Direct Synthesis of Branched Carboxylic Acid Functionalized Poly(1-octene) by α-Diimine Palladium Catalysts
Abstract
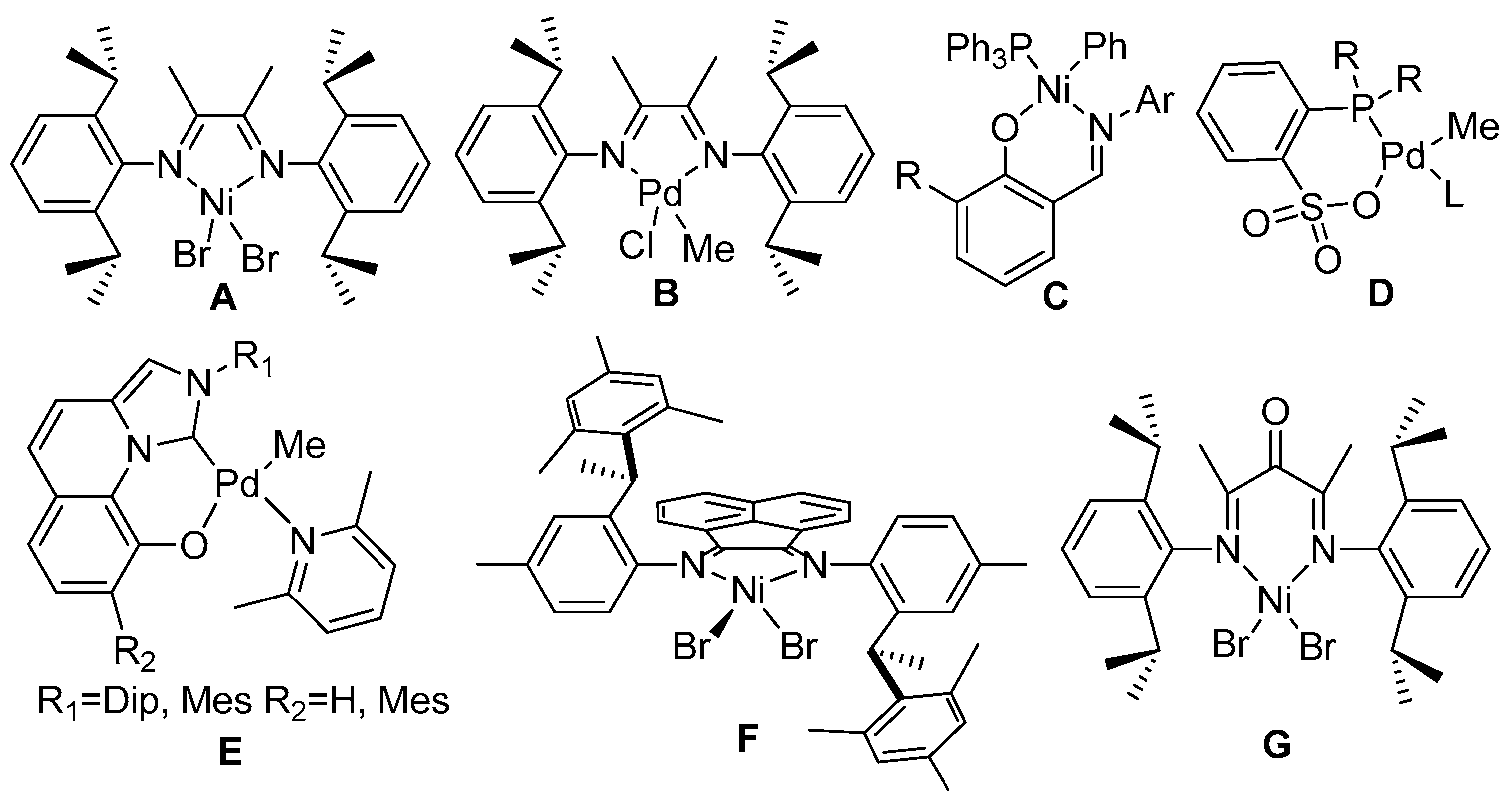
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. Polymerizations of Propylene
2.3. Copolymerization of 1-Octene and AA
3. Results and Discussion
3.1. Propylene Polymerization Studies
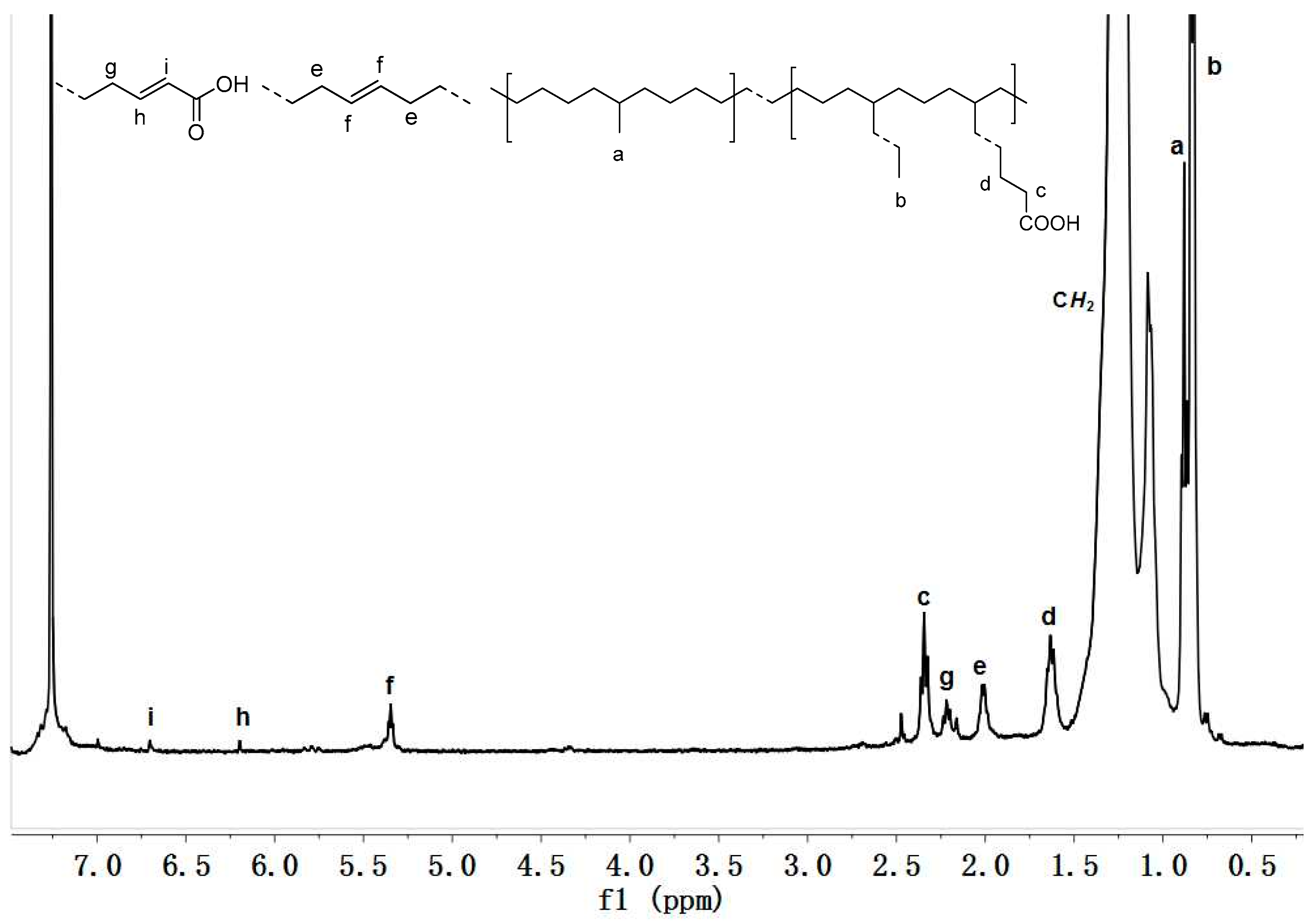
3.2. 1-Octene-Acrylic Acid (AA) Copolymerization Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd (II)- and Ni (II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Johnson, L.K.; Mecking, S.; Brookhart, M. Copolymerization of ethylene and propylene with functionalized vinyl monomers by palladium (II) catalysts. J. Am. Chem. Soc. 1996, 118, 267–268. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Popeney, C.S. Recent progress in late transition metal α-diimine catalysts for olefin polymerization. Top. Organomet. Chem. 2009, 26, 179–220. [Google Scholar]
- Dong, Z.; Ye, Z. Hyperbranched polyethylenes by chain walking polymerization: Synthesis, properties, functionalization, and applications. Polym. Chem. 2012, 3, 286–301. [Google Scholar] [CrossRef]
- Guo, L.H.; Dai, S.Y.; Sui, X.L.; Chen, C.L. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Guo, L.H.; Chen, C.L. (α-Diimine) palladium catalyzed ethylene polymerization and copolymerization with polar comonomers. Sci. China Chem. 2015, 58, 1663–1673. [Google Scholar] [CrossRef]
- Guan, Z.; Cotts, P.M.; McCord, E.F.; McLain, S.J. Chain walking: A new strategy to control polymer topology. Science 1999, 283, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Camacho, D.H.; Salo, E.V.; Ziller, J.W.; Guan, Z. Cyclophane-based highly active late-transition-metal catalysts for ethylene polymerization. Angew. Chem. Int. Ed. 2004, 43, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jordan, R.F. Palladium-catalyzed dimerization of vinyl ethers to acetals. J. Am. Chem. Soc. 2010, 132, 10254–10255. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, S.; Jordan, R.F. Cationic polymerization and insertion chemistry in the reactions of vinyl ethers with (α-diimine)PdMe+ species. J. Am. Chem. Soc. 2010, 132, 5273–5284. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Gao, H.; Guan, Q.; Hu, H.; Deng, J.; Liu, J.; Liu, F.; Wu, Q. Substituent effects of the backbone in α-diimine palladium catalysts on homo- and copolymerization of ethylene with methyl acrylate. Organometallics 2012, 31, 6054–6062. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A robust Ni(II) α-diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Dai, S.Y.; Chen, C.L. Investigations of the ligand electronic effects on α-diimine nickel(II) catalyzed ethylene polymerization. Polymers 2016, 8, 37. [Google Scholar] [CrossRef]
- Hu, H.; Gao, H.; Chen, D.; Li, G.; Tan, Y.; Liang, G.; Zhu, F.; Wu, Q. Ligand-directed regioselectivity in amine–imine nickel-catalyzed 1-hexene polymerization. ACS Catal. 2015, 5, 122–128. [Google Scholar] [CrossRef]
- Vaidya, T.; Klimovica, K.; LaPointe, A.M.; Keresztes, I.; Lobkovsky, E.B.; Daugulis, O.; Coates, G.W. Secondary alkene insertion and precision chain-walking: A new route to semicrystalline “polyethylene” from α-olefins by combining two rare catalytic events. J. Am. Chem. Soc. 2014, 136, 7213–7216. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Sui, X.L.; Pang, W.M.; Chen, C.L. Ethylene polymerization by xanthene-bridged dinuclear α-diimine NiII complexes. ChemCatChem 2016, 8, 434–440. [Google Scholar] [CrossRef]
- Wang, R.K.; Zhao, M.H.; Chen, C.L. Influence of ligand second coordination sphere effects on the olefin (co)polymerization properties of α-diimine Pd(II) catalysts. Polym. Chem. 2016, 7, 3933–3938. [Google Scholar] [CrossRef]
- Zou, W.P.; Chen, C.L. Influence of backbone substituents on the ethylene (co)polymerization properties of α-diimine Pd(II) and Ni(II) catalysts. Organometallics 2016, 35, 1794–1801. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, Z.S.; Pan, H.J.; Feng, W.; Chen, C.L.; Fan, Z.Q. Synthesis and application of binuclear α-diimine nickel/palladium catalysts with a conjugated backbone. Dalton Trans. 2014, 43, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Long, B.K.; Eagan, J.M.; Mulzer, M.; Coates, G.W. Semi-Crystalline polar polyethylene: Ester-functionalized linear polyolefins enabled by a functional-group-tolerant, cationic nickel catalyst. Angew. Chem. Int. Ed. 2016, 55, 7222–7226. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, W.J.; Daugulis, O.; Brookhart, M. Mechanistic studies of Pd(II)-catalyzed copolymerization of ethylene and vinylalkoxysilanes: Evidence for a β-silyl elimination chain transfer mechanism. J. Am. Chem. Soc. 2016, 138, 16120–16129. [Google Scholar] [CrossRef] [PubMed]
