Theoretical Study on Decomposition Mechanism of Insulating Epoxy Resin Cured by Anhydride
Abstract
:1. Introduction
2. Simulation Details
3. Results and Discussion
3.1. Simulation Results of PEP
3.2. Simulation Results of EPMHA
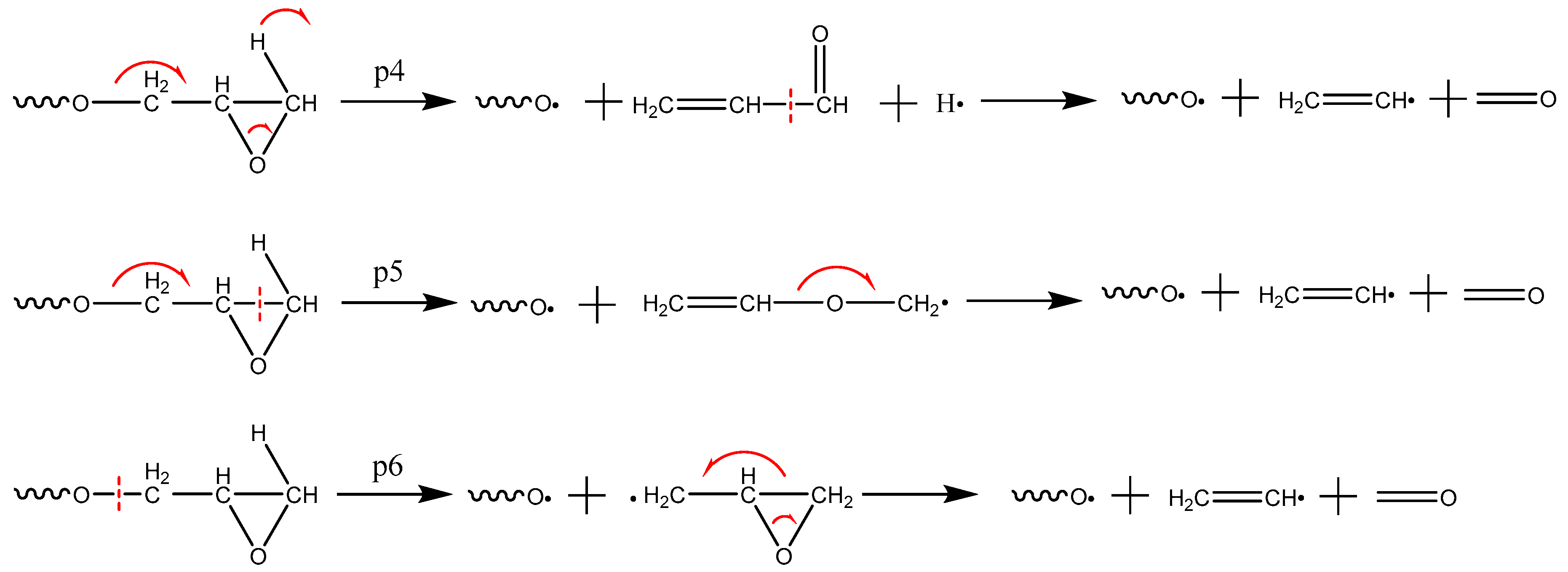
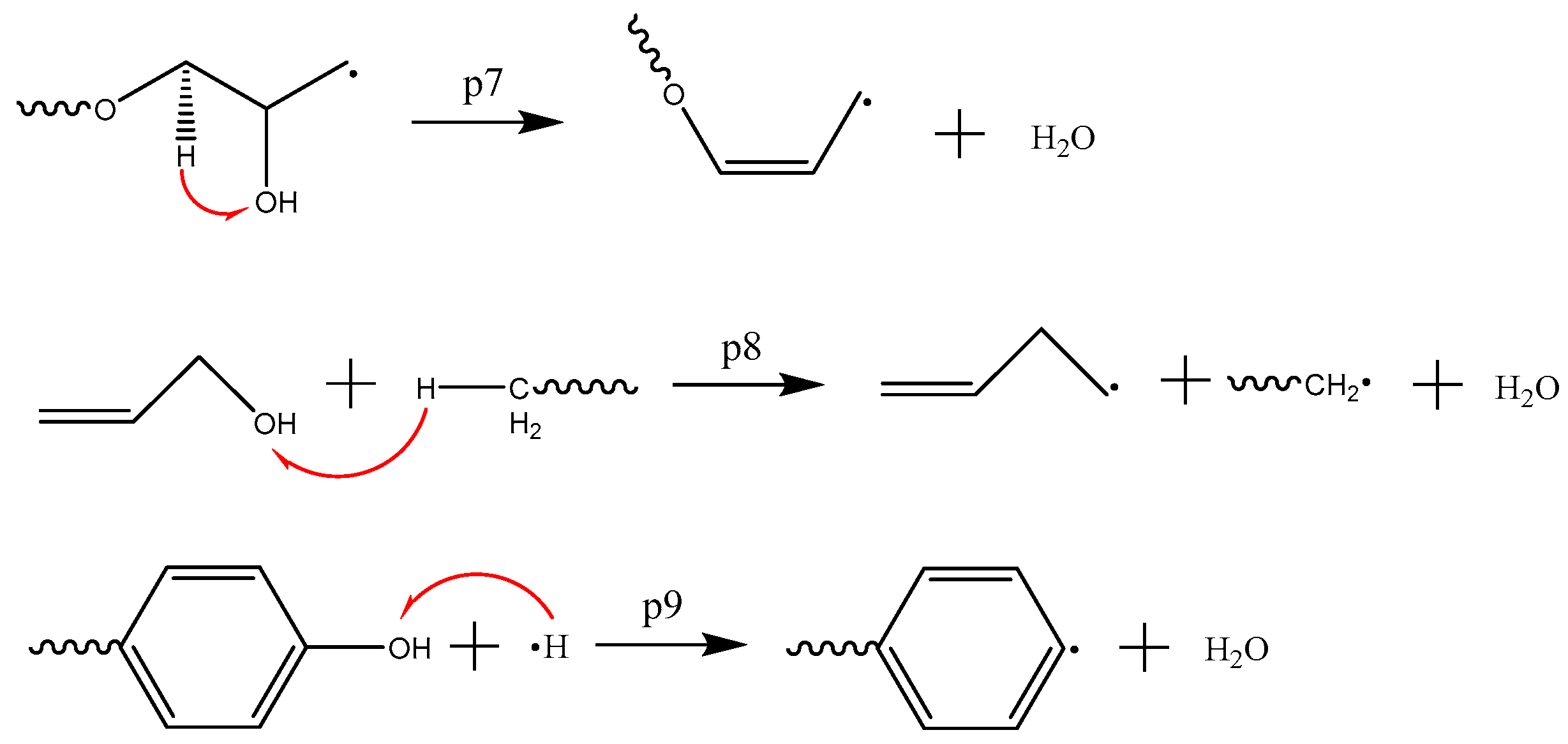
3.3. Formation Mechanism of Gas Products
4. Conclusions
- The initiation reaction of the decomposition of anhydride-cured epoxy resin is the cleavage of an ester bond;
- The first and the most abundant product is CO2. The other products were generated in the following sequence: CH2O, CO, and H2O. With respect to content, the following order was observed: CH2O, H2O, and CO.
- CO2 is produced from the carbonyl oxygen bond in anhydride; CH2O, from the decomposition of epoxy functional group; H2O, by free radical collision and dehydration.
