The Impact of Polymer Grafting from a Graphene Oxide Surface on Its Compatibility with a PDMS Matrix and the Light-Induced Actuation of the Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphene Oxide Preparation and Initiator Immobilization
2.3. Grafting Polymer Chains from the GO Surface
2.4. Composite Preparation
2.5. General Characterizations
2.6. Photoactuation
3. Results and Discussion
3.1. Synthesis of GO-Polymer-Modified Particles
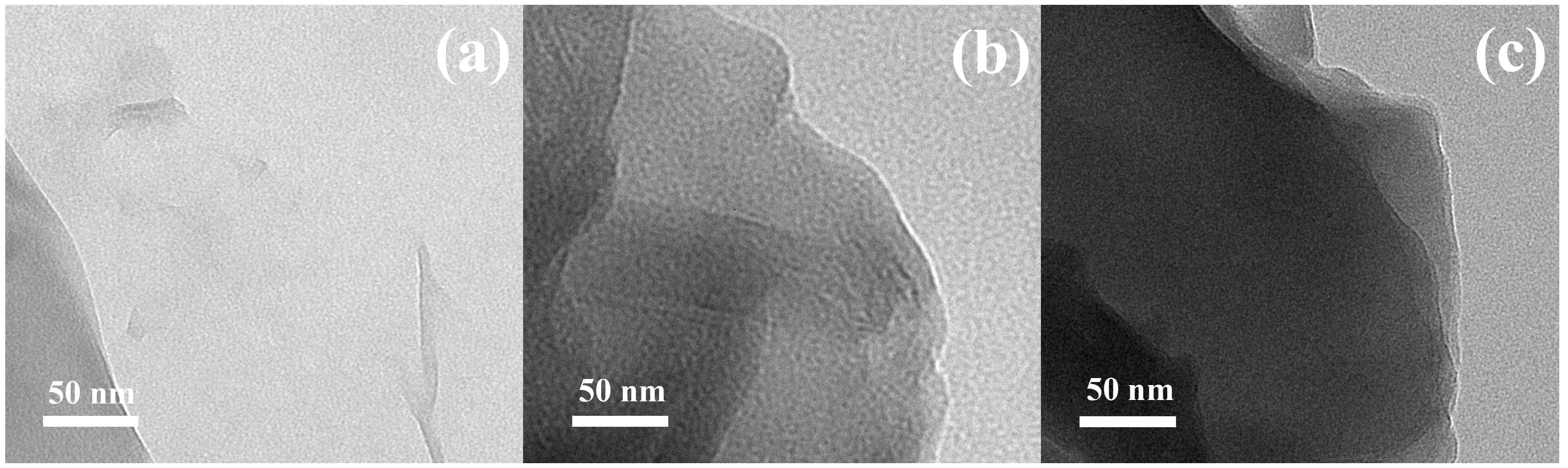
3.2. Transmission Electron Microscopy
3.3. Reduction of the GO Particles during the Synthesis
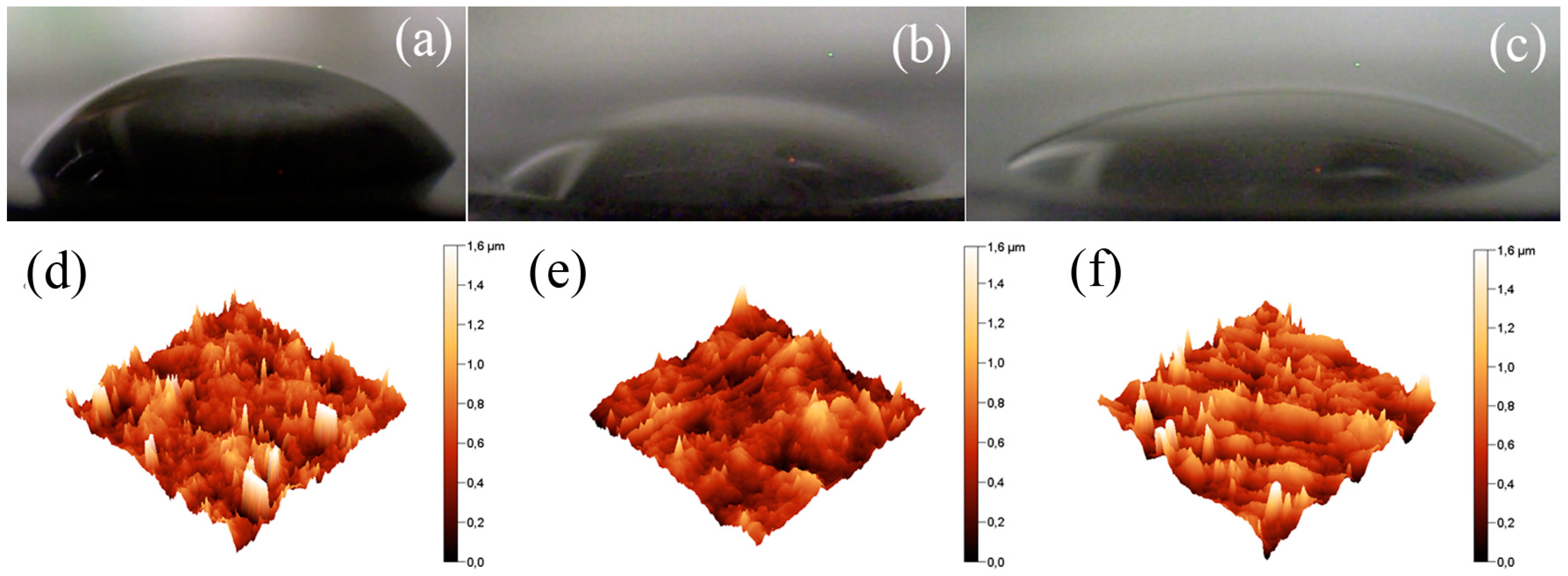
3.4. Compatibility of the Particles with the PDMS Matrix
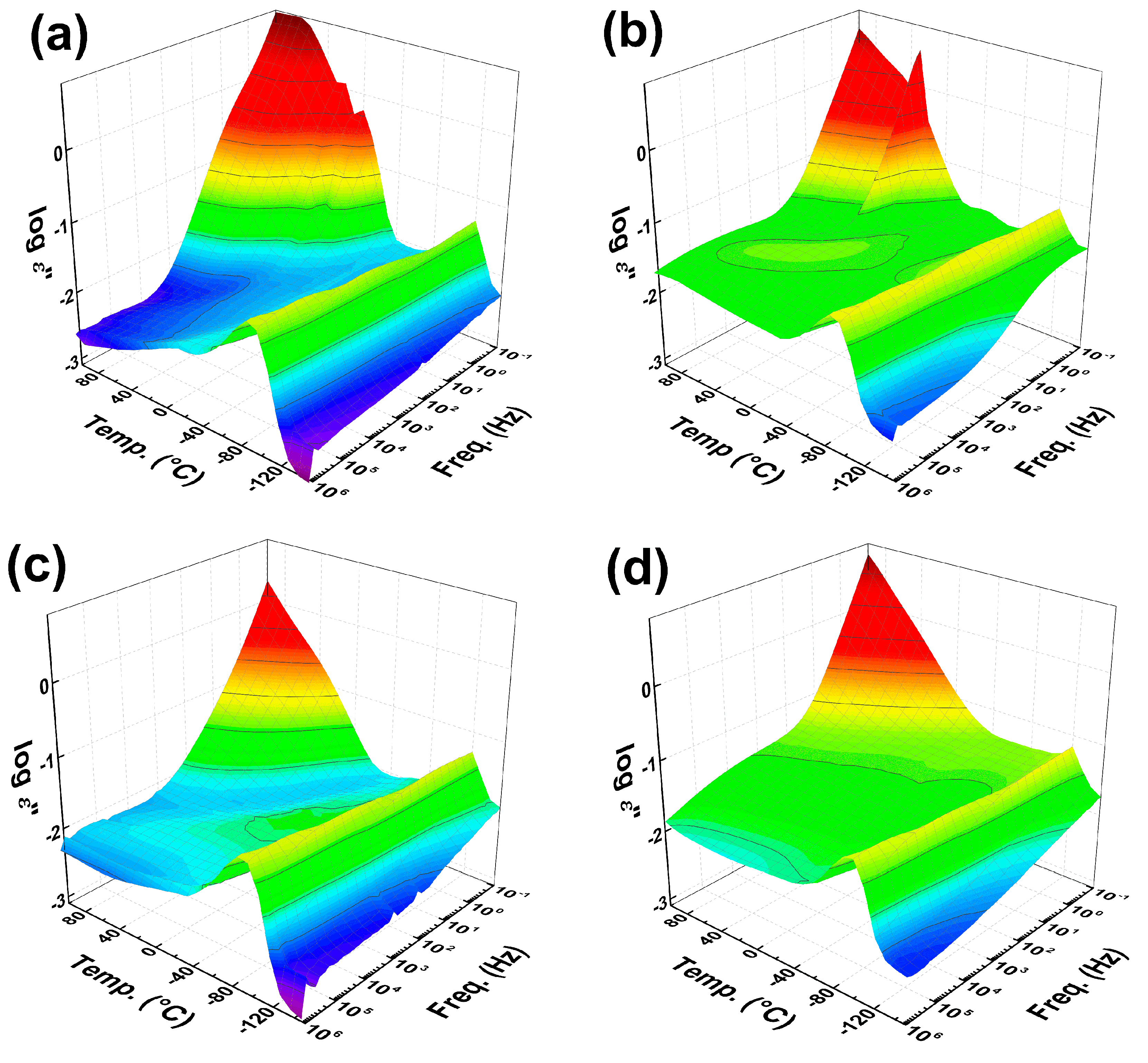
3.5. Dielectric Investigation of the Polymer Chain Dynamics
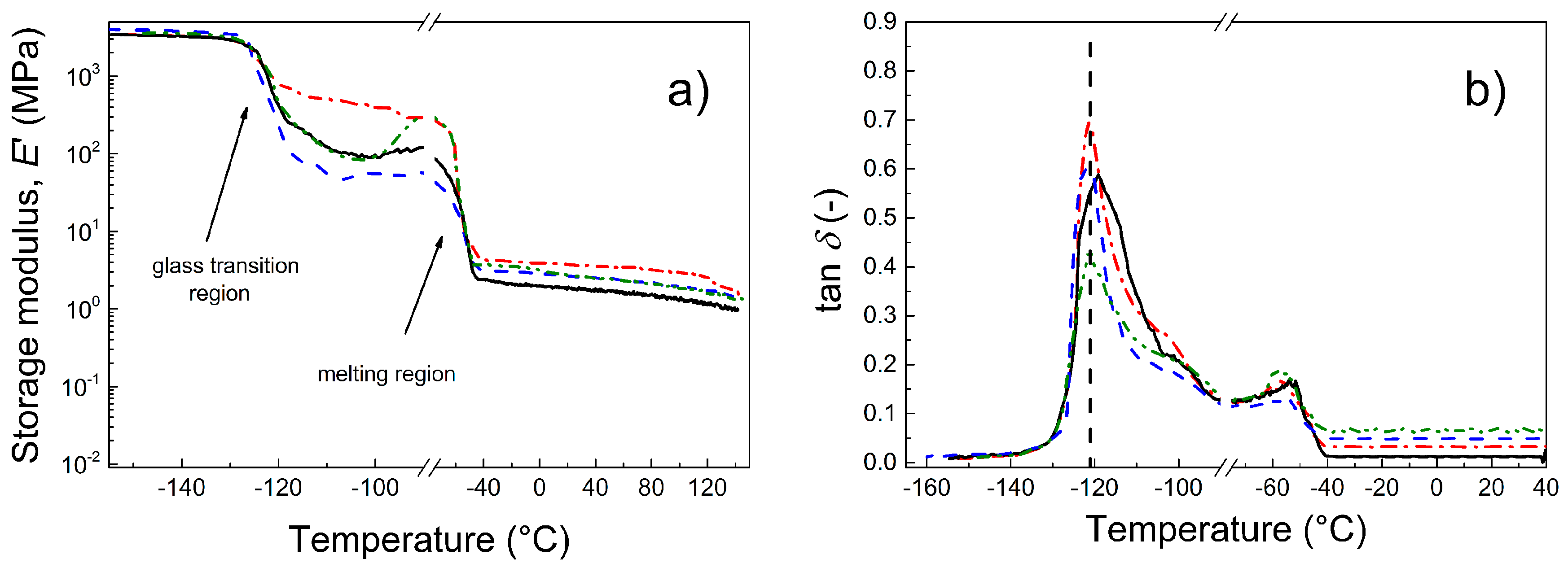
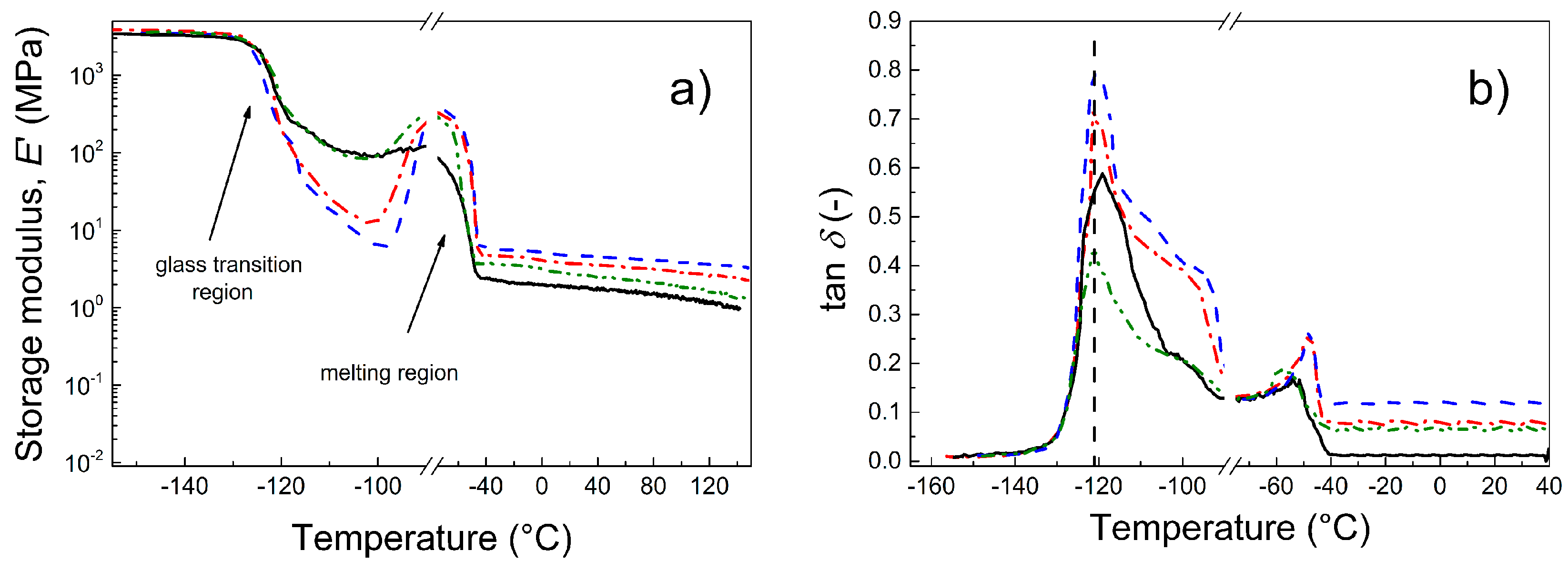
3.6. Dynamic Mechanical Analysis
3.7. Thermal Conductivity
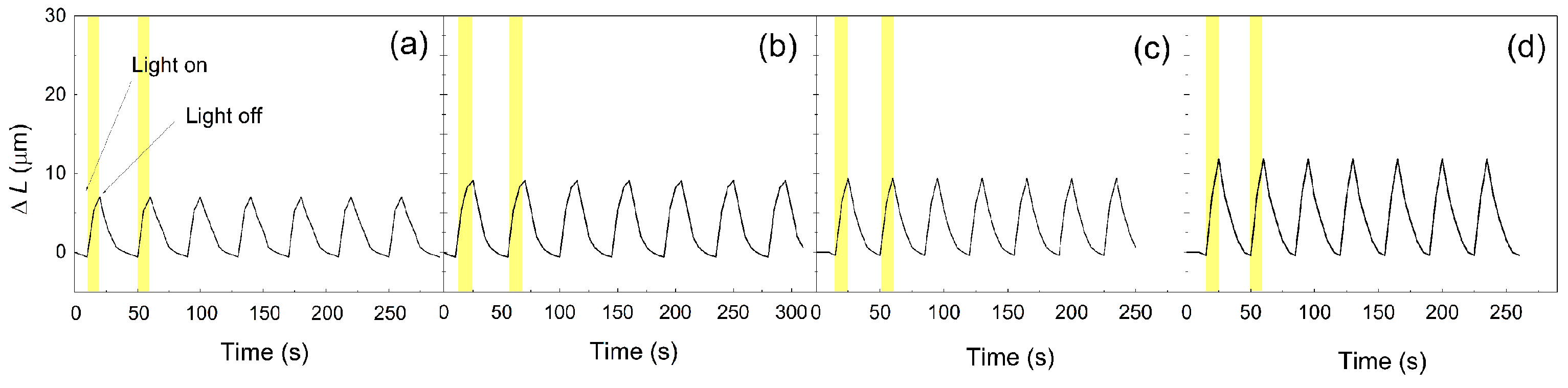
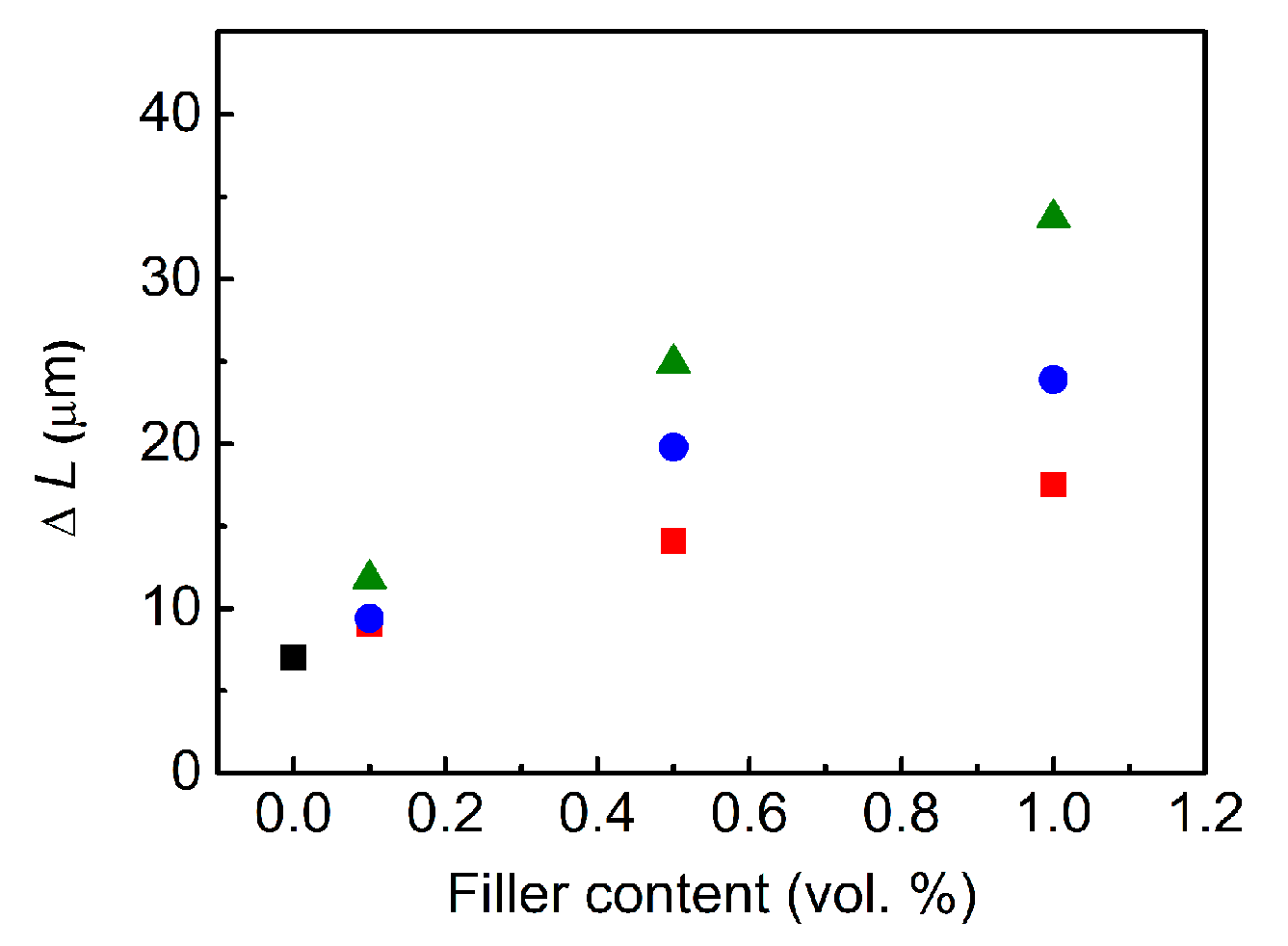
3.8. Photoactuation Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohn, R.; Panchapakesan, B. Spatially nonuniform heating and the nonlinear transient response of elastomeric photomechanical actuators. Actuators 2016, 5, 16. [Google Scholar] [CrossRef]