- Younkin, T.R.; Connor, E.F.; Henderson, J.I.; Friedrich, S.K.; Grubbs, R.H.; Bansleben, D.A. Neutral, Single-component nickel(II) polyolefin catalysts that tolerate heteroatoms. Science 2000, 287, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Weberski, M.P.; Chen, C.; Delferro, M.; Zuccaccia, C.; Macchioni, A.; Marks, T.J. Suppression of β-hydride chain transfer in nickel(II)-catalyzed ethylene polymerization via weak fluorocarbon ligand-product interactions. Organometallics 2012, 31, 3773–3789. [Google Scholar] [CrossRef]
- Weberski, M.P.; Chen, C.; Delferro, M.; Marks, T.J. Ligand steric and fluoroalkyl substituent effects on enchainment cooperativity and stability in bimetallic nickel(II) polymerization catalysts. Chem. Eur. J. 2012, 18, 10715–10732. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.J.; McInnis, J.P.; Chen, C.; Weberski, M.P.; Motta, A.; Delferro, M.; Marks, T.J. Ni(II) phenoxyiminato olefin polymerization catalysis: Striking coordinative modulation of hyperbranched polymer microstructure and stability by a proximate sulfonyl group. ACS Catal. 2014, 4, 999–1003. [Google Scholar] [CrossRef]
- Hu, X.H.; Dai, S.Y.; Chen, C.L. Ethylene polymerization by salicylaldimine nickel(II) complexes containing a dibenzhydryl moiety. Dalton Trans. 2016, 45, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.J.; Guo, L.H.; Hu, X.H.; Li, W.M. Ethylene polymerization by salicylaldimine nickel(II) complexes derived from arylnaphthylamine. J. Polym. Res. 2017, 24, 30. [Google Scholar] [CrossRef]
- Nakamura, A.; Anselment, T.M.J.; Claverie, J.; Goodall, B.; Jordan, R.F.; Mecking, S.; Rieger, B.; Sen, A.; Van Leeuwen, P.W.N.M.; Nozaki, K. Ortho-phosphinobenzenesulfonate: A superb ligand for palladium-catalyzed coordination–insertion copolymerization of polar vinyl monomers. Acc. Chem. Res. 2013, 46, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Ito, S.; Kuroda, J.; Okumura, Y.; Nozaki, K. Quantification of the Steric Influence of alkylphosphine-sulfonate ligands on polymerization, leading to high-molecular-weight copolymers of ethylene and polar monomers. J. Am. Chem. Soc. 2014, 136, 11898–11901. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.B.; Moritz, B.C.; Mecking, S. Suppression of chain transfer in catalytic acrylate polymerization via rapid and selective secondary insertion. J. Am. Chem. Soc. 2015, 137, 2836–2839. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, B.P.; Chen, C.L. Redox-controlled olefin (co)polymerization catalyzed by ferrocene-bridged phosphine-sulfonate palladium complexes. Angew. Chem. Int. Ed. 2015, 54, 15520–15744. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.L.; Dai, S.Y.; Chen, C.L. Ethylene polymerization and copolymerization with polar monomers by cationic phosphine phosphonic amide palladium complexes. ACS Catal. 2015, 5, 5932–5937. [Google Scholar] [CrossRef]
- Chen, M.; Zou, W.P.; Cai, Z.G.; Chen, C.L. Norbornene homopolymerization and copolymerization with ethylene by phosphine-sulfonate nickel catalysts. Polym. Chem. 2015, 6, 2669–2676. [Google Scholar] [CrossRef]
- Ota, Y.; Ito, S.; Kobayashi, M.; Kitade, S.; Sakata, K.; Tayano, T.; Nozaki, K. Crystalline isotactic polar polypropylene from the palladium-catalyzed copolymerization of propylene and polar monomers. Angew. Chem. Int. Ed. 2016, 55, 7505–7509. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Falivene, L.; Boffa, G.; Ortega Sánchez, S.; Caporaso, L.; Grassi, A.; Mecking, S. Direct synthesis of telechelic polyethylene by selective insertion polymerization. Angew. Chem. Int. Ed. 2016, 55, 14378–14595. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Jordan, R.F. Olefin Insertion into a Pd–F bond: Catalyst reactivation following β-F elimination in ethylene/vinyl fluoride copolymerization. Angew. Chem. Int. Ed. 2017, 56, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, C.L. Rational Design of High-performance phosphine sulfonate nickel catalysts for ethylene polymerization and copolymerization with polar monomers. ACS Catal. 2017, 7, 1308–1312. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Yu, H.; Zhao, Y.; Sun, R.; Jing, G.; Huang, J.; Khalid, H.; Abbasi, N.M.; Akram, M. Synthesis and application of polyethylene-based functionalized hyperbranched polymers. Prog. Polym. Sci. 2015, 45, 23–43. [Google Scholar] [CrossRef]