Author Contributions
Conflicts of Interest
References
- Uzunlar, E.; Schwartz, J.; Phillips, O.; Kohl, P.A. Decomposable and template polymers: Fundamentals and applications. J. Electron. Packag. 2016, 138, 15. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Zhang, X.; Meng, Q.H.; Zhou, J.B. Partial discharge recognition through an analysis of SF6 decomposition products part 1: Decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Ju, T.; Xu, Z.R.; Zhang, X.X.; Sun, C.X. GIS partial discharge quantitative measurements using UHF microstrip antenna sensors, Electrical Insulation and Dielectric Phenomena. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP 2007), Vancouver, BC, Canada, 14–17 October 2007. [Google Scholar] [CrossRef]
- Champion, J.V.; Dodd, S.J. The effect of voltage and material age on the electrical tree growth and breakdown characteristics of epoxy resins. J. Phys. D Appl. Phys. 1995, 28, 398–407. [Google Scholar] [CrossRef]
- Wu, K.; Dissado, L.A. Model for electrical tree initiation in epoxy resin. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 655–668. [Google Scholar]
- Morshuis, P.H.F. Degradation of solid dielectrics due to internal partial discharge: some thoughts on progress made and where to go now. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 905–913. [Google Scholar] [CrossRef]
- Sili, E.; Cambronne, J.P.; Naude, N.; Khazaka, R. Polyimide lifetime under partial discharge aging: Effects of temperature, pressure and humidity. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 435–442. [Google Scholar] [CrossRef]