- Torras, N.; Zinoviev, K.E.; Camargo, C.J.; Campo, E.M.; Campanella, H.; Esteve, J.; Marshall, J.E.; Terentjev, E.M.; Omastova, M.; Krupa, I.; et al. Tactile device based on opto-mechanical actuation of liquid crystal elastomers. Sens. Actuators A 2014, 208, 104–112. [Google Scholar] [CrossRef]
- Camargo, C.J.; Campanella, H.; Marshall, J.E.; Torras, N.; Zinoviev, K.; Terentjev, E.M.; Esteve, J. Batch fabrication of optical actuators using nanotube–elastomer composites towards refreshable braille displays. J. Micromech. Microeng. 2012, 22, 9. [Google Scholar] [CrossRef]
- Marshall, J.E.; Gallagher, S.; Terentjev, E.M.; Smoukov, S.K. Anisotropic colloidal micromuscles from liquid crystal elastomers. J. Am. Chem. Soc. 2014, 136, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Baer, G.M.; Small, W.; Wilson, T.S.; Benett, W.J.; Matthews, D.L.; Hartman, J.; Maitland, D.J. Fabrication and in vitro deployment of a laser-activated shape memory polymer vascular stent. Biomed. Eng. Online 2007, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Maitland, D.J.; Metzger, M.F.; Schumann, D.; Lee, A.; Wilson, T.S. Photothermal properties of shape memory polymer micro-actuators for treating stroke. Lasers Surg. Med. 2002, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.X.; Liu, Y.; Shao, N.; Panchapakesan, B. Nanotube micro-opto-mechanical systems. Nanotechnology 2007, 18, 065501. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z.B.; Wang, C.; Zarrouk, D.; Seo, J.W.T.; Cheng, J.C.; Buchan, A.D.; Takei, K.; Zhao, Y.; Ager, J.W.; et al. Photoactuators and motors based on carbon nanotubes with selective chirality distributions. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.M.; King, B.C.; Loomis, J.; Campo, E.M.; Hegseth, J.; Cohn, R.W.; Terentjev, E.; Panchapakesan, B. Nanotube liquid crystal elastomers: Photomechanical response and flexible energy conversion of layered polymer composites. Nanotechnology 2014, 25, 355501. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.Q.; Yang, Y.; Chen, Q.M.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Czanikova, K.; Torras, N.; Esteve, J.; Krupa, I.; Kasak, P.; Pavlova, E.; Racko, D.; Chodak, I.; Omastova, M. Nanocomposite photoactuators based on an ethylene vinyl acetate copolymer filled with carbon nanotubes. Sens. Actuator B 2013, 186, 701–710. [Google Scholar] [CrossRef]
- Czanikova, K.; Ilcikova, M.; Krupa, I.; Micusik, M.; Kasak, P.; Pavlova, E.; Mosnacek, J.; Chorvat, D.; Omastova, M. Elastomeric photo-actuators and their investigation by confocal laser scanning microscopy. Smart Mater. Struct. 2013, 22, 104001. [Google Scholar] [CrossRef]