- Chen, G.; Ma, X.S.; Guan, Z. Synthesis of functional olefin copolymers with controllable topologies using a chain-walking catalyst. J. Am. Chem. Soc. 2003, 125, 6697–6704. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P. Surface Grafting onto polypropylene—A survey of recent developments. Prog. Polym. Sci. 1992, 17, 251–281. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Modification of polymers under heterogeneous conditions. Prog. Polym. Sci. 1992, 17, 361–415. [Google Scholar] [CrossRef]
- Bergbreiter, D.E. Polyethylene surface chemistry. Prog. Polym. Sci. 1994, 19, 529–560. [Google Scholar] [CrossRef]
- Nakano, R.; Nozaki, K. Copolymerization of propylene and polar monomers using Pd/IzQO catalysts. J. Am. Chem. Soc. 2015, 137, 10934–10937. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.E.; Rose, J.M.; Lobkovsky, E.B.; Coates, G.W. A C2-symmetric, living α-diimine Ni(II) catalyst: Regioblock copolymers from propylene. J. Am. Chem. Soc. 2005, 127, 13770–13771. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.M.; Cherian, A.E.; Coates, G.W. Living polymerization of α-olefins with an α-diimine Ni(II) catalyst: Formation of well-defined ethylene-propylene copolymers through controlled chain-walking. J. Am. Chem. Soc. 2006, 128, 4186–4187. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, J.D.; Schneider, Y.; Galland, G.B.; Bazan, G.C. Living polymerization of ethylene and α-olefins using a nickel α-keto-β-diimine initiator. Chem. Commun. 2009, 6177–6179. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, J.D.; Gao, H.; Koretz, Z.A.; Kehr, G.; Erker, G.; Shimizu, F.; Galland, G.B.; Bazan, G.C. Propylene Polymerization with α-Keto-β-Diimine Initiators Proceeds via Enantiomorphic Site Control. Macromolecules 2012, 45, 4487–4493. [Google Scholar] [CrossRef]
- Shelden, R.A.; Fueno, T.; Tsunetsugu, T.; Furukawa, J. A one-parameter model for isotactic polymerization based on enantiomorphic catalyst sites. J. Polym. Sci. Polym. Lett. 1965, 3, 23–26. [Google Scholar] [CrossRef]
- Bovey, F.A.; Tiers, G.V.D. Polymer NSR spectroscopy. II. The high resolution spectra of methyl methacrylate polymers prepared with free radical and anionic initiators. J. Polym. Sci. 1996, 34, 711–720. [Google Scholar] [CrossRef]
