Ion Conduction and Its Activation in Hydrated Solid Polyelectrolyte Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Conductivity Measurements
3. Results
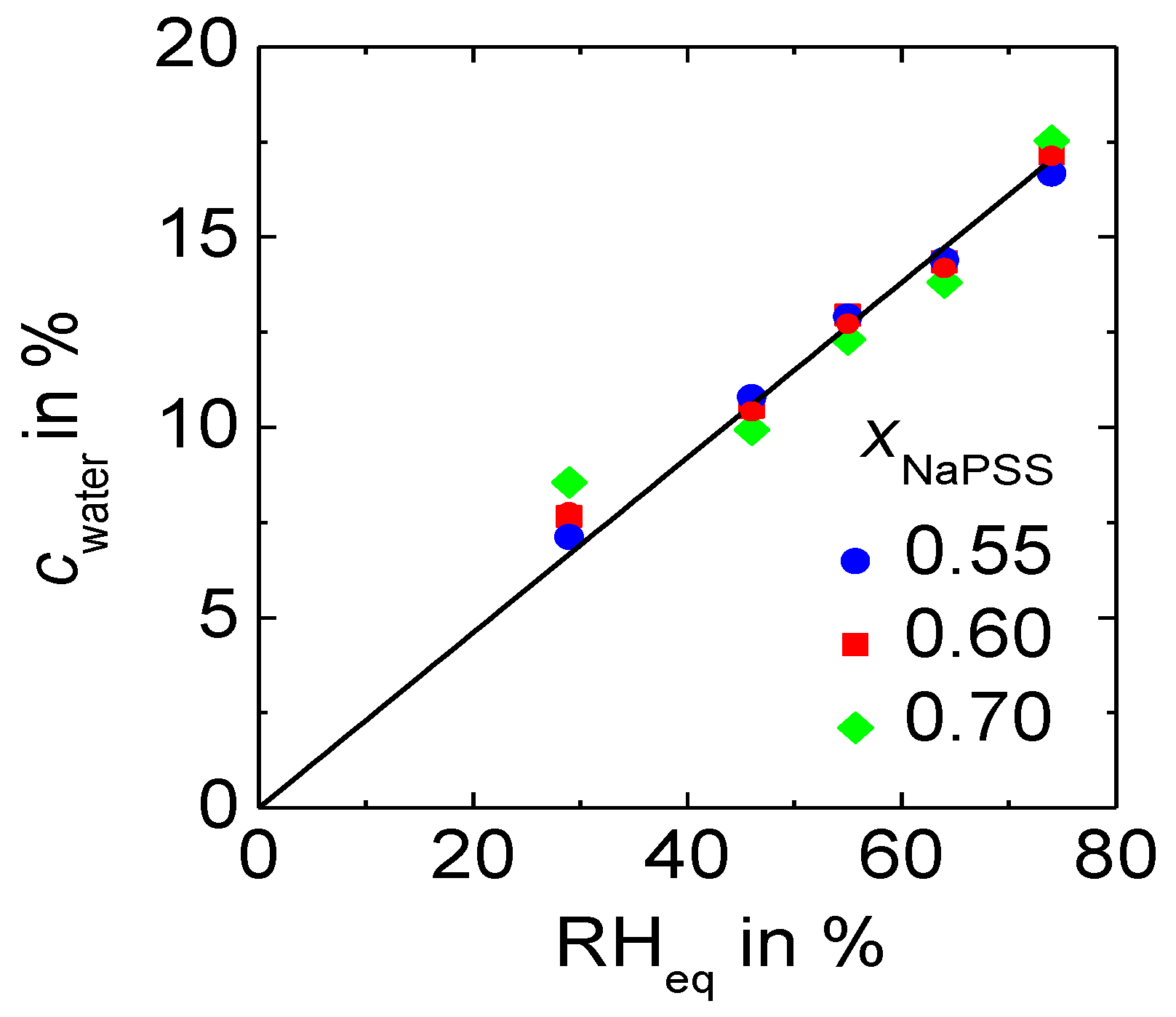
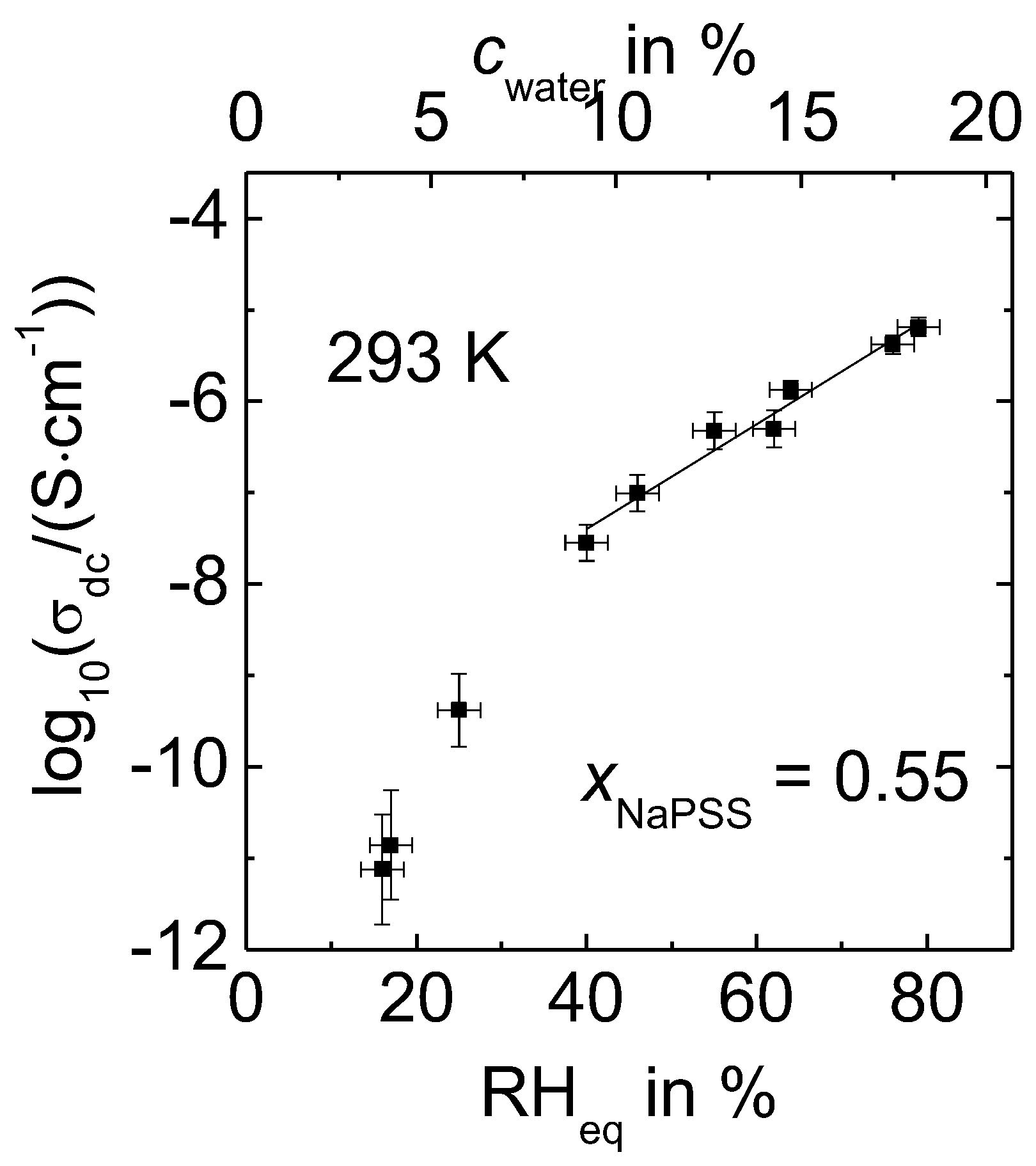
3.1. Water Content and Its Influence on the Conductivity