- Grassie, N.; Guy, M.I.; Tennent, N.H. Degradation of epoxy polymers: Part 1—Products of thermal degradation of bisphenol-A diglycidyl ether. Polym. Degrad. Stab. 1985, 12, 65–91. [Google Scholar] [CrossRef]
- Grassie, N.; Guy, M.I.; Tennent, N.H. Degradation of epoxy polymers: 2—Mechanism of thermal degradation of bisphenol-A diglycidyl ether. Polym. Degrad. Stab. 1985, 13, 11–20. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Choi, J.R.; Park, S.J. Thermal conductivity and thermo-physical properties of nanodiamond-attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. Part A Appl. Sci. Manuf. 2017, 101, 227–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Park, S.J. Nanodiamond nanocluster-decorated graphene oxide/epoxy nanocomposites with enhanced mechanical behavior and thermal stability. Compos. Part B 2017, 114, 111–120. [Google Scholar] [CrossRef]
- Vlastaras, A.S. Thermal degradation of an anhydride-cured epoxy resin by laser heating. J. Phys. Chem. 1970, 74, 2496–2501. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, W.; Liu, Z.; Huang, K.F.; Jin, H.Y.; Wu, C. Study on thermal aging characteristics of epoxy resin/inorganic filler composites for the fully casting bus bar, Electrical Insulation and Dielectric Phenomena. In Proceedings of the 2013 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Shenzhen, China, 20–23 October 2013. [Google Scholar] [CrossRef]
- Hudon, C.; Bartnikas, R.; Wertheimer, M.R. Chemical and physical degradation effects on epoxy surfaces exposed to partial discharges. In Proceedings of the International Conference on Properties and Applications of Dielectric Materials, Brisbane, Australia, 3–8 July 1994. [Google Scholar] [CrossRef]
