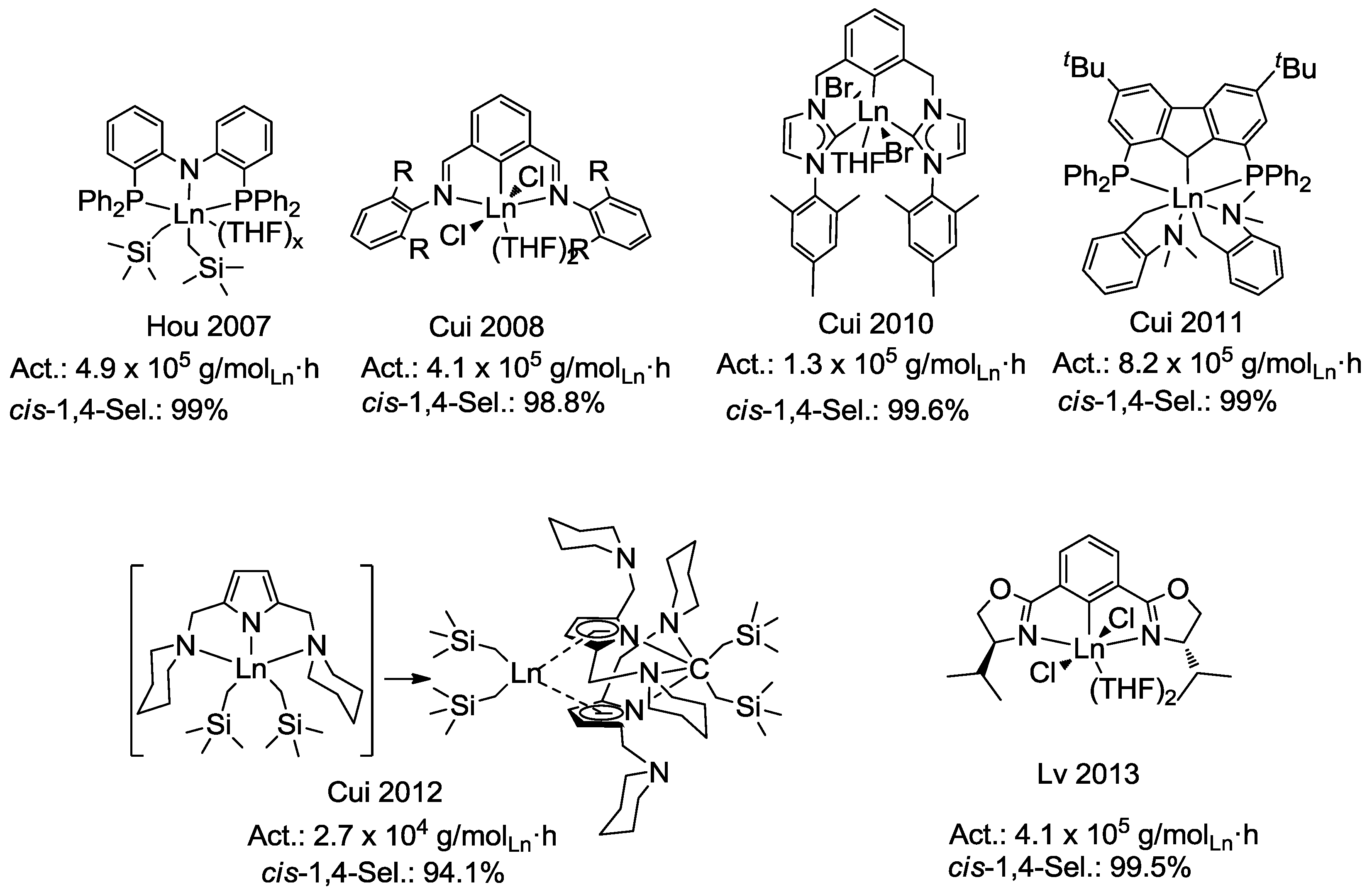
:1. Introduction
2. Experiment
2.1. Materials and Method
2.2. Synthesis of Chiral BOXMI-H-Ligated Rare-Earth Metal Dialkyl Complexes
2.3. A Typical Procedure for Isoprene (IP) Polymerization
3. Results and Discussion
3.1. Synthesis and Structural Characterization of BOXMI-Ligated Rare-Earth Metal Dialkyl Complexes 1–3
3.2. Single Crystals of Complexes 1 and 2
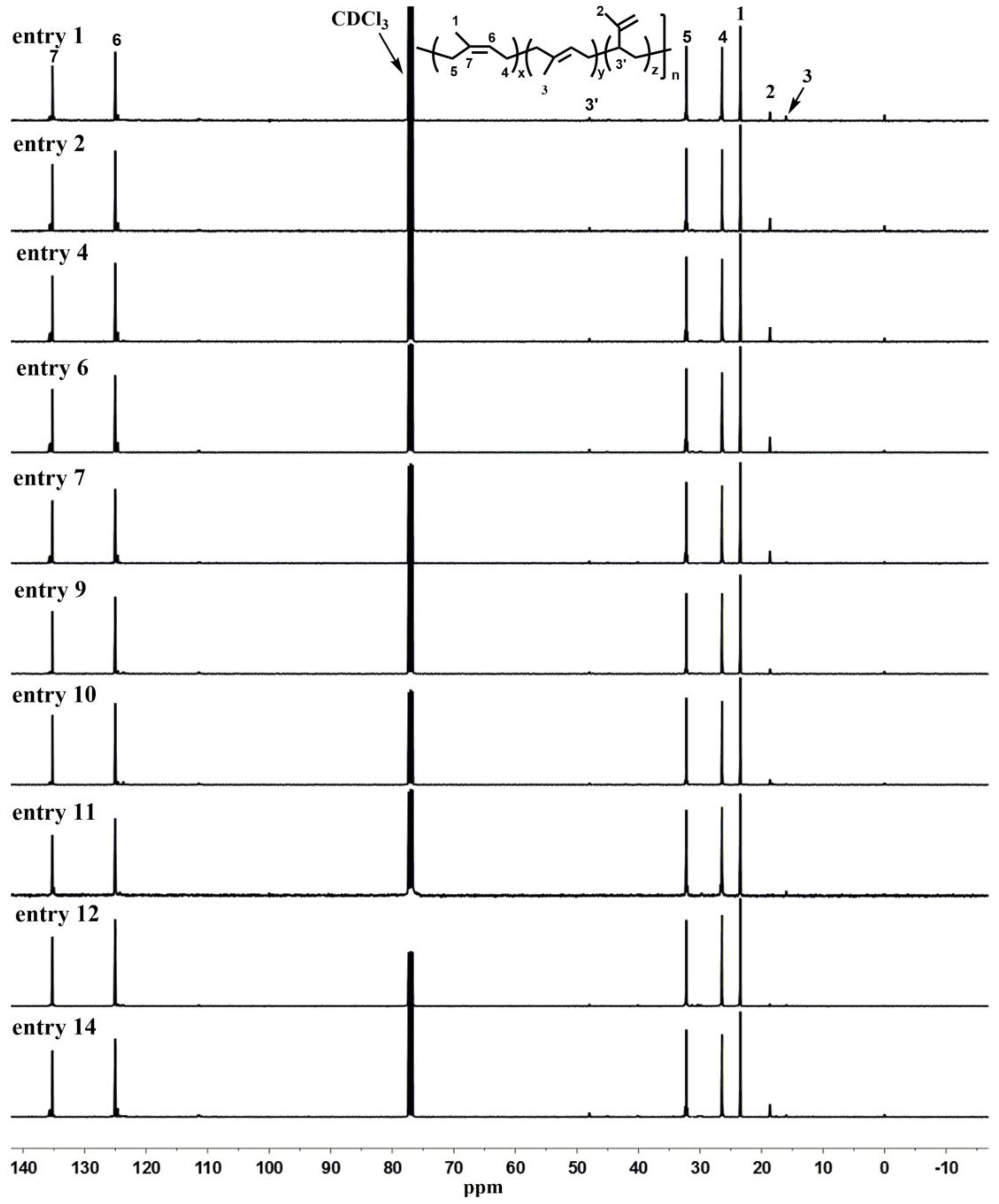
3.3. Cis-1,4-Polymerization of Isoprene by the Complexes 1–3/Activator/AlR3 Catalytic Systems
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, T.S.; Jenter, J.; Roesky, P.W. Rare Earth Metal Postmetallocene Catalysts with Chelating Amido Ligands. Struct. Bond. 2010, 137, 165–228. [Google Scholar]
- Piers, W.E.; Emslie, D.J.H. Non-cyclopentadienyl ancillaries in organogroup 3 metal chemistry: A fine balance in ligand design. Coord. Chem. Rev. 2002, 233, 131–155. [Google Scholar] [CrossRef]
- Gromada, J.; Carpentier, J.-F.; Mortreux, A. Group 3 metal catalysts for ethylene and α-olefin polymerization. Coord. Chem. Rev. 2004, 248, 397–410. [Google Scholar] [CrossRef]
- Kirillov, E.; Saillard, J.-Y.; Carpentier, J.-F. Groups 2 and 3 metal complexes incorporating fluorenyl ligands. Coord. Chem. Rev. 2005, 249, 1221–1248. [Google Scholar] [CrossRef]
- Zeimentz, P.M.; Arndt, S.; Elvidge, B.R.; Okuda, J. Cationic Organometallic Complexes of Scandium, Yttrium, and the Lanthanoids. Chem. Rev. 2006, 106, 2404–2433. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.T. Lanthanide amidinates and guanidinates in catalysis and materials science: A continuing success story. Chem. Soc. Rev. 2012, 41, 7657–7672. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, M.; Guo, F.; Hou, Z. Half-Sandwich rare earth catalyzed olefin polymerization, carbometalation, and hydroarylation. Acc. Chem. Res. 2015, 48, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.T. Lanthanides and actinides: Annual survey of their organometallic chemistry covering the year 2014. Coord. Chem. Rev. 2016, 306, 346–419. [Google Scholar] [CrossRef]
- Edelmann, F.T. Lanthanides and actinides: Annual survey of their organometallic chemistry covering the year 2015. Coord. Chem. Rev. 2016, 318, 29–130. [Google Scholar] [CrossRef]