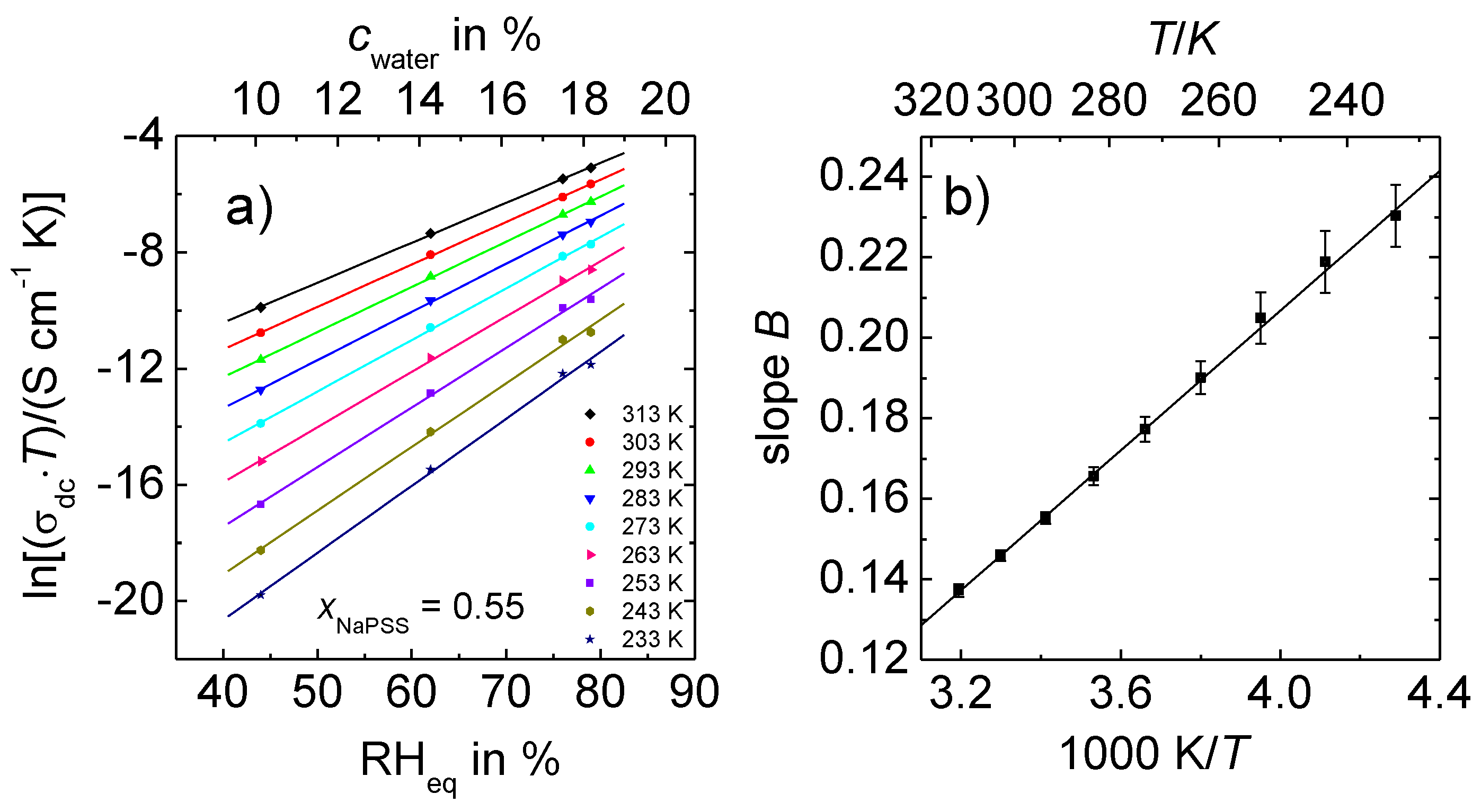
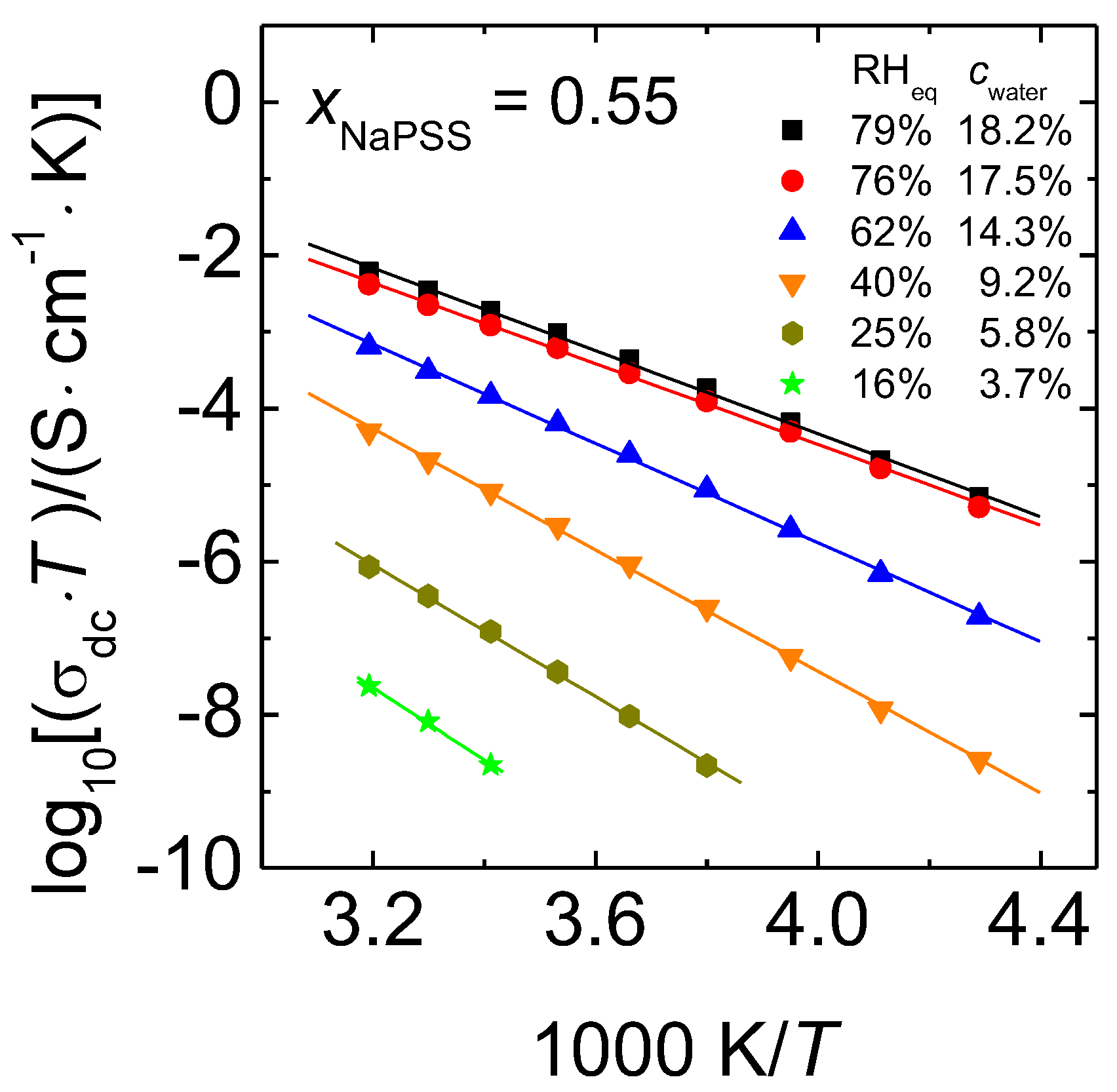
3.2. Arrhenius Representations of the DC Conductivity
