Micellization of Photo-Responsive Block Copolymers
Abstract
:1. Introduction and Scope
2. Block Copolymers in General
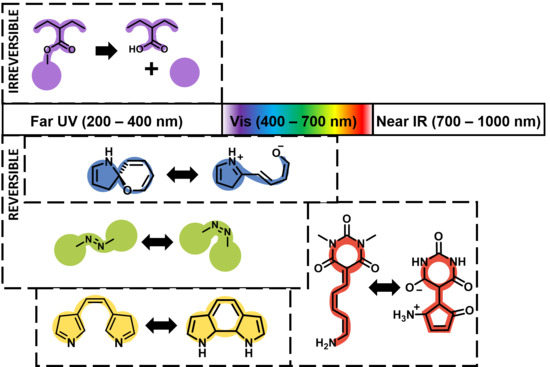
3. λ-Dependent Photo-Response in Block Copolymer Nanostructures in Solution
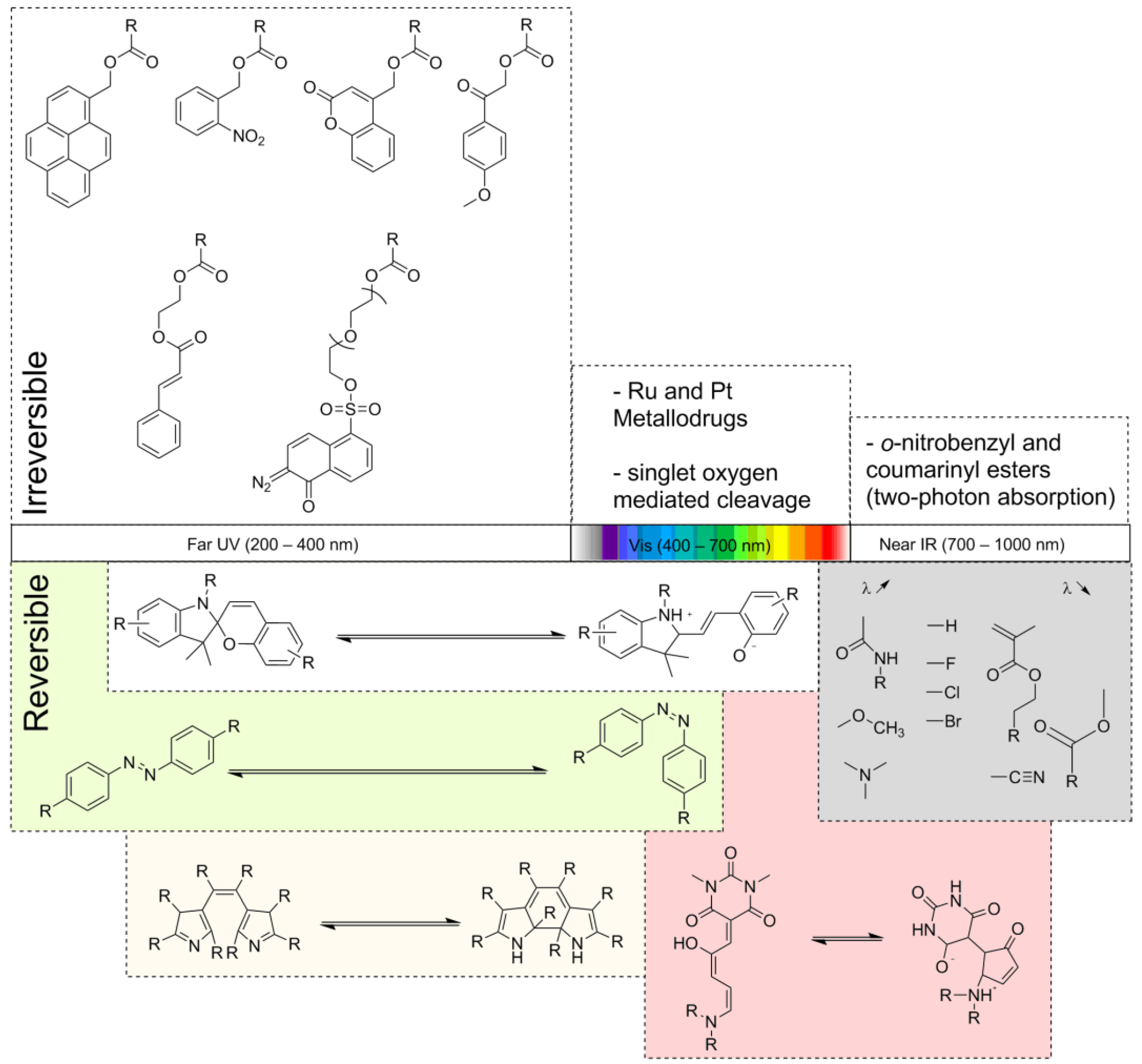