- Dai, S.Y.; Zhou, S.X.; Zhang, W.; Chen, C.L. Systematic investigations of ligand steric effects on α-diimine palladium catalyzed olefin polymerization and copolymerization. Macromolecules 2016, 49, 8855–8862. [Google Scholar] [CrossRef]
- Dai, S.Y.; Sui, X.L.; Chen, C.L. Highly robust palladium(II) α-diimine catalysts for slow-chain-walking polymerization of ethylene and copolymerization with methyl acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Chen, C.L. Direct synthesis of functionalized high-molecular-weight polyethylene by copolymerization of ethylene with polar monomers. Angew. Chem. Int. Ed. 2016, 55, 13281–13285. [Google Scholar] [CrossRef] [PubMed]
- McCord, E.F.; McLain, S.J.; Nelson, L.T.J.; Arthur, S.D.; Coughlin, E.B.; Ittel, S.D.; Johnson, L.K.; Tempel, D.; Killian, C.M.; Brookhart, M. 13C and 2D NMR analysis of propylene polymers made with α-diimine late metal catalysts. Macromolecules 2001, 34, 362–371. [Google Scholar] [CrossRef]
- McCord, E.F.; McLain, S.J.; Nelson, L.T.J.; Ittel, S.D.; Tempel, D.; Killian, C.M.; Johnson, L.K.; Brookhart, M. 13C-NMR analysis of α-olefin enchainment in poly(α-olefins) produced with nickel and palladium α-diimine catalysts. Macromolecules 2007, 40, 410–420. [Google Scholar] [CrossRef]
- Rose, J.M.; Deplace, F.; Lynd, N.A.; Wang, Z.; Hotta, A.; Lobkovsky, E.B.; Kramer, E.J.; Coates, G.W. C2-symmetric Ni(II) α-diimines featuring cumyl-derived ligands: Synthesis of improved elastomeric regioblock polypropylenes. Macromolecules 2008, 41, 9548–9555. [Google Scholar] [CrossRef]
- Runzi, T.; Frohlich, D.; Mecking, S. Direct synthesis of ethylene-acrylic acid copolymers by insertion polymerization. J. Am. Chem. Soc. 2010, 132, 17690–17691. [Google Scholar] [CrossRef] [PubMed]
- Daigle, J.C.; Piche, L.; Jerome, P. Claverie preparation of functional polyethylenes by catalytic copolymerization. Macromolecules 2011, 44, 1760–1762. [Google Scholar] [CrossRef]
- Contrella, N.D.; Sampson, J.R.; Jordan, R.F. Copolymerization of ethylene and methyl acrylate by cationic palladium catalysts that contain phosphine-diethyl phosphonate ancillary ligands. Organometallics 2014, 33, 3546–3555. [Google Scholar] [CrossRef]
- Mecking, S.; Johnson, L.K.; Wang, L.; Brookhart, M. Mechanistic Studies of the palladium-catalyzed copolymerization of ethylene and α-olefins with methyl acrylate. J. Am. Chem. Soc. 1998, 120, 888–899. [Google Scholar] [CrossRef]