- Hudon, C.; Bartnikas, R.; Wertheimer, M.R. Effect of physico-chemical degradation of epoxy resin on partial discharge behavior. IEEE Trans. Dielectr. Electr. Insul. 1995, 2, 1083–1094. [Google Scholar] [CrossRef]
- Hudon, C.; Bartnikas, R.; Wertheimer, M.R. Surface conductivity of epoxy specimens subjected to partial discharges. In Proceedings of the Conference Record of the 1990 IEEE International Symposium on Electrical Insulation, Toronto, ON, Canada, 3–6 June 1990. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Iii, W.A.G. ReaxFF: A Reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J. Phys. Chem. A 2008, 112, 1040. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.E.; van Duin, A.C.T.; Goddard, W.A. Application of the ReaxFF reactive force field to reactive dynamics of hydrocarbon chemisorption and decomposition. J. Phys. Chem. 2010, 114, 5675–5685. [Google Scholar] [CrossRef]
- Agrawalla, S.; van Duin, A.C.T. Development and application of a ReaxFF reactive force Field for hydrogen combustion. J. Phys. Chem. A 2011, 115, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Chenoweth, K.; Cheung, S.; van Duin, A.C.T.; Iii, W.A.G.; Kober, E.M. Simulations on the thermal decomposition of a poly(dimethylsiloxane) polymer using the ReaxFF reactive force field. J. ACS 2005, 127, 7192–7202. [Google Scholar] [CrossRef] [PubMed]
- Ballistreri, A.; Garozzo, D.; Montaudo, G. Mass spectral characterization and thermal decomposition mechanism of poly(dimethylsiloxane). Macromolecules 2015, 191, 1. [Google Scholar] [CrossRef]