- Czanikova, K.; Krupa, I.; Ilcikova, M.; Kasak, P.; Chorvat, D.; Valentin, M.; Slouf, M.; Mosnacek, J.; Micusik, M.; Omastova, M. Photo-actuating materials based on elastomers and modified carbon nanotubes. J. Nanophotonics 2012, 6, 063522. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Sedlacek, T.; Doroshenko, M.; Koynov, K.; Danko, M.; Mosnacek, J. Tailoring of viscoelastic properties and light-induced actuation performance of triblock copolymer composites through surface modification of carbon nanotubes. Polymer 2015, 72, 368–377. [Google Scholar] [CrossRef]
- Liang, J.J.; Xu, Y.F.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.F.; Li, F.F.; Guo, T.Y.; Chen, Y.S. Infrared-triggered actuators from graphene-based nanocomposites. J. Phys. Chem. C 2009, 113, 9921–9927. [Google Scholar] [CrossRef]
- Ahir, S.V.; Squires, A.M.; Tajbakhsh, A.R.; Terentjev, E.M. Infrared actuation in aligned polymer–nanotube composites. Phys. Rev. B 2006, 73, 085420. [Google Scholar] [CrossRef]
- Park, J.H.; Dao, T.D.; Lee, H.I.; Jeong, H.M.; Kim, B.K. Properties of graphene/shape memory thermoplastic polyurethane composites actuating by various methods. Materials 2014, 7, 1520–1538. [Google Scholar] [CrossRef]
