Synthesis and Study of Shape-Memory Polymers Selectively Induced by Near-Infrared Lights via In Situ Copolymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
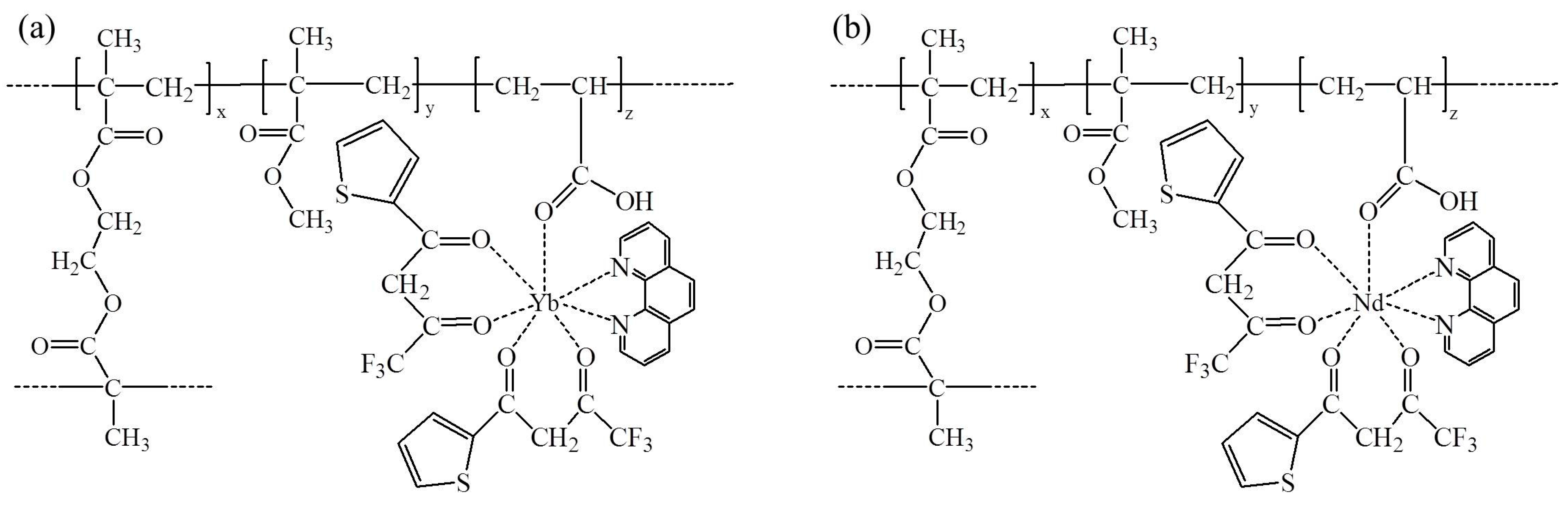
2.2. Synthesis of Yb/Nd(TTA)2AAPhen Complexes
2.3. In Situ Copolymerization of PMMA-Based Copolymers
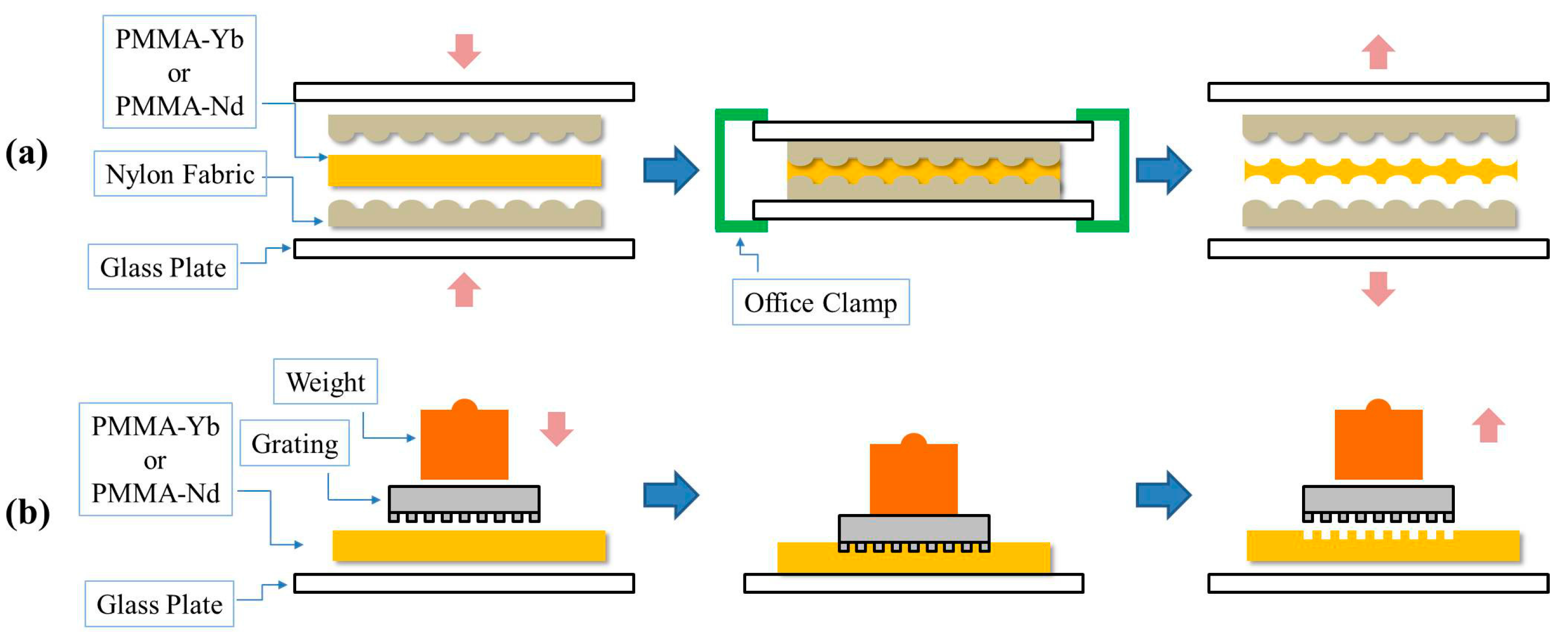
2.4. Creation of Temporary Microstructures
2.5. Characterizations
3. Results and Discussion
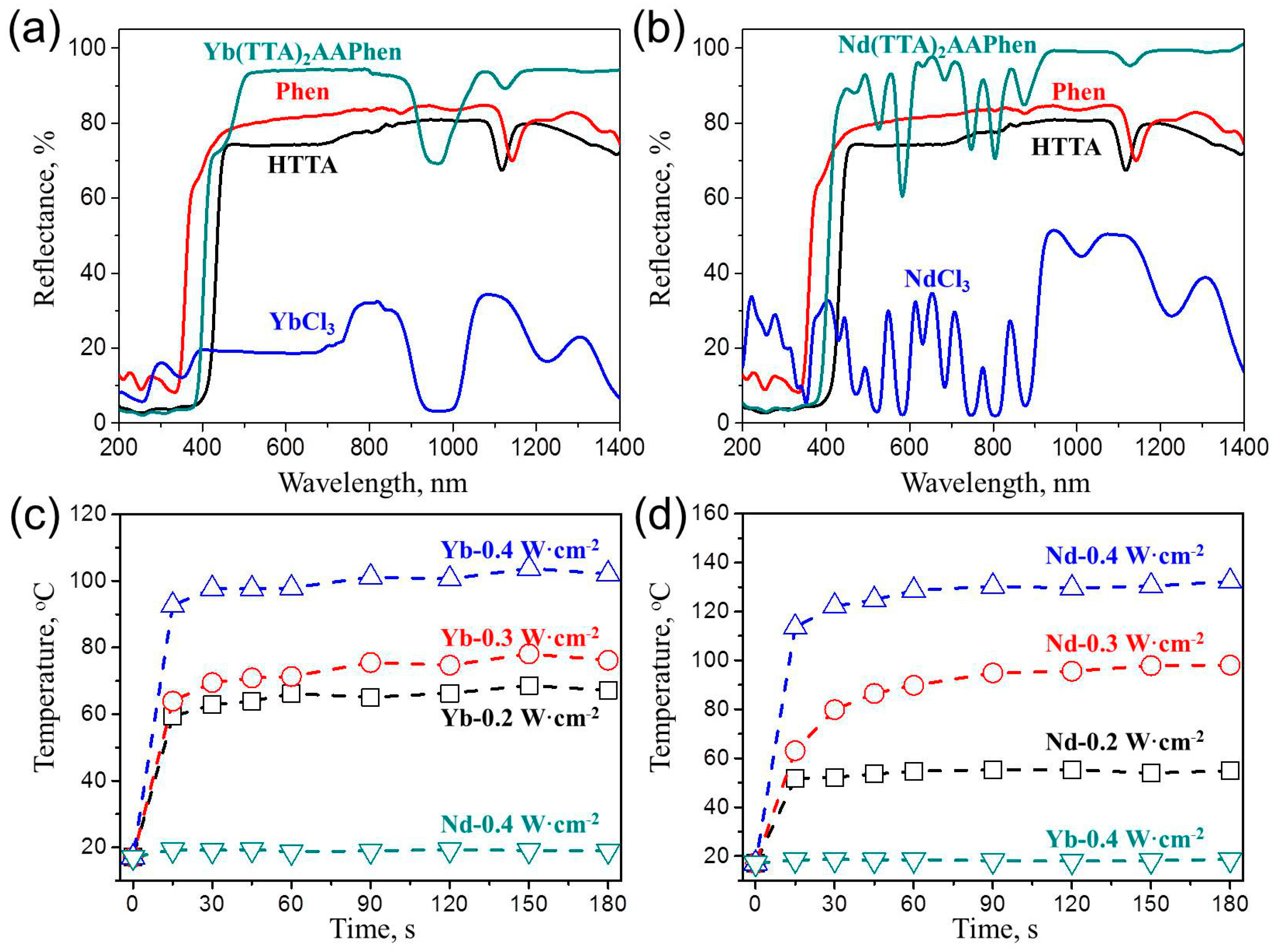
3.1. Structure and Properties of Yb(TTA)2AAPhen and Nd(TTA)2AAPhen
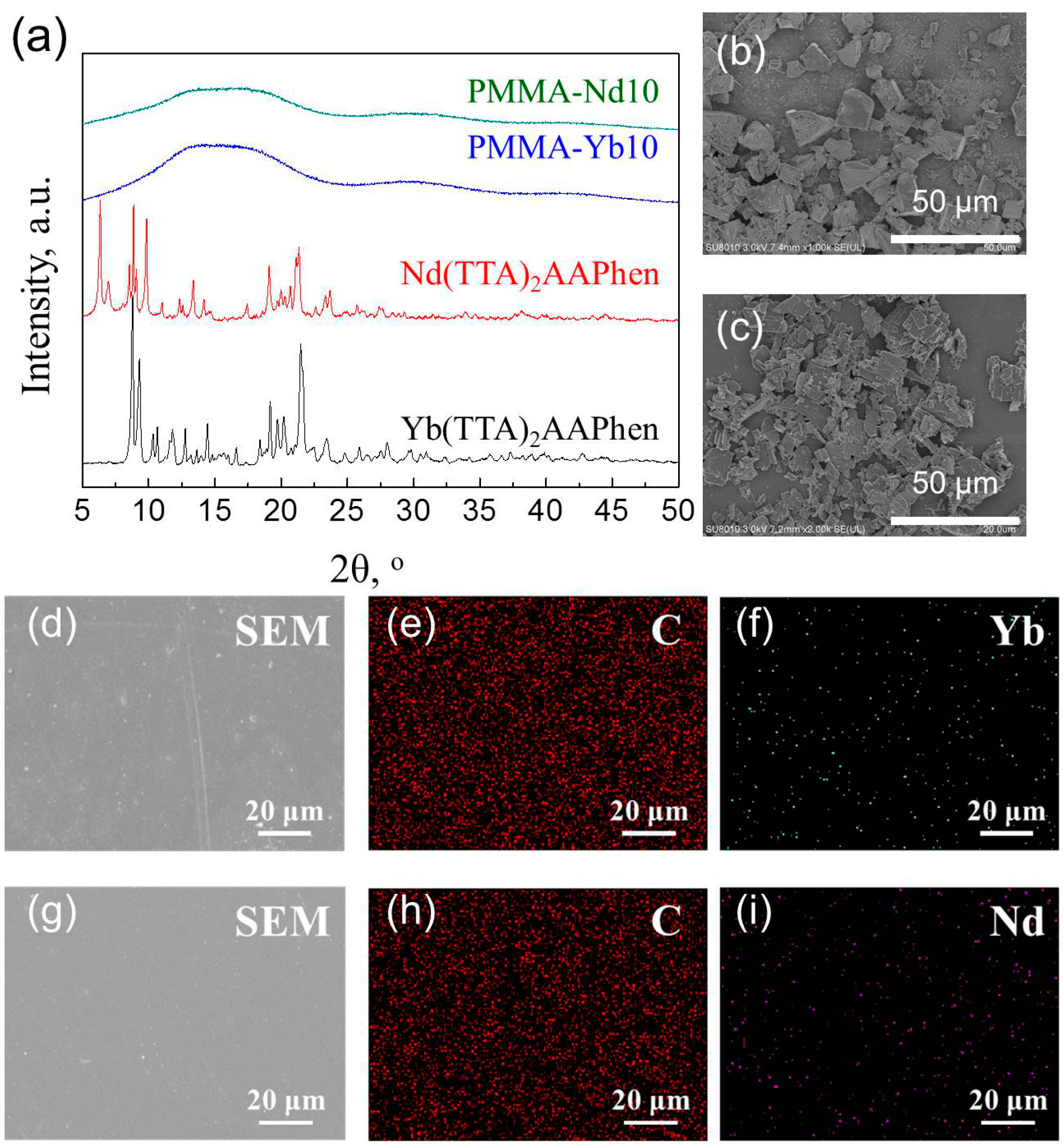
3.2. Structure and Properties of Light-Induced SMPs
3.3. Applications in Smart Optical Devices
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, H.; Kelch, S.; Lendlein, A. Polymers Move in Response to Light. Adv. Mater. 2006, 18, 1471–1475. [Google Scholar] [CrossRef]
- Razzaq, M.Y.; Behl, M.; Nöchel, U.; Lendlein, A. Magnetically controlled shape-memory effects of hybrid nanocomposites from oligo(ω-pentadecalactone) and covalently integrated magnetite nanoparticles. Polymer 2014, 55, 5953–5960. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-memory polymers and their composites: Stimulus methods and applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Luo, X.; Mather, P.T. Conductive shape memory nanocomposites for high speed electrical actuation. Soft Matter 2010, 6, 2146. [Google Scholar] [CrossRef]
- Huang, C.-L.; He, M.-J.; Huo, M.; Du, L.; Zhan, C.; Fan, C.-J.; Yang, K.K.; Chin, I.-J.; Wang, Y.-Z. A facile method to produce PBS-PEG/CNTs nanocomposites with controllable electro-induced shape memory effect. Polym. Chem. 2013, 4, 3987–3997. [Google Scholar] [CrossRef]