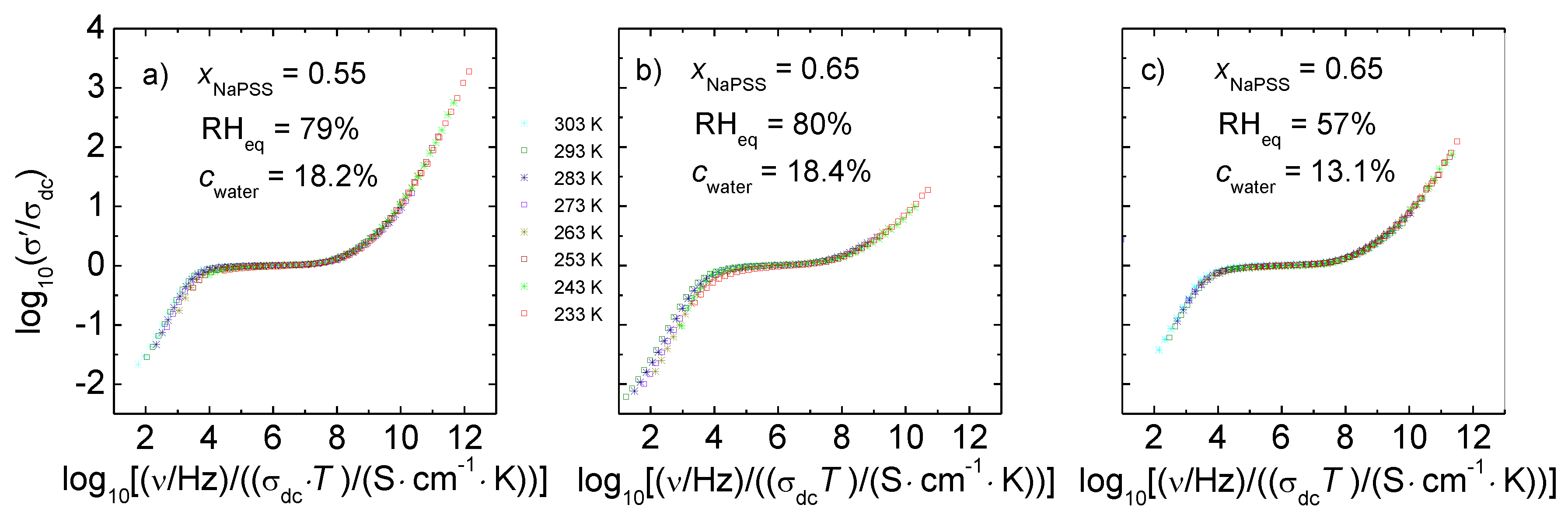
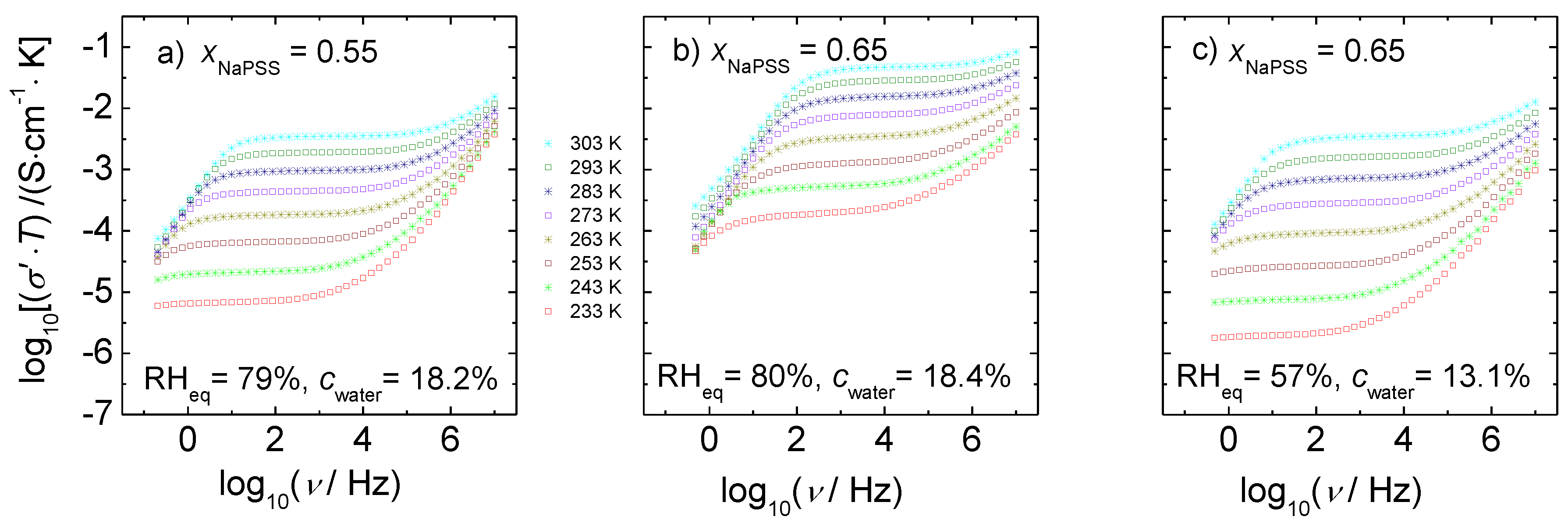
3.3. Scaling Behavior
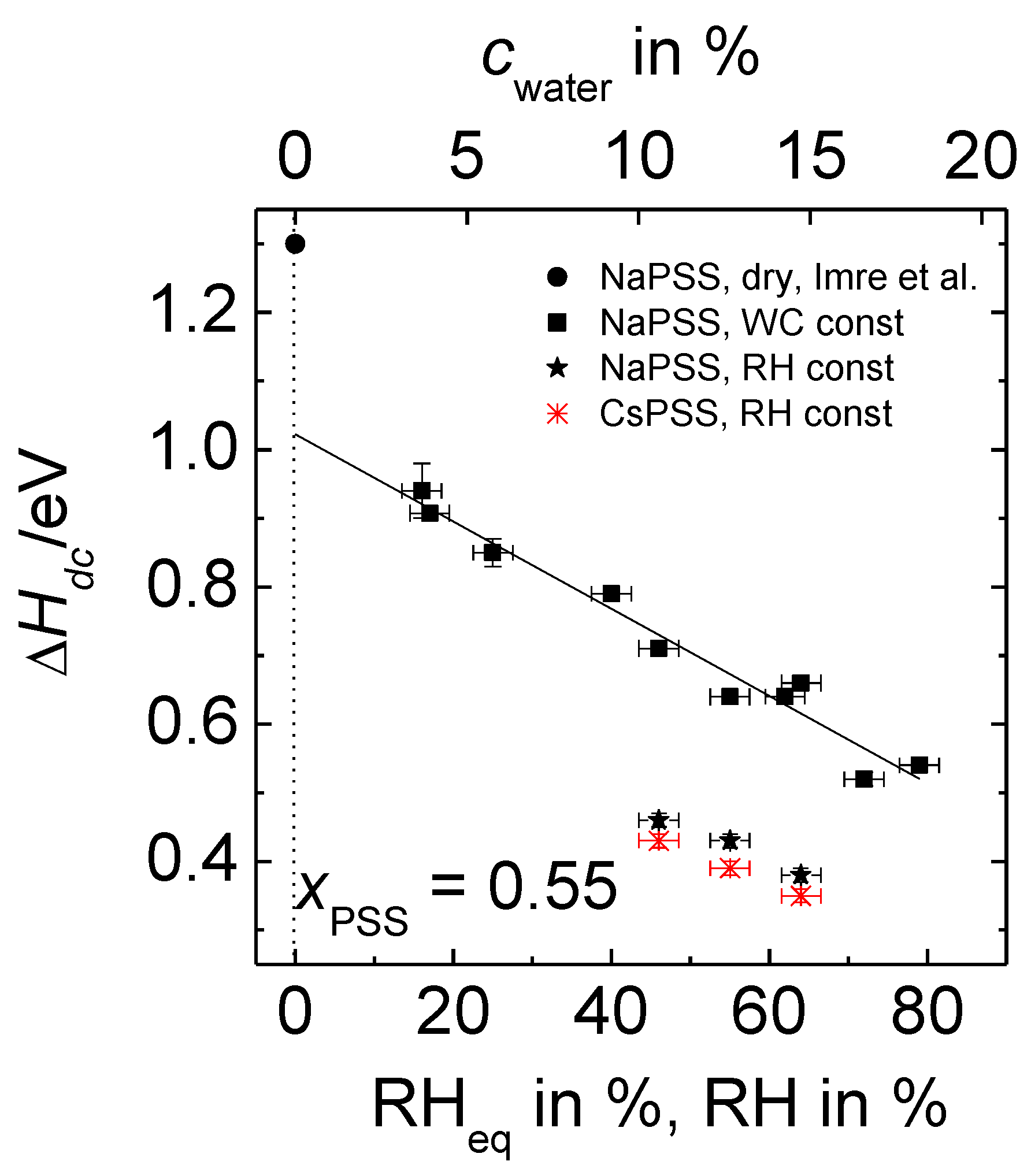
3.4. Comparison with Data Determined at Constant Relative Humidity
4. Discussion
- Under same external conditions PEC with NaPSS absorb more water than PEC with CsPSS.