- Chang, J.; Lian, P.; Wei, D.Q.; Chen, X.R.; Zhang, Q.M.; Gong, Z.Z. Thermal decomposition of the solid phase of nitromethane: ab initio molecular dynamics simulations. Phys. Rev. Lett. 2010, 105, 188302. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhang, H.; Cheng, X.L. Molecular dynamic simulations of solid nitromethane under high pressures. J. Theor. Comput. Chem. 2010, 9, 315–325. [Google Scholar] [CrossRef]
- Raymand, D.; van Duin, A.C.; Baudin, M.; Hermansson, K. A reactive force field (ReaxFF) for zinc oxide. Surf. Sci. 2008, 602, 1020–1031. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Persson, P.; Cheng, M.J.; Oxgaard, J.; Iii, W.A.G. Development and application of a ReaxFF reactive force field for oxidative dehydrogenation on vanadium oxide catalysts. J. Phys. Chem. A 2008, 112, 14645–14654. [Google Scholar] [CrossRef]
- Nielson, K.D.; van Duin, A.C.; Oxgaard, J.; Deng, W.Q.; Iii, W.A.G. Development of the ReaxFF reactive force field for describing transition metal catalyzed reactions, with application to the initial stages of the catalytic formation of carbon nanotubes. J. Phys. Chem. A 2005, 109, 493. [Google Scholar] [CrossRef] [PubMed]
- Iii, W.A.G.; Mueller, J.E.; Chenoweth, K.; van Duinc, A.C.T. ReaxFF Monte Carlo reactive dynamics: application to resolving the partial occupations of the M1 phase of the MoVNbTeO catalyst. Catal. Today 2010, 157, 71–76. [Google Scholar]
- Diao, Z.J.; Zhao, Y.M.; Chen, B.; Duan, C.L.; Song, S. ReaxFF reactive force field for molecular dynamics simulations of epoxy resin thermal decomposition with model compound. J. Anal. Appl. Pyrolysis 2013, 104, 618–624. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Li, J.L.; Wang, J.P.; Yang, X.S.; Shao, W.; Xiao, S.Q.; Wang, B.Z. Research on epoxy resin decomposition under microwave heating by using ReaxFF molecular dynamics simulations. RSC Adv. 2014, 4, 17083–17090. [Google Scholar] [CrossRef]