- Leng, J.; Wu, X.; Liu, Y. Infrared light-active shape memory polymer filled with nanocarbon particles. J. Appl. Polym Sci. 2009, 114, 2455–2460. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Liu, H.; Liu, H. UV light induced plasticization and light activated shape memory of spiropyran doped ethylene-vinyl acetate copolymers. Soft Matter 2014, 10, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y. Polymers with Dual Light-Triggered Functions of Shape Memory and Healing Using Gold Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 13069–13075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, H.; Zhao, Y. Light-Controlled Complex Deformation and Motion of Shape-Memory Polymers Using a Temperature Gradient. ACS Macro Lett. 2014, 3, 940–943. [Google Scholar] [CrossRef]
- Fang, L.; Chen, S.; Fang, T.; Fang, J.; Lu, C.; Xu, Z. Shape-memory polymer composites selectively triggered by near-infrared light of two certain wavelengths and their applications at macro-/microscale. Compos. Sci. Technol. 2017, 138, 106–116. [Google Scholar] [CrossRef]
- Yamada, M.; Kondo, M.; Mamiya, J.-I.; Yu, Y.; Kinoshita, M.; Barrett, C.J.; Ikeda, T. Photomobile Polymer Materials: Towards Light-Driven Plastic Motors. Angew. Chem. Int. Ed. 2008, 47, 4986–4988. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kondo, M.; Miyasato, R.; Naka, Y.; Mamiya, J.-I.; Kinoshita, M.; Shishido, A.; Yu, Y.; Barrett, C.J.; Ikeda, T. Photomobile polymer materials—Various three-dimensional movements. J. Mater. Chem. 2009, 19, 60–62. [Google Scholar] [CrossRef]