- At fixed temperature and humidity, CsPEC show a higher conductivity than NaPEC.
- The dc conductivity increases as a function of temperature, humidity and/or water content.
- The dc conductivity obtained at constant water content and at constant RH increases with increasing PSS content.
- The temperature dependence of the dc conductivities of all humid PEC follows the Arrhenius law.
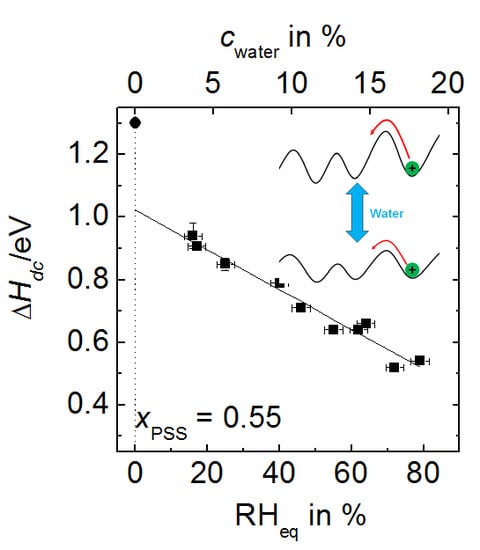
- The activation enthalpy values for humid PEC are significantly lower than those of completely dried complexes as reported in Reference [26]. For the composition and temperature range investigated, the activation enthalpies determined at fixed relative humidity are lower than those determined at fixed water content. Moreover, in case of fixed humidity measurements, PEC prepared from CsPSS possess lower activation enthalpies than PEC with NaPSS under same temperature and humidity condition.
- The time-temperature superposition principle is valid for the conductivity spectra of humidified PEC and the conductivity spectra can be scaled with the help of the Summerfield scaling.
- If proton transport dominated the dc conductivity, one would expect that the conductivity in the cRH-series should increase with temperature because of the additional absorption of water into the PEC.
- An increasing water content in the PEC should go hand in hand with an increasing number of mobile charge carries, which would result in deviations from the Summerfield scaling concept. By contrast, the number density of mobile ions is not T-dependent, indicating that hydrated alkali ions are the major charge carriers.
- PEC prepared from CsPSS show under same conditions a higher conductivity than PEC with NaPSS. This also confirms that the alkali ions in their hydrations shell determine the ion transport properties.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bungenberg de Jong, H.G.; Kruyt, H.R. Coacervation (partial miscibility in colloid systems). Proc. Acad. Sci. (Amsterdam) 1929, 32, 849–856. [Google Scholar]
- Fuoss, R.M.; Sadek, H. Mutual Interaction of Polyelectrolytes. Science 1949, 110, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Bucur, C.B.; Sui, Z.; Schlenoff, J.B. Ideal Mixing in Polyelectrolyte Complexes and Multilayers: Entropy Driven Assembly. J. Am. Chem. Soc. 2006, 128, 13690–13691. [Google Scholar] [CrossRef] [PubMed]
- Gopinadhan, M.; Ivanova, O.; Ahrens, H.; Gunther, J.U.; Steitz, R.; Helm, C.A. The Influence of Secondary Interactions during the Formation of Polyelectrolyte Multilayers: Layer Thickness; Bound Water and Layer Interpenetration. J. Phys. Chem. B 2007, 111, 8426–8434. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.; Kabanov, A.V. DNA Complexes with Polycations for the Delivery of Genetic Material into Cells. Bioconj. Chem. 1995, 6, 7–20. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Lin, H.; Zhang, G.; Zhang, Y.; Ni, J.; Ma, W.; Na, H. Design of a stable and methanol resistant membrane with cross-linked multilayered polyelectrolyte complexes for direct methanol fuel cells. J. Power Sources 2011, 196, 5432–5437. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Polyelectrolyte Complexes of Chitosan and Poly(acrylic acid) As Proton Exchange Membranes for Fuel Cells. Macromolecules 2004, 37, 2233–2239. [Google Scholar] [CrossRef]
- Yilmaztürk, S.; Deligoz, H.; Yilmazoglu, M.; Damyan, H.; Oksuzomer, F.; Koc, S.N.; Durmus, A.; Gurkaynak, M.A. Self-assembly of highly charged polyelectrolyte complexes with superior proton conductivity and methanol barrier properties for fuel cells. J. Power Sources 2010, 195, 703–709. [Google Scholar] [CrossRef]