- Edelmann, F.T. Lanthanides and actinides: Annual survey of their organometallic chemistry covering the year 2016. Coord. Chem. Rev. 2017, 338, 27–140. [Google Scholar] [CrossRef]
- Nishiura, M.; Hou, Z. Novel polymerization catalysts and hydride clusters from rare earth metal dialkyls. Nat. Chem. 2010, 2, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Ouyang, J.; Wang, F.; Hu, Z.; Yu, F.; Qian, B.J. The characteristics of lanthanide coordination catalysts and the cis-polydienes prepared therewith. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 3345–3357. [Google Scholar]
- Kwag, G.; Lee, H.; Kim, S. First in-Situ Observation of Pseudoliving Character and Active Site of Nd-Based Catalyst for 1,3-Butadiene Polymerization Using Synchrotron X-ray Absorption and UV-Visible Spectroscopies. Macromolecules 2001, 34, 5367–5369. [Google Scholar] [CrossRef]
- Evans, W.J.; Giarikos, D.G.; Ziller, J.W. Lanthanide Carboxylate Precursors for Diene Polymerization Catalysis: Syntheses, Structures, and Reactivity with Et2AlCl. Organometallics 2001, 20, 5751–5758. [Google Scholar] [CrossRef]
- Evans, W.J.; Giarikos, D.G. Chloride Effects in Lanthanide Carboxylate Based Isoprene Polymerization. Macromolecules 2004, 37, 5130–5132. [Google Scholar] [CrossRef]
- Fischbach, A.; Klimpel, M.G.; Widenmeyer, M.; Herdtweck, E.; Scherer, W.; Anwander, R. Stereospecific polymerization of isoprene with molecular and MCM-48-grafted lanthanide(III) tetraalkylaluminates. Angew. Chem. Int. Ed. 2004, 43, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Beckerle, K.; Zeimentz, P.M.; Spaniol, T.P.; Okuda, J. Cationic yttrium methyl complexes as functional models for polymerization catalysts of 1,3-dienes. Angew. Chem. Int. Ed. 2005, 44, 7473–7477. [Google Scholar] [CrossRef] [PubMed]
- Ajellal, N.; Furlan, L.; Thomas, C.M.; Casagrande, O.L.; Carpentier, J.-F. Mixed Aluminum-Magnesium-Rare Earth Allyl Catalysts for Controlled Isoprene Polymerization: Modulation of Stereocontrol. Macromol. Rapid Commun. 2006, 27, 338–343. [Google Scholar] [CrossRef]
- Fischbach, A.; Perdih, F.; Herdtweck, E.; Anwander, R. Structure-Reactivity Relationships in Rare-Earth Metal Carboxylate-Based Binary Ziegler-Type Catalysts. Organometallics 2006, 25, 1626–1642. [Google Scholar] [CrossRef]