- Yu, Y.; Nakano, M.; Ikeda, T. Photomechanics: Directed bending of a polymer film by light. Nature 2003, 425, 145. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yin, R.; Zhang, Y.; Yen, C.-C.; Yu, Y. Fully plastic microrobots which manipulate objects using only visible light. Soft Matter 2010, 6, 3447. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Cheng, Y.; Cui, X.; Lian, H.; Liang, Y.; Chen, F.; Wang, H.; Guo, W.; Li, H.; et al. A Bioinspired Swimming and Walking Hydrogel Driven by Light-Controlled Local Density. Adv. Sci. 2015, 2, 1500084. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-A.; Liu, Y.; Wei, J.; Chen, E.; Qin, L.; Yu, Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Sershen, S.R.; Mensing, G.A.; Ng, M.; Halas, N.J.; Beebe, D.J.; West, J.L. Independent optical control of microfluidic valves formed from optomechanically responsive nanocomposite hydrogels. Adv. Mater. 2005, 17, 1366–1368. [Google Scholar] [CrossRef]
- White, T.J.; Tabiryan, N.V.; Serak, S.V.; Hrozhyk, U.A.; Tondiglia, V.P.; Koerner, H.; Vaia, R.A.; Bunning, T.J. A high frequency photodriven polymer oscillator. Soft Matter 2008, 4, 1796. [Google Scholar] [CrossRef]
- Kumar, K.; Knie, C.; Bléger, D.; Peletier, M.A.; Friedrich, H.; Hecht, S.; Broer, D.J.; Debije, M.G.; Schenning, A.P.H.J. A chaotic self-oscillating sunlight-driven polymer actuator. Nat. Commun. 2016, 7, 11975. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.S.; Burdick, J.A. Light-responsive biomaterials: Development and applications. Macromol. Biosci. 