- Loomis, J.; King, B.; Burkhead, T.; Xu, P.; Bessler, N.; Terentjev, E.; Panchapakesan, B. Graphene-nanoplatelet-based photomechanical actuators. Nanotechnology 2012, 23, 045501. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Qin, M.M.; Guo, H.Q.; Yoshino, K.; Feng, W. Infrared-actuated recovery of polyurethane filled by reduced graphene oxide/carbon nanotube hybrids with high energy density. ACS Appl. Mater. Interf. 2013, 5, 10882–10888. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.M.; Khosravi, F.; Rahneshin, V.; Shanmugam, M.; Loeian, M.; Jasinski, J.; Cohn, R.W.; Terentjev, E.; Panchapakesan, B. MoS2 actuators: Reversible mechanical responses of MoS2–polymer nanocomposites to photons. Nanotechnology 2015, 26, 261001. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.Y.; Zhu, W.C.; Sun, S.T.; Wu, P.Y. MoS2-based dual-responsive flexible anisotropic actuators. Nanoscale 2016, 8, 18800–18807. [Google Scholar] [CrossRef] [PubMed]
- Ilcikova, M.; Mrlik, M.; Sedlacek, T.; Chorvat, D.; Krupa, I.; Slouf, M.; Koynov, K.; Mosnacek, J. Viscoelastic and photo-actuation studies of composites based on polystyrene-grafted carbon nanotubes and styrene-b-isoprene–b–styrene block copolymer. Polymer 2014, 55, 211–218. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Danko, M.; Mosnacek, J. Preparation of functionalized graphene sheets. Curr. Org. Chem. 2011, 15, 1133–1150. [Google Scholar] [CrossRef]