- Meermann, C.; Tornroos, K.W.; Nerdal, W.; Anwander, R. Rare-earth metal mixed chloro/methyl compounds: heterogeneous-homogeneous borderline catalysts in 1,3-diene polymerization. Angew. Chem. Int. Ed. 2007, 46, 6508–6513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nishiura, M.; Yuki, M.; Luo, Y.; Hou, Z. Isoprene polymerization with yttrium amidinate catalysts: switching the regio- and stereoselectivity by addition of AlMe3. Angew. Chem. Int. Ed. 2008, 47, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Cui, D.; Hou, Z.; Li, X. Living catalyzed-chain-growth polymerization and block copolymerization of isoprene by rare earth metal allyl precursors bearing a constrained-geometry-conformation ligand. Chem. Commun. 2010, 46, 3022–3024. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, C.; Liu, D.; Li, S.; Cui, D. Binuclear Rare-Earth-Metal Alkyl Complexes Ligated by Phenylene-Bridged β-Diketiminate Ligands: Synthesis, Characterization, and Catalysis toward Isoprene Polymerization. Organometallics 2013, 32, 3203–3209. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Z.; Gao, W.; Xin, L.; Zhang, L.; Mu, Y. Y, Lu, and Gd complexes of NCO/NCS pincer ligands: synthesis, characterization, and catalysis in the cis-1,4-selective polymerization of isoprene. Chem. Asian J. 2013, 8, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, S.; Zhou, S.; Wei, Y.; Guo, L.; Zhu, X.; Zhang, L.; Gu, X.; Mu, X. Synthesis and Reactivity of Rare-Earth-Metal Monoalkyl Complexes Supported by Bidentate Indolyl Ligands and Their High Performance in Isoprene 1,4-cis Polymerization. Organometallics 2015, 34, 4251–4261. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, Y.; Guo, L.; Zhu, X.; Wang, S.; Zhou, S.; Mu, X. Dinuclear rare-earth metal alkyl complexes supported by indolyl ligands in μ-η2:η1:η1 hapticities and their high catalytic activity for isoprene 1,4-cis-polymerization. Chem. Eur. J. 2015, 21, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Deng, B.; Wang, S.; Wei, Y.; Zhou, S.; Zhu, X.; Huang, Z.; Mu, X. Di and trinuclear rare-earth metal complexes supported by 3-amido appended indolyl ligands: synthesis, characterization and catalytic activity towards isoprene 1,4-cis polymerization. Dalton Trans. 2016, 45, 15445–15456. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, A.A.; Lyubov, D.M. A quarter-century long story of bis(alkyl) rare-earth (III) complexes. Coord. Chem. Rev. 2017, 340, 10–61. [Google Scholar] [CrossRef]