2010, 10, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, X.; Li, J.; Hao, X.; Lin, J.; Cheng, D.; Lu, Y. Thermosensitive Behavior and Antibacterial Activity of Cotton Fabric Modified with a Chitosan-poly(N-isopropylacrylamide) Interpenetrating Polymer Network Hydrogel. Polymers 2016, 8, 110. [Google Scholar] [CrossRef]
- Zhu, C.-H.; Lu, Y.; Peng, J.; Chen, J.-F.; Yu, S.-H. Photothermally Sensitive Poly(N-isopropylacrylamide)/Graphene Oxide Nanocomposite Hydrogels as Remote Light-Controlled Liquid Microvalves. Adv. Funct. Mater. 2012, 22, 4017–4022. [Google Scholar] [CrossRef]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion-contraction of photoresponsive artificial muscle regulated by host-guest interactions. Nat. Commun. 2012, 3, 1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Desai, M.S.; Lee, S.W. Light-controlled graphene-elastin composite hydrogel actuators. Nano Lett. 2013, 13, 2826–2830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, Z.; Wang, C.; Zarrouk, D.; Seo, J.W.; Cheng, J.C.; Buchan, A.D.; Takei, K.; Zhao, Y.; Ager, J.W.; et al. Photoactuators and motors based on carbon nanotubes with selective chirality distributions. Nat. Commun. 2014, 5, 2983. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Gao, Z.; Jia, S.; Wang, F.; Wang, Y. Graphene-Based Polymer Bilayers with Superior Light-Driven Properties for Remote Construction of 3D Structures. Adv. Sci. 2017. [Google Scholar] [CrossRef]
- Ji, M.; Jiang, N.; Chang, J.; Sun, J. Near-Infrared Light-Driven, Highly Efficient Bilayer Actuators Based on Polydopamine-Modified Reduced Graphene Oxide. Adv. Funct. Mater. 2014, 24, 5412–5419. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A review of shape memory polymer composites and blends. Compos. A Appl. Sci. Manuf. 2009, 40, 1661–1672. [Google Scholar] [CrossRef]