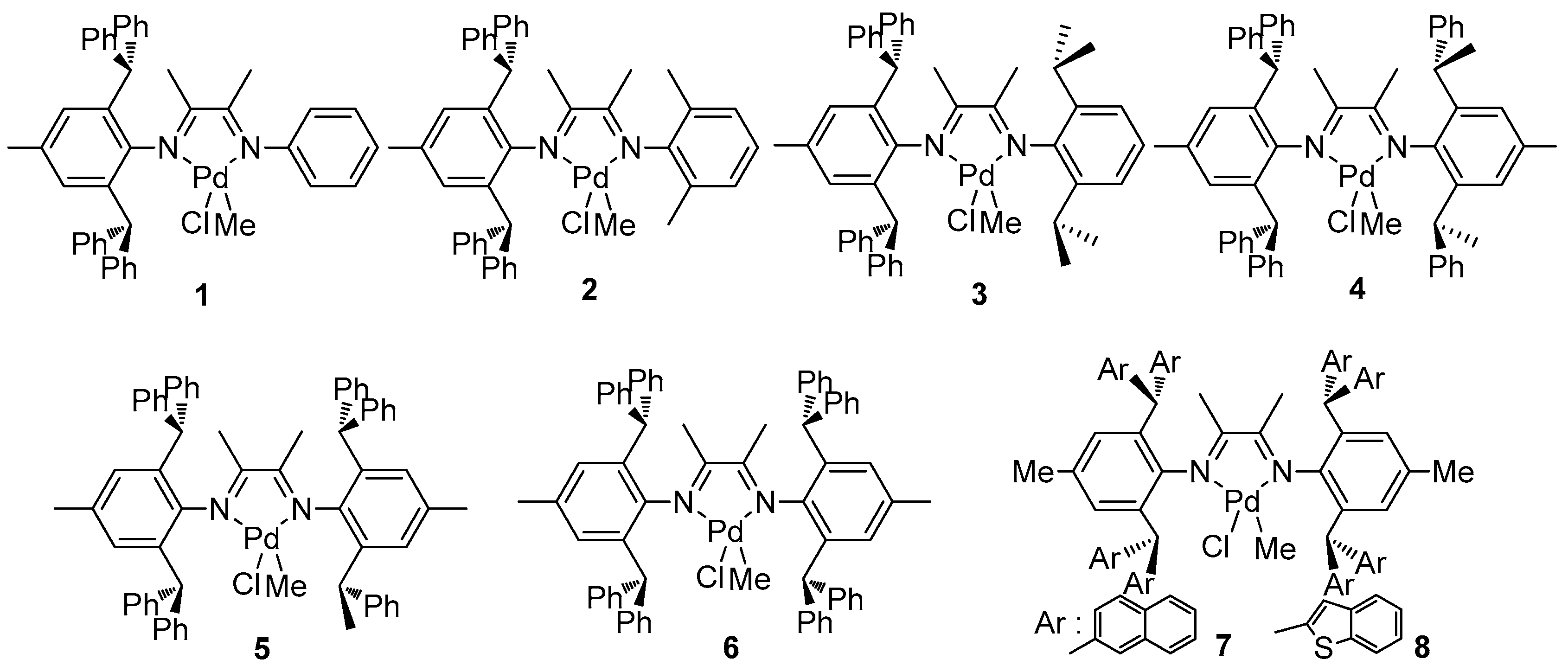
| Entry | Cat. | Yield (g) | TOF b (h−1) | Mn c (×10−3) | PDI c | B d | 3,1-Ins. e (mol %) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.82 | 650 | 0.7 | 1.43 | - | - |
| 2 | 2 | 1.49 | 1180 | 56.6 | 1.67 | 279 | 16.3 |
| 3 | 3 | 1.78 | 1410 | 86.0 | 1.59 | 235 | 29.5 |
| 4 | 4 | 0.53 | 420 | 59.3 | 1.74 | 174 | 47.8 |
| 5 | 5 | 0.41 | 325 | 51.6 | 1.99 | 159 | 52.3 |
| 6 | 6 | 0.36 | 285 | 27.7 | 1.50 | 153 | 54.2 |
| 7 | 7 | 0.22 | 174 | 30.5 | 1.46 | 169 | 49.3 |
| 8 | 8 | 0.12 | 95 | 4.3 | 1.37 | 201 | 39.7 |
| Entry | Cat. | [Oct] (M) | [AA] (M) | Yield (mg) | Act. b | XAA c (%) | Mn d (×10−3) | PDI d | B e | Tm f |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 1 | 201 | 6.7 | 15.7 | 0.5 | 3.78 | 93 | - |
| 2 | 2 | 2 | 1 | 141 | 4.7 | 3.59 | 2.3 | 2.34 | 85 | - |
| 3 | 3 | 2 | 1 | 253 | 8.4 | 2.77 | 40.4 | 2.03 | 98 | 14.1 |
| 4 | 4 | 2 | 1 | 231 | 7.7 | 1.95 | 35.7 | 1.37 | 66 | 35.7 |
| 5 | 5 | 2 | 1 | 165 | 5.5 | 1.28 | 28.2 | 1.41 | 58 | 56.0 |
| 6 | 6 | 2 | 1 | 203 | 6.8 | 1.07 | 34.8 | 1.52 | 60 | 72.0 |
| 7 | 7 | 2 | 1 | 48 | 1.6 | 1.82 | 26.3 | 1.30 | 79 | 25.2 |
| 8 | 8 | 2 | 1 | 102 | 3.4 | 1.26 | 8.6 | 1.60 | 84 | - |
| 9 | 2 | 2 | 2 | 50 | 1.7 | 7.24 | 1.1 | 3.23 | 95 | - |
| 10 | 3 | 2 | 2 | 156 | 5.2 | 5.89 | 8.5 | 1.59 | 80 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Zou, C.; Dai, S.; Chen, C. Direct Synthesis of Branched Carboxylic Acid Functionalized Poly(1-octene) by α-Diimine Palladium Catalysts. Polymers 2017, 9, 122. https://doi.org/10.3390/polym9040122
Guo L, Zou C, Dai S, Chen C. Direct Synthesis of Branched Carboxylic Acid Functionalized Poly(1-octene) by α-Diimine Palladium Catalysts. Polymers. 2017; 9(4):122. https://doi.org/10.3390/polym9040122
Chicago/Turabian StyleGuo, Lihua, Chen Zou, Shengyu Dai, and Changle Chen. 2017. "Direct Synthesis of Branched Carboxylic Acid Functionalized Poly(1-octene) by α-Diimine Palladium Catalysts" Polymers 9, no. 4: 122. https://doi.org/10.3390/polym9040122
APA StyleGuo, L., Zou, C., Dai, S., & Chen, C. (2017). Direct Synthesis of Branched Carboxylic Acid Functionalized Poly(1-octene) by α-Diimine Palladium Catalysts. Polymers, 9(4), 122. https://doi.org/10.3390/polym9040122