- Peng, D.; Yan, X.; Yu, C.; Zhang, S.; Li, X. Transition metal complexes bearing tridentate ligands for precise olefin polymerization. Polym. Chem. 2016, 7, 2601–2634. [Google Scholar] [CrossRef]
- Dagorne, S.; Fliedel, C. Modern Organoaluminium Reagents. Top. Organomet. Chem. 2013, 41, 125–172. [Google Scholar]
- Zhang, L.; Suzuki, T.; Luo, Y.; Nishiura, M.; Hou, Z. Cationic alkyl rare earth metal complexes bearing an ancillary bis(phosphinophenyl)amido ligand: A catalytic system for living cis-1,4-polymerization and copolymerization of isoprene and butadiene. Angew. Chem. Int. Ed. 2007, 46, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Cui, D. Highly cis-1,4 Selective Polymerization of Dienes with Homogeneous Ziegler-Natta Catalysts Based on NCN-Pincer Rare Earth Metal Dichloride Precursors. J. Am. Chem. Soc. 2008, 130, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Cui, D. CCC-Pincer Bis(carbene) Lanthanide Dibromides. Catalysis on Highly cis-1,4-Selective Polymerization of Isoprene and Active Species. Organometallics 2010, 29, 2987–2993. [Google Scholar] [CrossRef]
- Wang, L.; Cui, D.; Hou, Z.; Li, W.; Li, Y. Highly Cis-1,4-Selective Living Polymerization of 1,3-Conjugated Dienes and Copolymerization with ε-Caprolactone by Bis(phosphino)carbazolide Rare-Earth-Metal Complexes. Organometallics 2011, 30, 760–767. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Cui, D. NNN-Tridentate Pyrrolyl Rare-Earth Metal Complexes: Structure and Catalysis on Specific Selective Living Polymerization of Isoprene. Organometallics 2012, 31, 6014–6021. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, T.; Yang, G.W.; Jin, K.; Lu, X.B. Bis(oxazolinyl)phenyl-ligated rare-earth-metal complexes: highly regioselective catalysts for cis-1,4-polymerization of isoprene. Inorg. Chem. 2013, 52, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Cui, D.; Chen, X.; Jing, X. Pyrrolide-Ligated Organoyttrium Complexes. Synthesis, Characterization, and Lactide Polymerization Behavior. Organometallics 2007, 26, 671–678. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Liu, Z.; Lin, Z.; Du, G.; Zhang, S.; Li, X. Quasi-Living trans-1,4-Polymerization of Isoprene by Cationic Rare Earth Metal Alkyl Species Bearing a Chiral (S,S)-Bis(oxazolinylphenyl)amido Ligand. Macromolecules 2013, 46, 3257–3265. [Google Scholar] [CrossRef]