- Mirtschin, N.; Pretsch, T. Programming of one- and two-step stress recovery in a Poly(ester urethane). Polymers 2017, 9, 98. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape-memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Lu, H.; Yao, Y.; Huang, W.M.; Leng, J.; Hui, D. Significantly improving infrared light-induced shape recovery behavior of shape memory polymeric nanocomposite via a synergistic effect of carbon nanotube and boron nitride. Compos. B Eng. 2014, 62, 256–261. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Q.; Lv, X.; Gao, L.; Fang, S.; Yu, H. Photoinduced triple shape memory polyurethane enabled by doping with azobenzene and GO. J. Mater. Chem. C 2016, 4, 9993–9997. [Google Scholar] [CrossRef]
- Leng, J.; Zhang, D.; Liu, Y.; Yu, K.; Lan, X. Study on the activation of styrene-based shape memory polymer by medium-infrared laser light. Appl. Phys. Lett. 2010, 96, 111905. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Q.; Sun, J.; Li, C.; Zou, C.; He, Z.; Wang, Z.; Zhang, L.; Yang, H. Multi-shape-memory effects in a wavelength-selective multicomposite. J. Mater. Chem. A 2015, 3, 13953–13961. [Google Scholar] [CrossRef]
- He, Z.; Satarkar, N.; Xie, T.; Cheng, Y.T.; Hilt, J.Z. Remote controlled multishape polymer nanocomposites with selective radiofrequency actuations. Adv. Mater. 2011, 23, 3192–3196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shaw, B.; Dickey, M.D.; Genzer, J. Sequential self-folding of polymer sheets. Sci. Adv. 2017, 3, e1602417. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, B.P.; Yang, H. A plant tendril mimic soft actuator with phototunable bending and chiral twisting motion modes. Nat. Commun. 2016, 7, 13981. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, T.; Li, X.; Zhang, Y.; Yu, H. NIR-Vis-UV Light-Responsive Actuator Films of Polymer-Dispersed Liquid Crystal/Graphene Oxide Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 27494–27501. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, C.; Wang, S.; Malyarchuk, V.; Xie, T.; Rogers, J.A. Deformable, Programmable, and Shape-Memorizing Micro-Optics. Adv. Funct. Mater. 2013, 23, 3299–3306. [Google Scholar] [CrossRef]
- Nam, S.; Song, M.; Kim, D.-H.; Cho, B.; Lee, H.-M.; Kwon, J.-D.; Park, S.-G.; Nam, K.-S.; Jeong, Y.; Kwon, S.-H.; et al. Ultrasmooth, extremely deformable and shape recoverable Ag nanowire embedded transparent electrode. Sci. Rep. 2014, 4, 4788. [Google Scholar] [CrossRef] [PubMed]
- Espinha, A.; Serrano, M.C.; Blanco, Á.; López, C. Thermoresponsive Shape-memory photonic nanostructures. Adv. Opt. Mater. 2014, 2, 516–521. [Google Scholar] [CrossRef]
- Liu, L.; He, L.; Yang, C.; Zhang, W.; Jin, R.-G.; Zhang, L.-Q. In situ Reaction and Radiation Protection Properties of Gd(AA)3/NR Composites. Macromol. Rapid Commun. 2004, 25, 1197–1202. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, X.; Hu, S.; Zhang, L.; Liu, L. Influence of in-situ reaction on luminescent properties of samarium-complex/hydrogenated acrylonitrile-butadiene composites. Polymer 2009, 50, 3269–3274. [Google Scholar] [CrossRef]