3.1. Far UV (200–400 nm)
3.1.1. Reversible
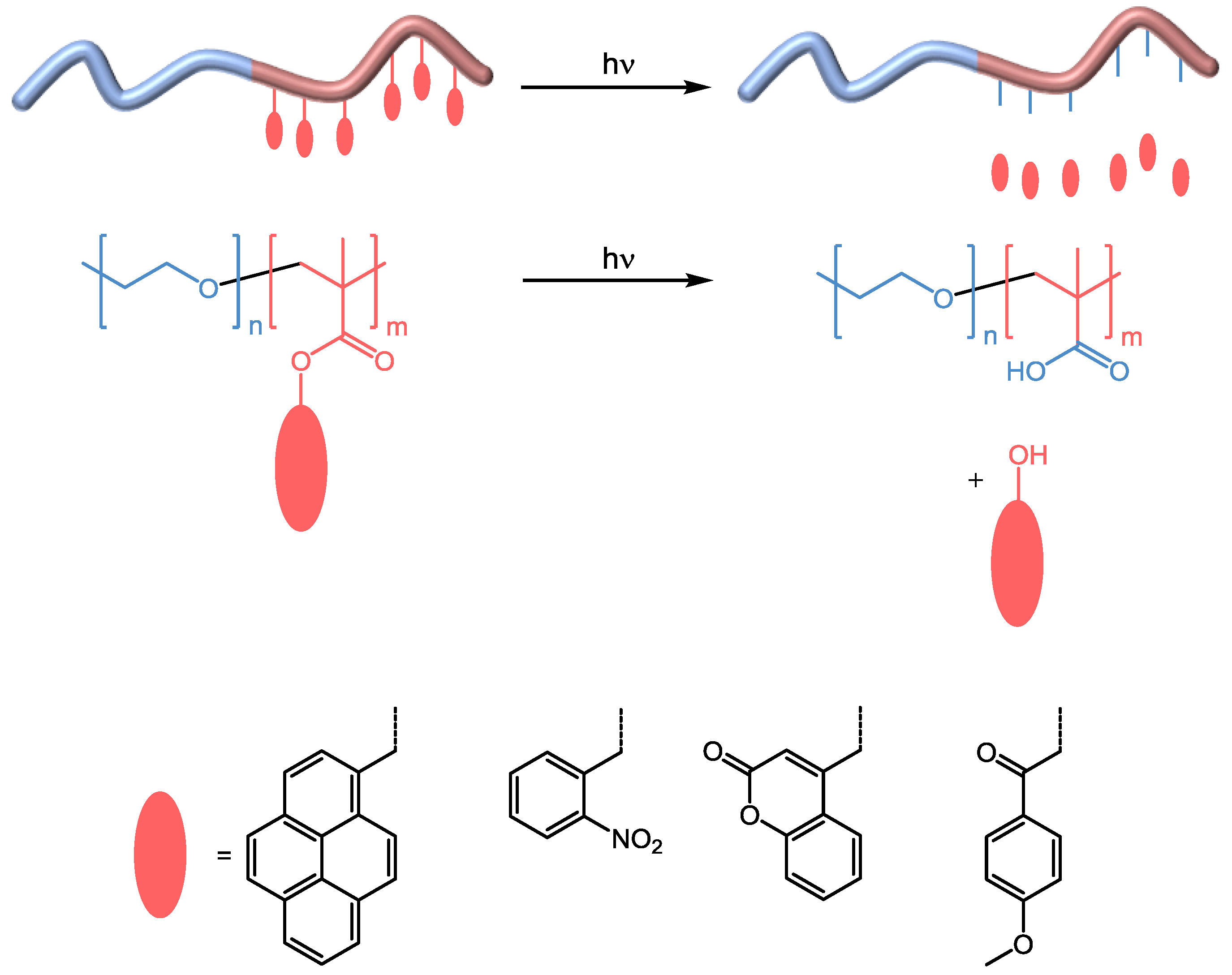
3.1.2. Irreversible
3.2. Vis (400–600 nm)
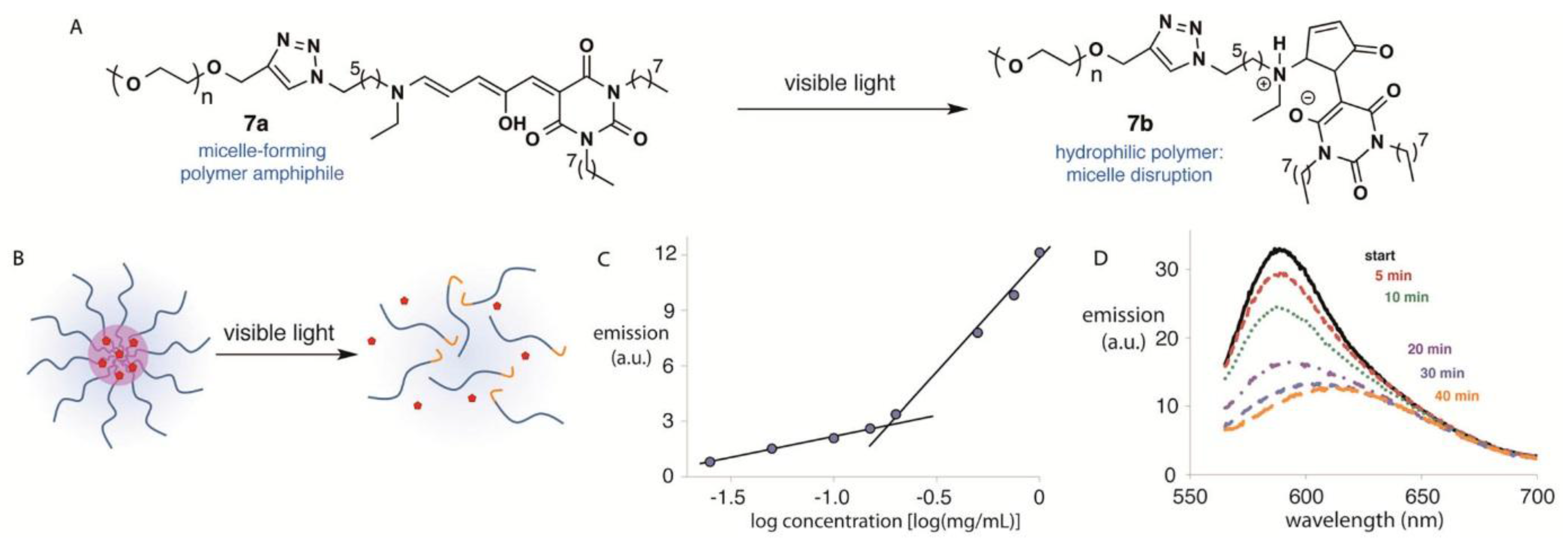
3.2.1. Reversible
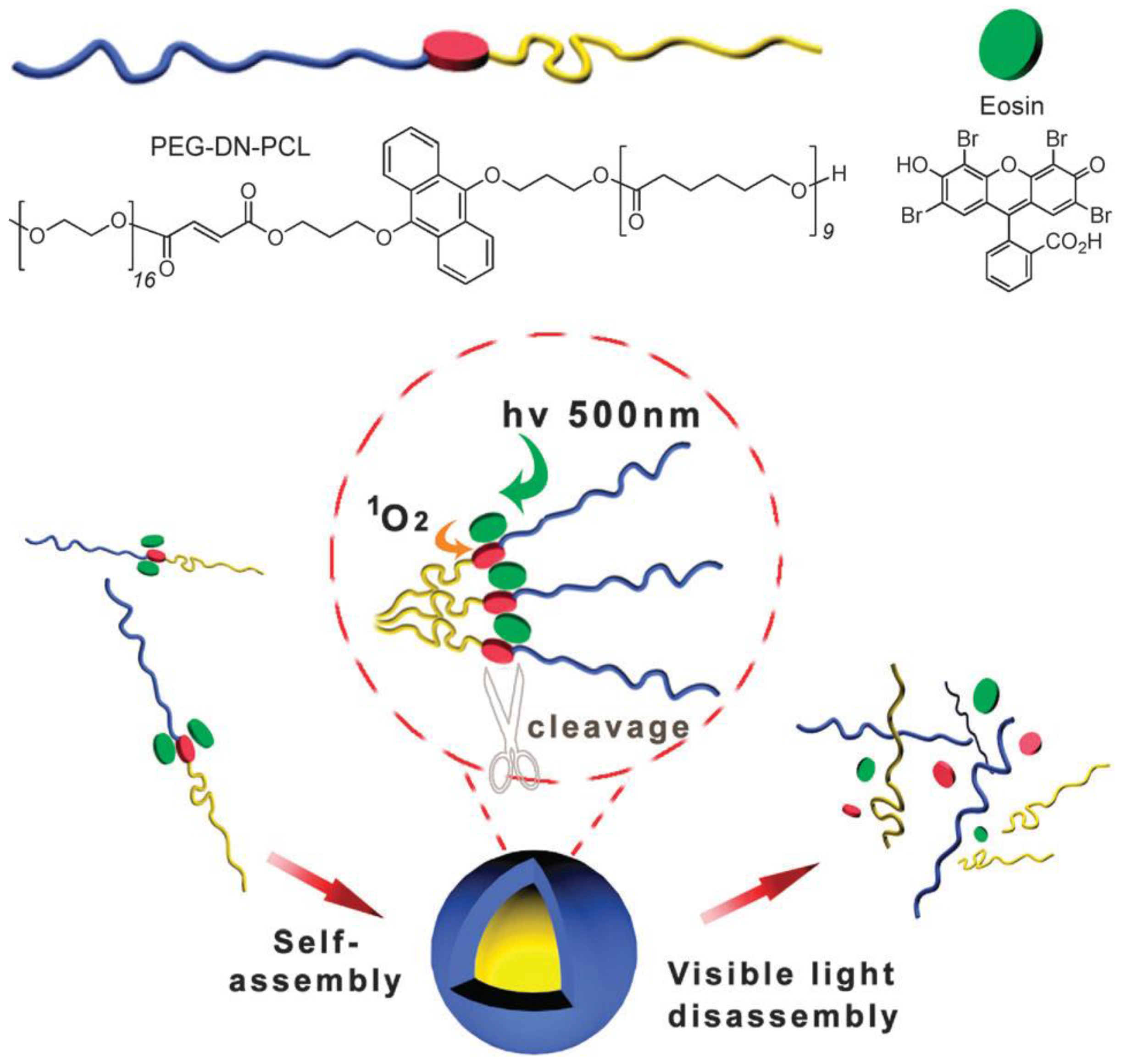
3.2.2. Irreversible
3.3. Near IR (700–1000 nm)
3.3.1. Irreversible