- Zhang, P.; Liao, H.; Wang, H.; Li, X.; Yang, F.; Zhang, S. Cis-1,4-Polymerization of Isoprene Catalyzed by 1,3-Bis(2-pyridylimino)isoindoline-Ligated Rare-Earth-Metal Dialkyl Complexes. Organometallics 2017, 36, 2446–2451. [Google Scholar] [CrossRef]
- Deng, Q.H.; Wadepohl, H.; Gade, L.H. The Synthesis of a New Class of Chiral Pincer Ligands and Their Applications in Enantioselective Catalytic Fluorinations and the Nozaki–Hiyama–Kishi Reaction. Chem. Eur. J. 2011, 17, 14922–14928. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.H.; Wadepohl, H.; Gade, L.H. Highly enantioselective copper-catalyzed alkylation of beta-ketoesters and subsequent cyclization to spirolactones/bi-spirolactones. J. Am. Chem. Soc. 2012, 134, 2946–2949. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.H.; Bleith, T.; Wadepohl, H.; Gade, L.H. Enantioselective iron-catalyzed azidation of beta-keto esters and oxindoles. J. Am. Chem. Soc. 2013, 135, 5356–5359. [Google Scholar] [CrossRef] [PubMed]
- Bleith, T.; Wadepohl, H.; Gade, L.H. Iron Achieves Noble Metal Reactivity and Selectivity: Highly Reactive and Enantioselective Iron Complexesas Catalysts in the Hydrosilylation of Ketones. J. Am. Chem. Soc. 2015, 137, 2456–2459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nishiura, M.; Hu, L.; Mori, k.; Hou, Z. Alternating and Random Copolymerization of Isoprene and Ethylene Catalyzed by Cationic Half-Sandwich Scandium Alkyls. J. Am. Chem. Soc. 2009, 131, 13870–13882. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Wei, Y.; Ai, L.; Chen, Y.; Xu, Q.; Liu, X.; Zhang, S.; Hou, Z.; Li, X. Living 3,4-Polymerization of Isoprene by Cationic Rare Earth Metal Alkyl Complexes Bearing Iminoamido Ligands. Organometallics 2011, 30, 160–170. [Google Scholar] [CrossRef]


| Bond Lengths and Bond Angles | 1 | 2 |
|---|---|---|
| Ln−N1 | 2.272(4) | 2.345(4) |
| Ln−N2 | 2.228(3) | 2.313(4) |
| Ln−N3 | 2.274(4) | 2.351(4) |
| Ln−C29 | 2.235(4) | 2.329(5) |
| Ln−C33 | 2.241(4) | 2.333(5) |
| N1−Ln−N3 | 166.9(1) | 161.8(1) |
| N2−Ln−N1 | 83.8(1) | 81.1(1) |
| N2−Ln−N3 | 83.9(1) | 81.4(1) |
| N2−Ln−C29 | 118.9(1) | 118.9(1) |
| N2−Ln−C33 | 126.3(2) | 126.3(4) |
| C29−Ln−N1 | 98.3(2) | 100.5(2) |
| C29−Ln−N3 | 91.6(2) | 92.2(3) |
| C29−Ln−C33 | 114.8(2) | 114.7(2) |
| C33−Ln−N1 | 88.3(1) | 89.1(2) |
| C33−Ln−N3 | 95.4(1) | 97.4(8) |