- Fan, D.; Fei, X.; Tian, J.; Zhi, H.; Xu, L.; Wang, X.; Wang, Y. Synthesis and investigation of a novel luminous hydrogel. Polym. Chem. 2016, 7, 3766–3772. [Google Scholar] [CrossRef]
- Polgar, L.M.; van Duin, M.; Broekhuis, A.A.; Picchioni, F. Use of diels-alder chemistry for thermoreversible cross-linking of rubbers: The next step toward recycling of rubber products? Macromolecules 2015, 48, 7096–7105. [Google Scholar] [CrossRef]
- Gómez, C.M.; Figueruelo, J.E.; Campos, A. Evaluation of thermodynamic parameters for blends of polyethersulfone and Poly(methyl methacrylate) of polystyrene in dimethylformamide. Polymer 1998, 39, 4023–4032. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, L.; Li, W.; Li, L. Preparation and fluorescent property of Eu (TTA) 3phen incorporated in polycarbonate resin. Polym. J. 2006, 38, 523. [Google Scholar] [CrossRef]
- Yao, L.; Wen, S.; Duan, X.; Hu, X.; Che, M.; Jing, W.; Liu, H.; Liu, L. Self-polymerization of Eu (TTA) 3 AA in rubber and their fluorescence effect. J. Rare Earth 2013, 31, 1130–1136. [Google Scholar] [CrossRef]
- Kratz, K.; Madbouly, S.A.; Wagermaier, W.; Lendlein, A. Temperature-memory polymer networks with crystallizable controlling units. Adv. Mater. 2011, 23, 4058–4062. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Wang, X. Hydrogel diffraction grating as sensor: A tool for studying volume phase transition of thermos-responsive hydrogel. Sens. Actuators B 2014, 204, 611–616. [Google Scholar] [CrossRef]

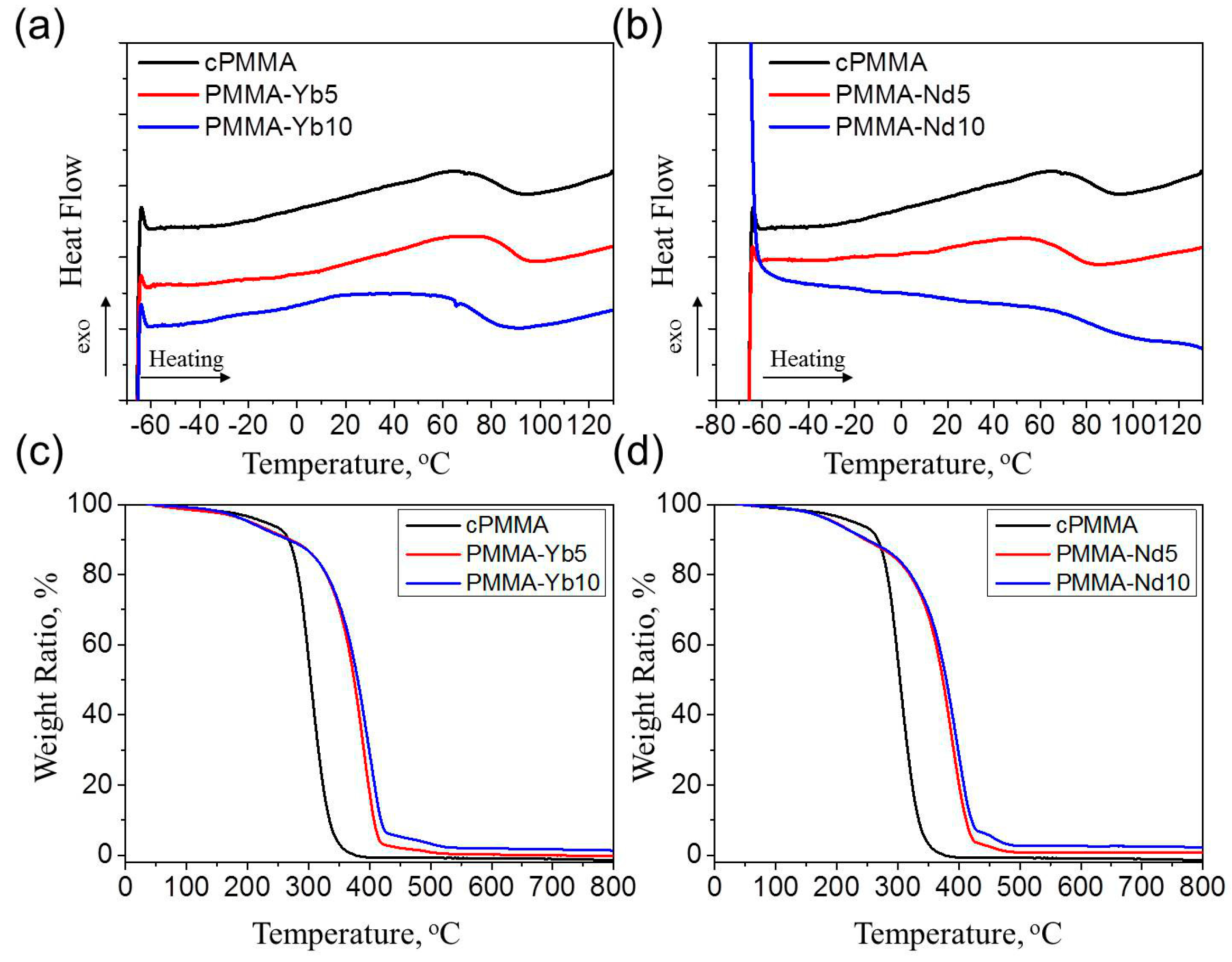
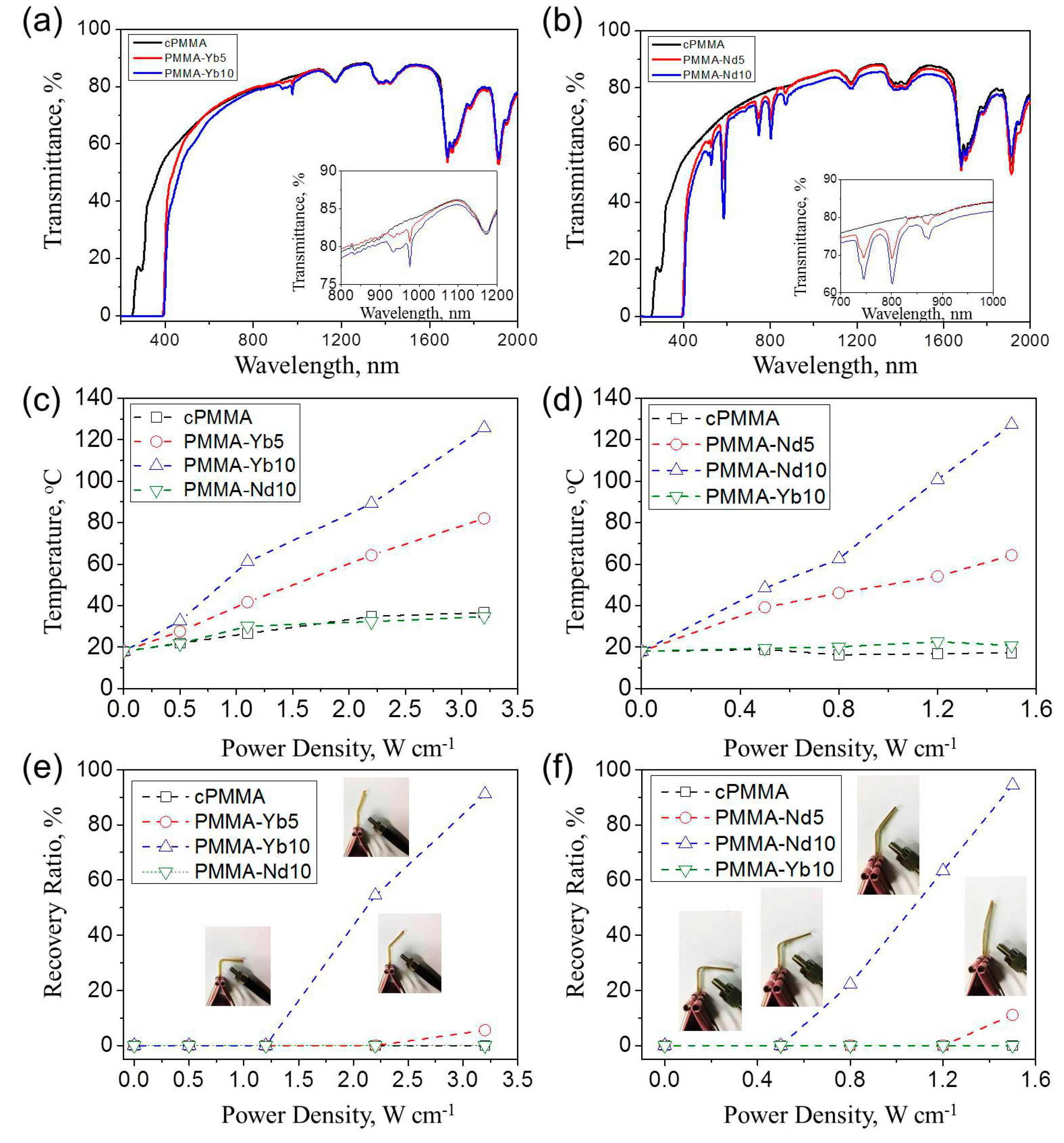