- Hui, C.M.; Pietrasik, J.; Schmitt, M.; Mahoney, C.; Choi, J.; Bockstaller, M.R.; Matyjaszewski, K. Surface-initiated polymerization as an enabling tool for multifunctional (nano-)engineered hybrid materials. Chem. Mater. 2014, 26, 745–762. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mosnacek, J.; Mrlik, M.; Sedlacek, T.; Csomorova, K.; Czanikova, K.; Krupa, I. Influence of surface modification of carbon nanotubes on interactions with polystyrene–b–polyisoprene-b-polystyrene matrix and its photo-actuation properties. Polym. Adv. Technol. 2014, 25, 1293–1300. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Sedlacek, T.; Slouf, M.; Zhigunov, A.; Koynov, K.; Mosnacek, J. Synthesis of photoactuating acrylic thermoplastic elastomers containing diblock copolymer-grafted carbon nanotubes. ACS Macro Lett. 2014, 3, 999–1003. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Spitalsky, Z.; Micusik, M.; Csomorova, K.; Sasinkova, V.; Kleinova, A.; Mosnacek, J. A tertiary amine in two competitive processes: Reduction of graphene oxide vs. Catalysis of atom transfer radical polymerization. RSC Adv. 2015, 5, 3370–3376. [Google Scholar] [CrossRef]
- Mrlik, M.; Ilcikova, M.; Plachy, T.; Pavlinek, V.; Spitalsky, Z.; Mosnacek, J. Graphene oxide reduction during surface-initiated atom transfer radical polymerization of glycidyl methacrylate: Controlling electro-responsive properties. Chem. Eng. J. 2016, 283, 717–720. [Google Scholar] [CrossRef]