| Entry | Comp. | Borate b | AlR3 | t (h) | T (°C) | Y (%) | A c | Microstructure (%) d | Mn e (104) | Mw/Mn e | Tg f (°C) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c-1,4- | t-1,4- | 3,4- | |||||||||||
| 1 | 1 | A | AliBu3 | 0.5 | 25 | 85 | 23 | 87 | 2 | 11 | 71 | 2.00 | −58 |
| 2 | 1 | B | AliBu3 | 4 | 25 | 96 | 3 | 87 | 0 | 13 | 32 | 2.75 | −60 |
| 3 | 1 | C | AliBu3 | 12 | 25 | — | — | — | — | — | — | — | — |
| 4 | 1 | A | AlEt3 | 4 | 25 | 93 | 3 | 88 | 0 | 12 | 16 | 3.18 | −59 |
| 5 | 1 | B | AlEt3 | 4 | 25 | 86 | 3 | 88 | 0 | 12 | 13 | 3.21 | −62 |
| 6 | 1 | A | AlMe3 | 6 | 25 | 92 | 2 | 89 | 1 | 10 | 8 | 4.31 | −58 |
| 7 | 2 | A | AliBu3 | 4 | 25 | 93 | 3 | 87 | 0 | 13 | 11 | 3.72 | −59 |
| 8 | 2 | B | AliBu3 | 4 | 25 | 85 | 3 | 88 | 0 | 12 | 8 | 4.51 | — |
| 9 | 3 | A | AliBu3 | 2 | 25 | 92 | 6 | 93 | 0 | 7 | 16 | 1.87 | −63 |
| 10 | 3 | B | AliBu3 | 4 | 25 | 85 | 3 | 90 | 0 | 10 | 10 | 3.36 | −61 |
| 11 | 3 | A | AliBu3 | 2 | 0 | 82 | 6 | 95 | 0 | 5 | 25 | 2.32 | −62 |
| 12 | 3 | A | AliBu3 | 2 | −20 | 80 | 5 | 97 | 1 | 2 | 35 | 1.83 | −65 |
| 13 | 3 | A | AliBu3 | 0.5 | 50 | 93 | 25 | 87 | 0 | 13 | 16 | 2.87 | −61 |
| 14 | 3 | A | AliBu3 | 0.5 | 70 | 99 | 68 | 85 | 2 | 13 | 20 | 3.31 | −59 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Zhou, D.; Yan, X.; Gao, F.; Zhang, L.; Zhang, S.; Li, X. Cis-1,4-Polymerization of Isoprene by 1,3-Bis(oxazolinymethylidene)isoindoline-Ligated Rare-Earth Metal Dialkyl Complexes. Polymers 2017, 9, 531. https://doi.org/10.3390/polym9100531
Yu C, Zhou D, Yan X, Gao F, Zhang L, Zhang S, Li X. Cis-1,4-Polymerization of Isoprene by 1,3-Bis(oxazolinymethylidene)isoindoline-Ligated Rare-Earth Metal Dialkyl Complexes. Polymers. 2017; 9(10):531. https://doi.org/10.3390/polym9100531
Chicago/Turabian StyleYu, Chao, Dahai Zhou, Xiangqian Yan, Fei Gao, Li Zhang, Shaowen Zhang, and Xiaofang Li. 2017. "Cis-1,4-Polymerization of Isoprene by 1,3-Bis(oxazolinymethylidene)isoindoline-Ligated Rare-Earth Metal Dialkyl Complexes" Polymers 9, no. 10: 531. https://doi.org/10.3390/polym9100531
APA StyleYu, C., Zhou, D., Yan, X., Gao, F., Zhang, L., Zhang, S., & Li, X. (2017). Cis-1,4-Polymerization of Isoprene by 1,3-Bis(oxazolinymethylidene)isoindoline-Ligated Rare-Earth Metal Dialkyl Complexes. Polymers, 9(10), 531. https://doi.org/10.3390/polym9100531