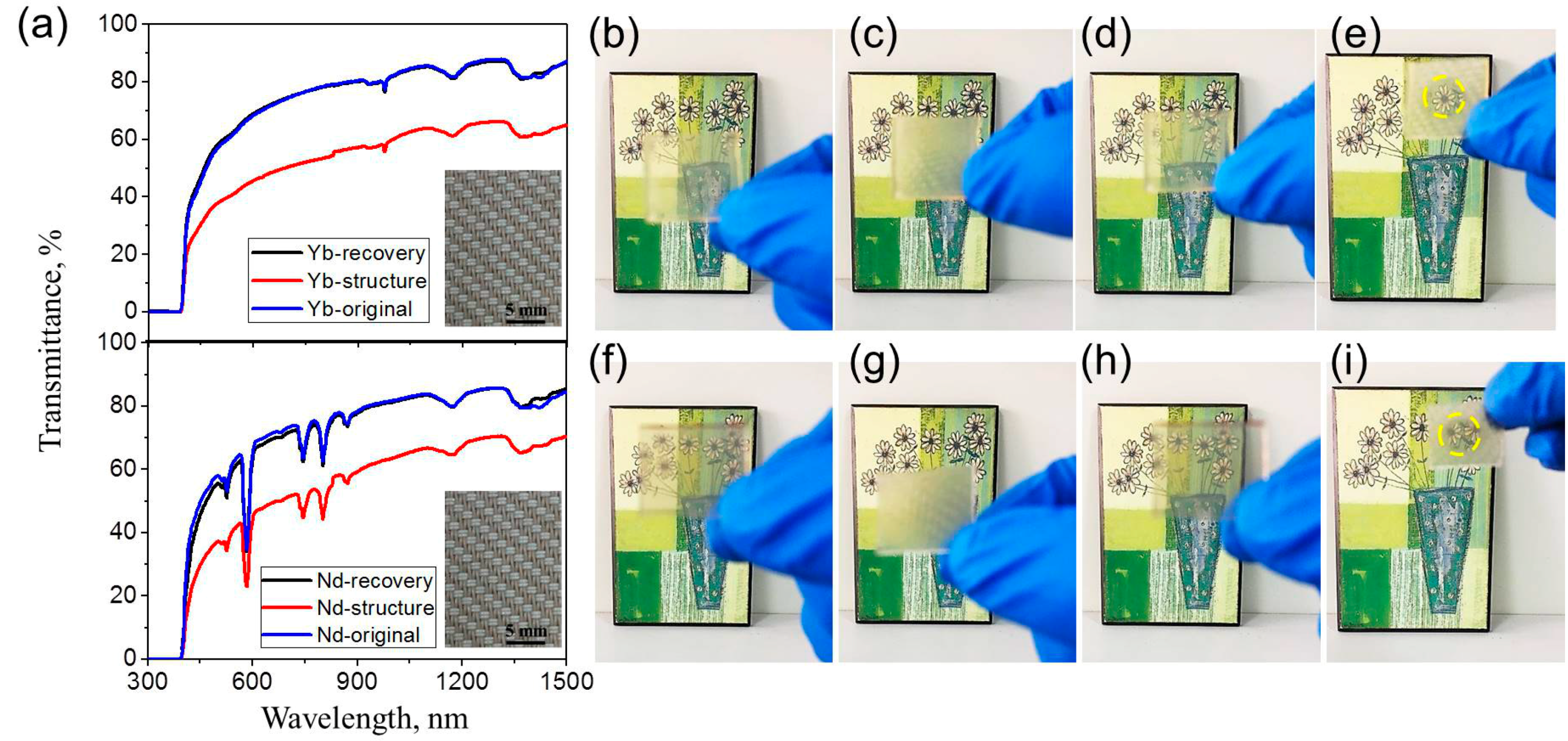
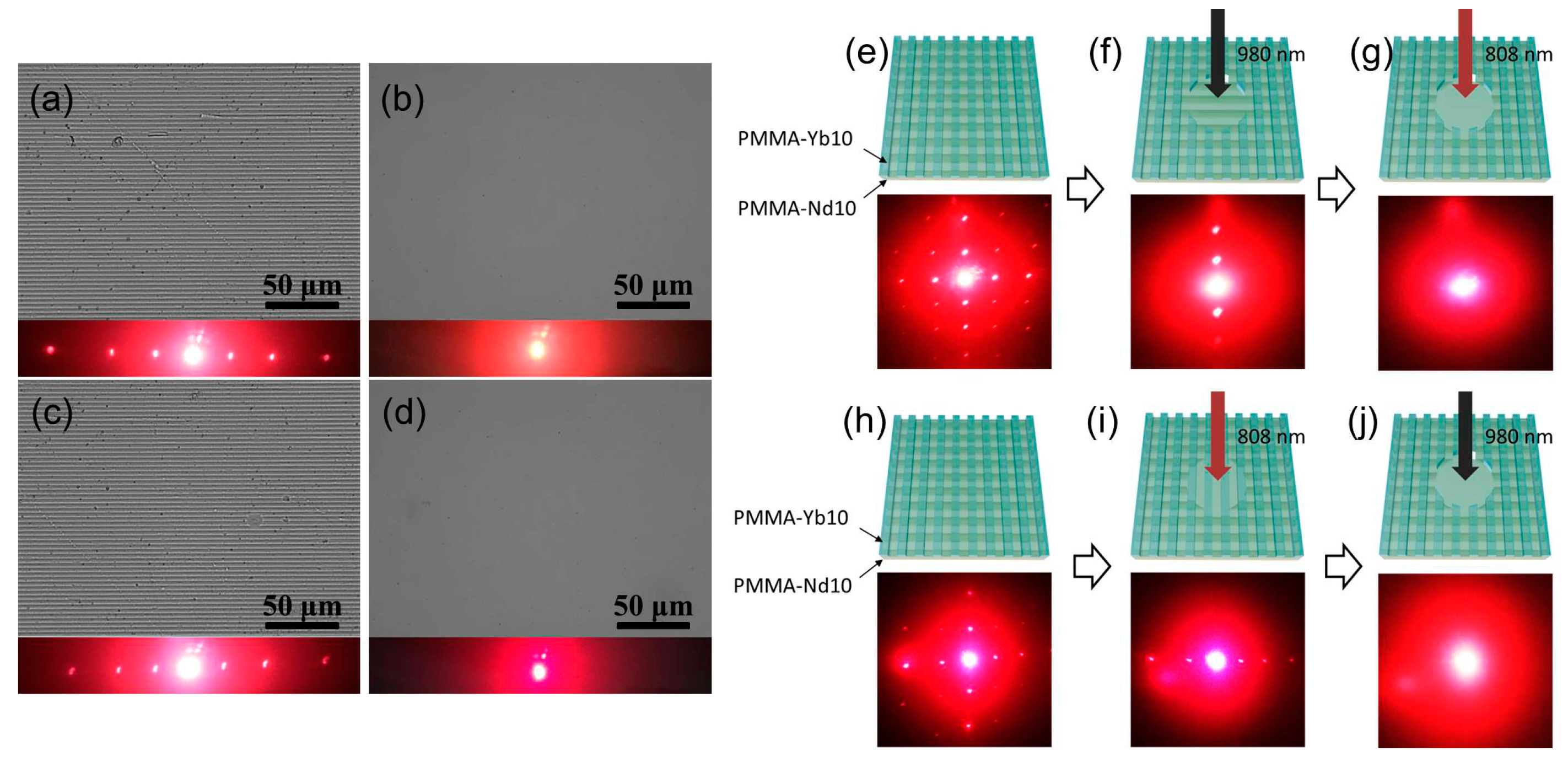
| Sample name | σ a | E b | Tg c | G d | Q e | [XLD]s f | Td,5 g | Td,50 h | Td,90 i |
|---|---|---|---|---|---|---|---|---|---|
| MPa | GPa | °C | % | % | mmol cm−3 | °C | °C | °C | |
| cPMMA | 45.5 ± 2.4 | 2.1 ± 0.1 | 86 | 99.3 ± 0.2% | 169 ± 3% | 0.62 ± 0.06 | 227 | 304 | 335 |
| PMMA-Yb5 | 41.4 ± 2.9 | 1.9 ± 0.2 | 87 | 94.7 ± 0.8% | 198 ± 13% | 0.26 ± 0.07 | 206 | 374 | 400 |
| PMMA-Yb10 | 33.5 ± 5.2 | 1.6 ± 0.2 | 78 | 92.5 ± 1.2% | 207 ± 2% | 0.19 ± 0.02 | 202 | 380 | 418 |
| PMMA-Nd5 | 35.3 ± 3.9 | 1.6 ± 0.2 | 73 | 94.9 ± 0.5% | 202 ± 5% | 0.23 ± 0.02 | 194 | 374 | 412 |
| PMMA-Nd10 | 29.4 ± 2.1 | 1.3 ± 0.2 | 82 | 93.1 ± 0.6% | 213 ± 23% | 0.19 ± 0.10 | 195 | 379 | 421 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, T.; Fang, L.; Chen, S.; Li, L.; Huang, H.; Lu, C.; Xu, Z. Synthesis and Study of Shape-Memory Polymers Selectively Induced by Near-Infrared Lights via In Situ Copolymerization. Polymers 2017, 9, 181. https://doi.org/10.3390/polym9050181
Fang T, Fang L, Chen S, Li L, Huang H, Lu C, Xu Z. Synthesis and Study of Shape-Memory Polymers Selectively Induced by Near-Infrared Lights via In Situ Copolymerization. Polymers. 2017; 9(5):181. https://doi.org/10.3390/polym9050181
Chicago/Turabian StyleFang, Tianyu, Liang Fang, Shunping Chen, Lingyu Li, Hengming Huang, Chunhua Lu, and Zhongzi Xu. 2017. "Synthesis and Study of Shape-Memory Polymers Selectively Induced by Near-Infrared Lights via In Situ Copolymerization" Polymers 9, no. 5: 181. https://doi.org/10.3390/polym9050181