- Rodriguez, L.N.J.; De Paul, S.M.; Barrett, C.J.; Reven, L.; Spiess, H.W. Fast Magic-Angle Spinning and Double-Quantum 1H Solid-State NMR Spectroscopy of Polyelectrolyte Multilayers. Adv. Mater. 2000, 12, 1934–1938. [Google Scholar] [CrossRef]
- Sukhishvili, S.A.; Kharlampieva, E.; Izumrudov, V. Where Polyelectrolyte Multilayers and Polyelectrolyte Complexes Meet. Macromolecules 2006, 39, 8873–8881. [Google Scholar] [CrossRef]
- Kovacevic, D.; van der Burgh, S.; de Keizer, A.; Cohen Stuart, M.A. Kinetics of Formation and Dissolution of Weak Polyelectrolyte Multilayers: Role of Salt and Free Polyions. Langmuir 2002, 18, 5607–5612. [Google Scholar] [CrossRef]
- Qiao, B.; Cerdà, J.J.; Holm, C. Poly(styrenesulfonate)–Poly(dialllyldimethylammonium) Mixtures: Toward the Understanding of Polyelectrolyte Complexes and Multilayers via Atomistic Simulations. Macromolecules 2010, 43, 7828–7838. [Google Scholar] [CrossRef]
- Vögele, M.; Holm, C.; Smiatek, J. Cooarse-grained simulations of polyelectrolyte complexes: MARTINI models for poly(styrene sulfonate) an poly(diallyldimethylammonuium). J. Chem. Phys. 2015, 143, 243151-1–243151-8. [Google Scholar] [CrossRef] [PubMed]
- Durstock, M.F.; Rubner, M.F. Dielectric Properties of Polyelectrolyte Multilayers. Langmuir 2001, 17, 7865–7872. [Google Scholar] [CrossRef]
- DeLongchamp, D.M.; Hammond, P.T. Fast Ion Conduction in Layer-By-Layer Polymer Films. Chem. Mater. 2003, 15, 1165–1173. [Google Scholar] [CrossRef]
- DeLongchamp, D.M.; Hammond, P.T. Highly Ion Conductive Poly(ethylene oxide)-Based Solid Polymer Electrolytes from Hydrogen Bonding Layer-by-Layer Assembly. Langmuir 2004, 20, 5403–5411. [Google Scholar] [CrossRef] [PubMed]
- Lutkenhaus, J.L.; Hammond, P.T. Electrochemically enabled polyelectrolyte multilayer devices: From fuel cells to sensors. Soft Matter 2007, 3, 804–816. [Google Scholar] [CrossRef]
- Farhat, T.; Yassinn, G.; Dubas, S.T.; Schlenoff, J.B. Water and Ion Pairing in Polyelectrolyte Multilayers. Langmuir 1999, 15, 6621–6623. [Google Scholar] [CrossRef]
- Ghostine, R.A.; Markarian, M.Z.; Schlenoff, J.B. Asymmetric Growth in Polyelectrolyte Multilayers. J. Am. Chem. Soc. 2013, 135, 7636–7646. [Google Scholar] [CrossRef] [PubMed]
- Akgöl, A.; Cramer, C.; Hofmann, C.; Karatas, Y.; Wiemhöfer, H.D.; Schönhoff, M. Humidity-Dependent DC Conductivity of Polyelectrolyte Multilayers: Protons or Other Small Ions as Charge Carriers? Macromolecules 2010, 43, 7282–7287. [Google Scholar] [CrossRef]
- Cramer, C.; Schönhoff, M. Ion Conduction in Solid Polyelectrolyte Complex Materials. In Polyelectrolyte Complexes in the Dispersed and Solid State I; Müller, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 255, pp. 97–138, ISBN 978-3-642-40733-8 (Print), 978-3-642-40734-5 (Online). [Google Scholar]
- Michaels, A.S.; Miekka, R.G. Polycation-Polyanion Complexes: Preparation and Properties of Poly-(Vinyl-benzyltrimethylammonium) Poly-(Styrenesulfonate). J. Phys. Chem. 1961, 65, 1765–1773. [Google Scholar] [CrossRef]
- Michaels, A.S.; Falkenstein, G.L.; Schneider, N.S. Dielectric Properties of Polyanion—Polycation Complexes. J. Phys. Chem. 1965, 69, 1456–1465. [Google Scholar] [CrossRef]
- Philipp, B.; Dautzenberg, H.; Linow, K.J.; Koetz, J.; Davydoff, W. Polyelectrolyte complexes—Recent developments and open problems. Prog. Polym. Sci. 1989, 14, 91–172. [Google Scholar] [CrossRef]
- Van der Gucht, J.; Spruijt, E.; Lemmers, M.; Cohen Stuart, M.A. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Imre, Á.W.; Schönhoff, M.; Cramer, C. A conductivity study and calorimetric analysis of dried poly(sodium 4-styrene sulfonate)/poly(diallyldimethylammonium chloride) polyelectrolyte complexes. J. Chem. Phys. 2008, 128, 134905-1–134905-9. [Google Scholar] [CrossRef] [PubMed]