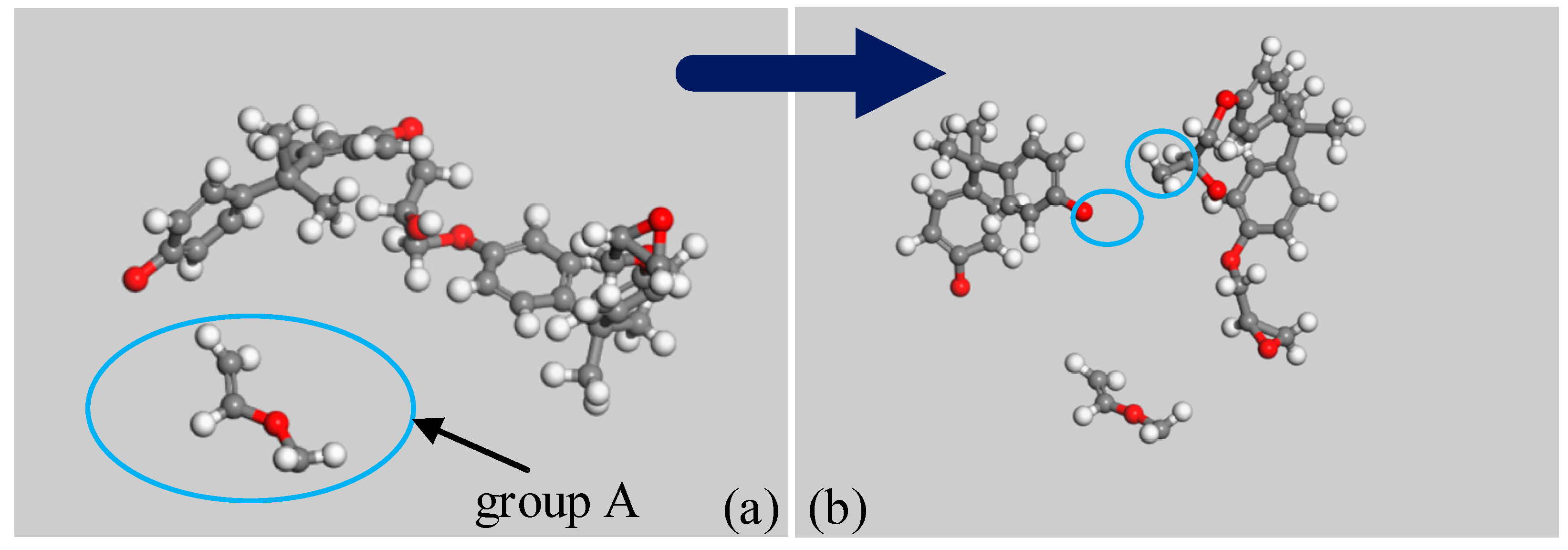
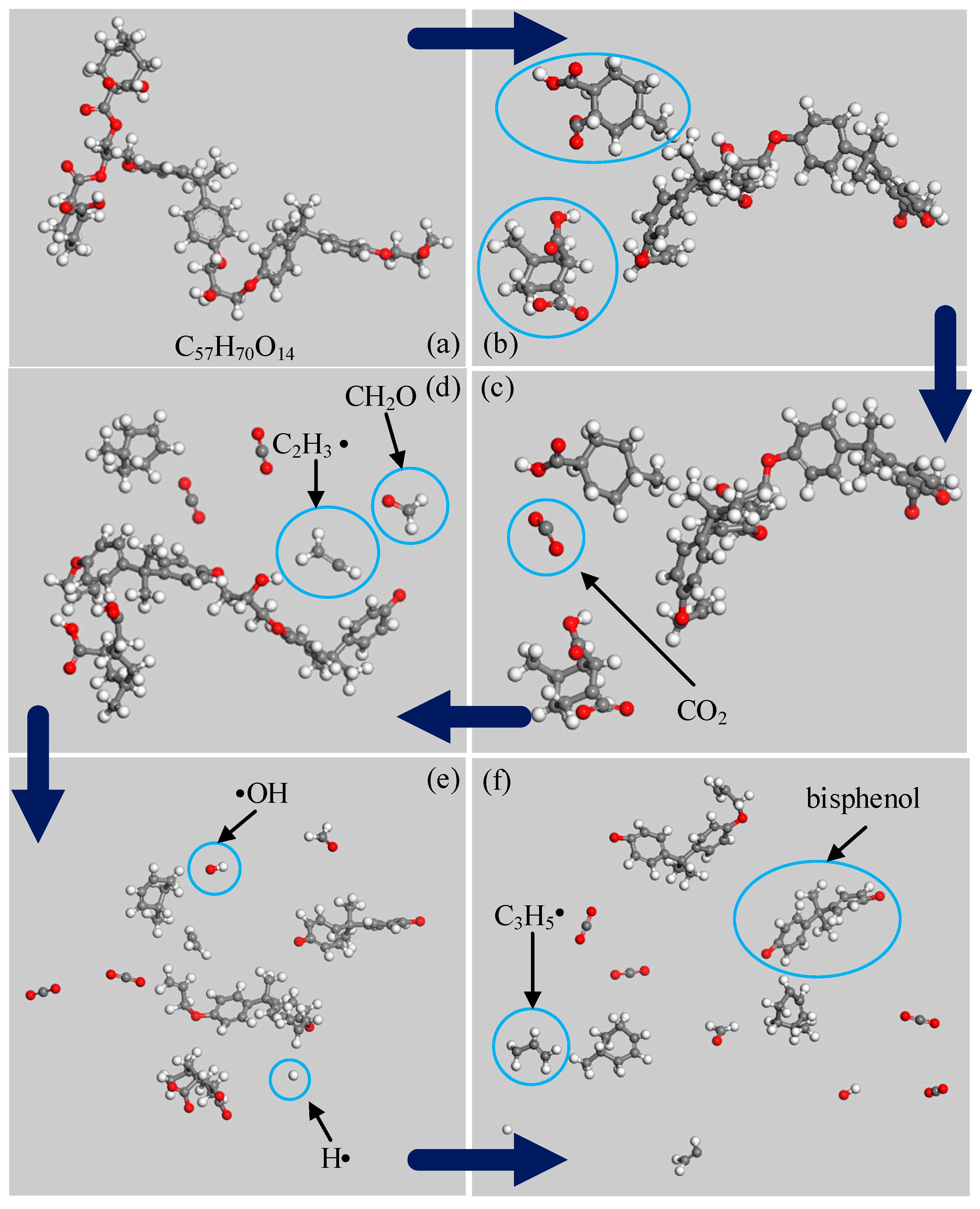

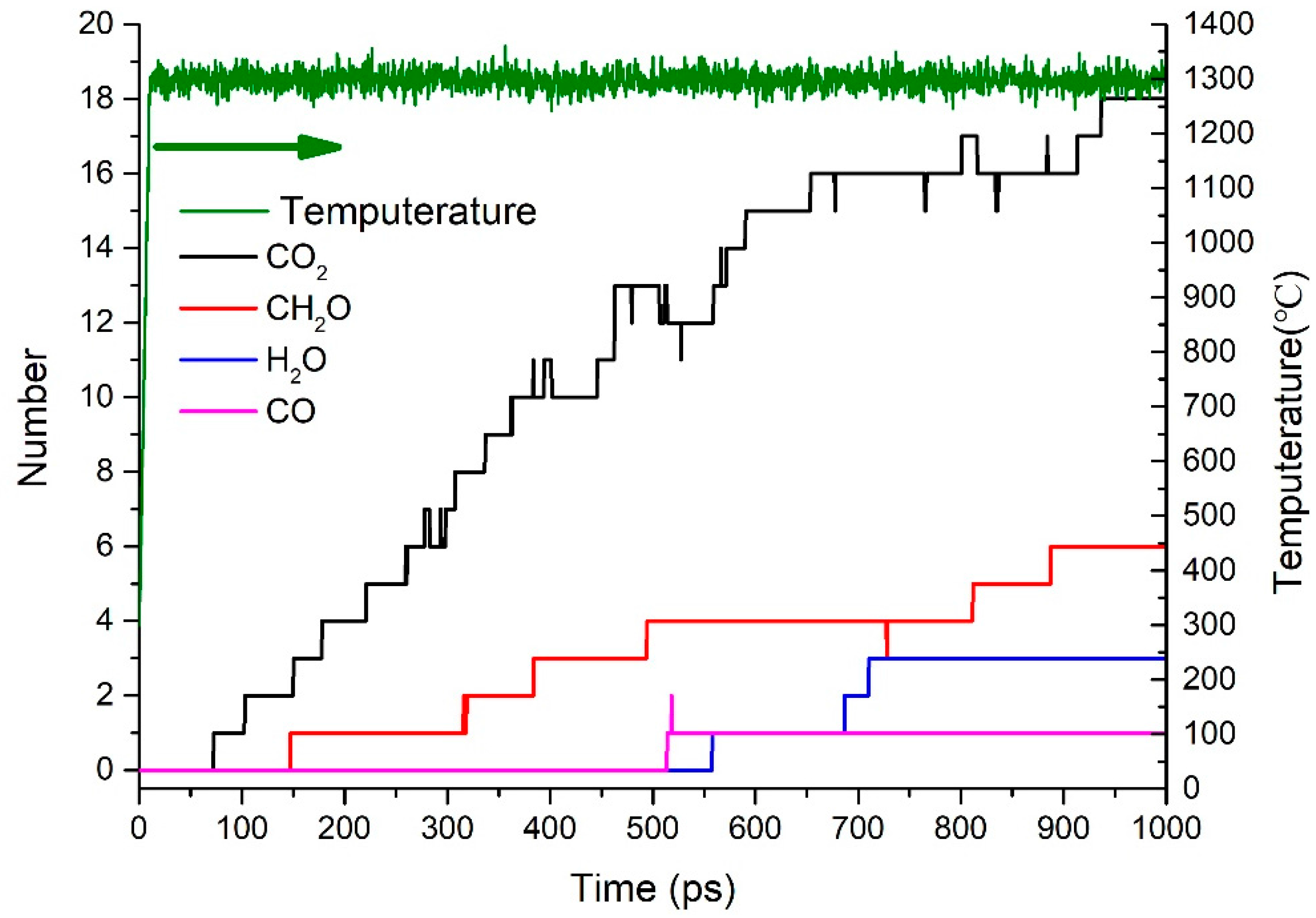
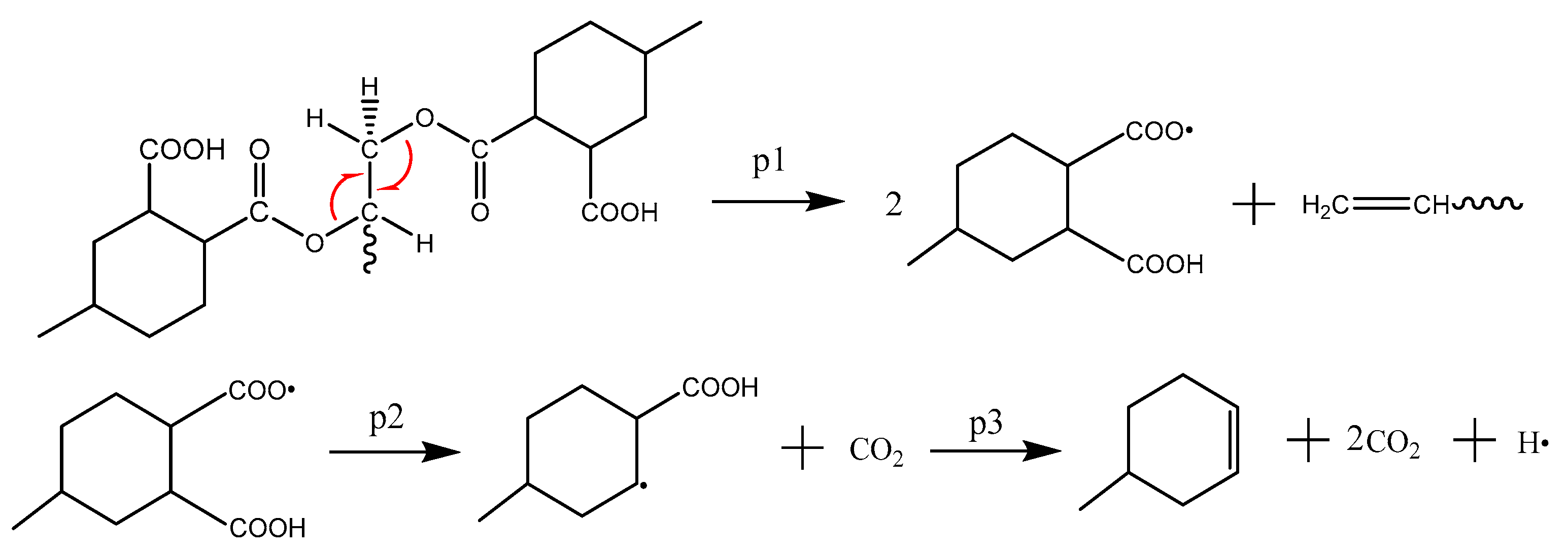
| Model | Material | Molecular formula | Number | Initial density g/cm3 | Final density g/cm3 |
|---|---|---|---|---|---|
| Model 1 | PEP | C39H44O7 | 1 | 0.5 | 1.13 |
| Model 2 | EPMHA | C57H70O14 | 1 | 0.5 | 1.13 |
| Model 3 | EPMHA | 15*C57H70O14 | 15 | 0.5 | 1.17 |
| Molecule | Breakage of bond | Activation energy (kJ/mol) |
|---|---|---|
| PEP | ① | −232.64 |
| ② | −332.53 | |
| ③ | −320.62 | |
| ④ | −261.75 | |
| ⑤ | −276.54 | |
| EPMHA | ① | −192.27 |
| ② | −186.58 | |
| ③ | −256.81 | |
| ④ | −258.14 | |
| ⑤ | −273.92 | |
| ⑥ | −252.38 | |
| ⑦ | −230.78 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wu, Y.; Chen, X.; Wen, H.; Xiao, S. Theoretical Study on Decomposition Mechanism of Insulating Epoxy Resin Cured by Anhydride. Polymers 2017, 9, 341. https://doi.org/10.3390/polym9080341
Zhang X, Wu Y, Chen X, Wen H, Xiao S. Theoretical Study on Decomposition Mechanism of Insulating Epoxy Resin Cured by Anhydride. Polymers. 2017; 9(8):341. https://doi.org/10.3390/polym9080341
Chicago/Turabian StyleZhang, Xiaoxing, Yunjian Wu, Xiaoyu Chen, Hao Wen, and Song Xiao. 2017. "Theoretical Study on Decomposition Mechanism of Insulating Epoxy Resin Cured by Anhydride" Polymers 9, no. 8: 341. https://doi.org/10.3390/polym9080341
APA StyleZhang, X., Wu, Y., Chen, X., Wen, H., & Xiao, S. (2017). Theoretical Study on Decomposition Mechanism of Insulating Epoxy Resin Cured by Anhydride. Polymers, 9(8), 341. https://doi.org/10.3390/polym9080341