- Yoon, J.T.; Lee, S.C.; Jeong, Y.G. Effects of grafted chain length on mechanical and electrical properties of nanocomposites containing polylactide-grafted carbon nanotubes. Compos. Sci. Technol. 2010, 70, 776–782. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlik, M.; Ilcikova, M.; Mosnacek, M.; Munster, L.; Pavlínek, V. Synthesis of silicone elastomers containing silyl-based polymer-grafted carbonyl iron particles: An efficient way to improve magnetorheological, damping, and sensing performances. Macromolecules 2017, 50, 2189–2200. [Google Scholar] [CrossRef]
- Rabindranath, R.; Bose, H. On the mobility of iron particles embedded in elastomeric silicone matrix. J. Phys. Conf. Ser. 2013, 412, 012034. [Google Scholar] [CrossRef]


| Sample Code | Activation Energy (kJ·mol−1) |
|---|---|
| pure PDMS | 45.70 |
| 0.1 vol % neat GO PDMS | 36.57 |
| 0.5 vol % neat GO PDMS | 34.97 |
| 1 vol % neat GO PDMS | 20.35 |
| 0.1 vol % GO–PMMA PDMS | 35.99 |
| 0.5 vol % GO–PMMA PDMS | 23.58 |
| 1 vol % GO–PMMA PDMS | 10.16 |
| 0.1 vol % GO–PBMA PDMS | 25.39 |
| 0.5 vol % GO–PBMA PDMS | 22.79 |
| 1 vol % GO–PBMA PDMS | 9.49 |
| Sample Code | Thermal Conductivity (W·mK−1) |