- Imre, Á.W.; Schönhoff, M.; Cramer, C. Unconventional Scaling of Electrical Conductivity Spectra for PSS-PDADMAC Polyelectrolyte Complexes. Phys. Rev. Lett. 2009, 102, 255901-1–255901-4. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Cramer, C.; Schönhoff, M. Humidity Dependence of the Ionic Conductivity of Polyelectrolyte Complexes. Macromolecules 2011, 44, 8936–8943. [Google Scholar] [CrossRef]
- Cramer, C.; De, S.; Schönhoff, M. Time-humidity-superposition principle in electrical conductivity spectra of ion-conducting polymers. Phys. Rev. Lett. 2011, 107, 028301-1–028301-4. [Google Scholar] [CrossRef] [PubMed]
- Funke, K.; Cramer, C. Conductivity Spectroscopy. Curr. Opin. Solid State Mater. Sci. 1997, 2, 483–490. [Google Scholar] [CrossRef]
- Young, J.F. Humidity control in the laboratory using salt solutions—A review. J. Appl. Chem. 1967, 17, 241–245. [Google Scholar] [CrossRef]
- Bhide, A.; Schönhoff, M.; Cramer, C. Cation conductivity in dried poly(4-styrene sulfonate) poly(diallyl-dimethylammonium chloride) based polyelectrolyte complexes. Solid State Ion. 2012, 214, 13–18. [Google Scholar] [CrossRef]
- Summerfield, S. Universal low-frequency behaviour in the a.c. hopping conductivity of disordered systems. Philos. Mag. B 1985, 52, 9–22. [Google Scholar] [CrossRef]
- Summerfield, S.; Butcher, P.N. Universal behaviour of AC hopping conductivity in disordered systems. J. Non-Cryst. Solids 1985, 77–78, 135–138. [Google Scholar] [CrossRef]
- Macovez, R.; Zachariah, M.; Romanini, M.; Zygouri, P.; Gournis, D.; Tamarit, J.L. Hopping Conductivity and Polarization Effects in a Fullerene Derivative Salt. J. Phys. Chem. C 2014, 118, 12170–12175. [Google Scholar] [CrossRef]
- Ioanid, A.; Dafinei, A.S. AC conductivity spectra of KCl crystals: Temperature dependent deviations from the Summerfield scaling. J. Optoelectron. Adv. Mater. 2004, 6, 465–469. [Google Scholar]
- Roling, B.; Happe, A.; Funke, K.; Ingram, M.D. Carrier Concentrations and Relaxation Spectroscopy: New Information from Scaling Properties of Conductivity Spectra in Ionically Conducting Glasses. Phys. Rev. Lett. 1997, 78, 2160–2163. [Google Scholar] [CrossRef]
- Sidebottom, D.L. Universal Approach for Scaling the a.c. Conductivity in Ionic Glasses. Phys. Rev. Lett. 1999, 82, 3653–3656. [Google Scholar] [CrossRef]
- Šantić, A.; Wrobel, W.; Mutke, M.; Banhatti, R.D.; Funke, K. Frequency-dependent fluidity and conductivity of an ionic liquid. Phys. Chem. Chem. Phys. 2009, 11, 5930–5934. [Google Scholar] [CrossRef] [PubMed]
- Schrøder, T.B.; Dyre, J.C. Scaling and universality of ac conduction in disordered solids. Phys. Rev. Lett. 2000, 84, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Dyre, J.C.; Maass, P.; Roling, B.; Sidebottom, D.L. Fundamental questions relating to ion conduction in disordered solids. Rep. Prog. Phys. 2009, 72, 046501/1–046501/15. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Baranovskii, S.D.; Cordes, H. On the conduction mechanism in ionic glasses. J. Chem. Phys. 1999, 111, 7546–7557. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De, S.; Ostendorf, A.; Schönhoff, M.; Cramer, C. Ion Conduction and Its Activation in Hydrated Solid Polyelectrolyte Complexes. Polymers 2017, 9, 550. https://doi.org/10.3390/polym9110550
De S, Ostendorf A, Schönhoff M, Cramer C. Ion Conduction and Its Activation in Hydrated Solid Polyelectrolyte Complexes. Polymers. 2017; 9(11):550. https://doi.org/10.3390/polym9110550
Chicago/Turabian StyleDe, Souvik, Annika Ostendorf, Monika Schönhoff, and Cornelia Cramer. 2017. "Ion Conduction and Its Activation in Hydrated Solid Polyelectrolyte Complexes" Polymers 9, no. 11: 550. https://doi.org/10.3390/polym9110550