|---|---|
| pure PDMS | 0.071 |
| 0.1 vol % neat GO/PDMS | 0.144 |
| 0.5 vol % neat GO/PDMS | 0.156 |
| 1 vol % neat GO/PDMS | 0.161 |
| 0.1 vol % GO–PMMA/PDMS | 0.153 |
| 0.5 vol % GO–PMMA/PDMS | 0.174 |
| 1 vol % GO–PMMA/PDMS | 0.209 |
| 0.1 vol % GO–PBMA/PDMS | 0.151 |
| 0.5 vol % GO–PBMA/PDMS | 0.186 |
| 1 vol % GO–PBMA/PDMS | 0.250 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osicka, J.; Ilčíková, M.; Mrlik, M.; Minařík, A.; Pavlinek, V.; Mosnáček, J. The Impact of Polymer Grafting from a Graphene Oxide Surface on Its Compatibility with a PDMS Matrix and the Light-Induced Actuation of the Composites. Polymers 2017, 9, 264. https://doi.org/10.3390/polym9070264
Osicka J, Ilčíková M, Mrlik M, Minařík A, Pavlinek V, Mosnáček J. The Impact of Polymer Grafting from a Graphene Oxide Surface on Its Compatibility with a PDMS Matrix and the Light-Induced Actuation of the Composites. Polymers. 2017; 9(7):264. https://doi.org/10.3390/polym9070264
Chicago/Turabian StyleOsicka, Josef, Markéta Ilčíková, Miroslav Mrlik, Antonín Minařík, Vladimir Pavlinek, and Jaroslav Mosnáček. 2017. "The Impact of Polymer Grafting from a Graphene Oxide Surface on Its Compatibility with a PDMS Matrix and the Light-Induced Actuation of the Composites" Polymers 9, no. 7: 264. https://doi.org/10.3390/polym9070264