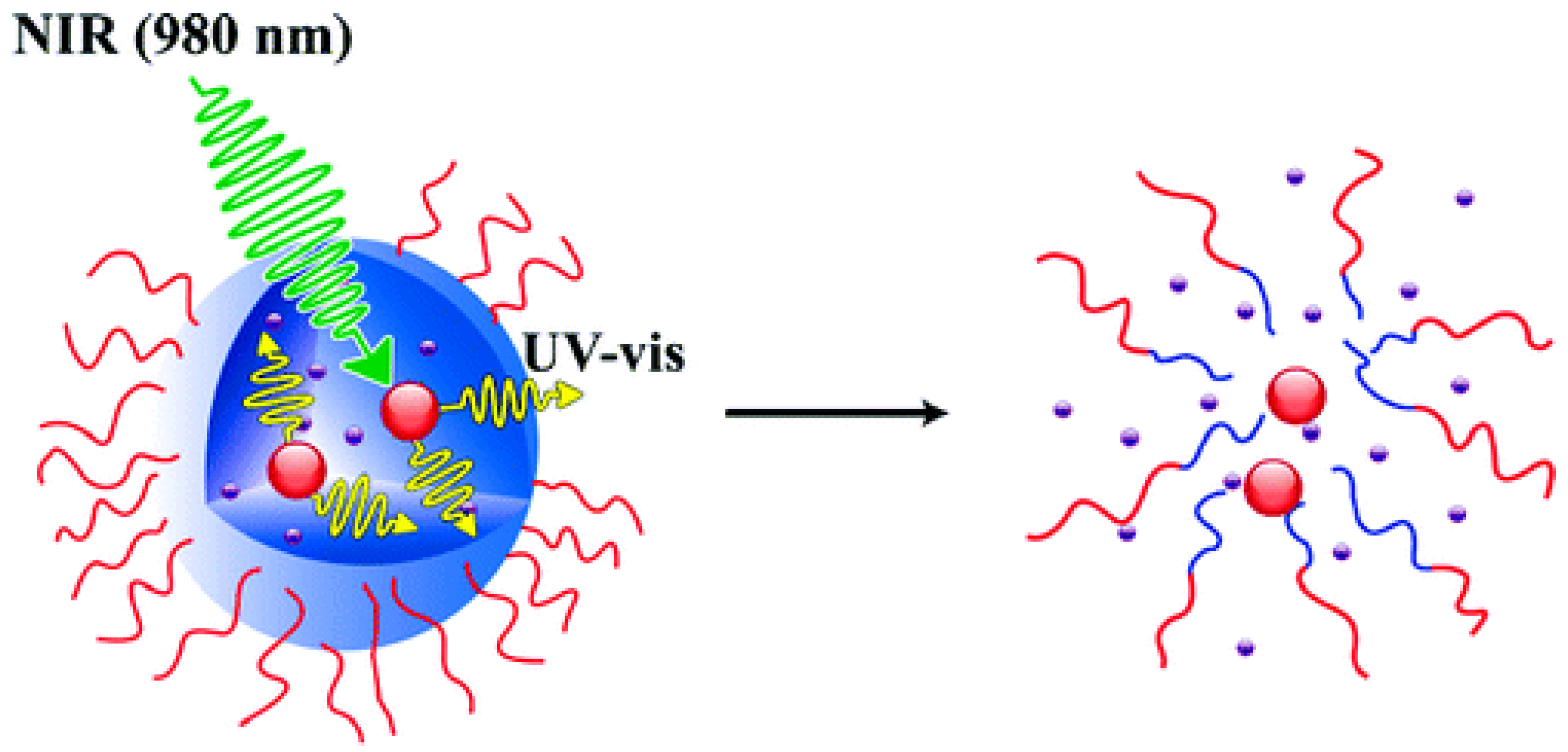
3.3.2. Upconversion
4. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotech. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.R.; Matthias, K.; Matthias, E.; Mark, C. Near-infrared optical properties of ex vivo human skin and subcutaneous tissues measured using the Monte Carlo inversion technique. Phys. Med. Biol. 1998, 43, 2465–2478. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Light-Responsive Block Copolymer Micelles. Macromolecules 2012, 45, 3647–3657. [Google Scholar] [CrossRef]
- Gohy, J.-F.; Zhao, Y. Photo-responsive block copolymer micelles: Design and behavior. Chem. Soc. Rev. 2013, 42, 7117–7129. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, O.; Gohy, J.-F. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2017, 8, 52–73. [Google Scholar] [CrossRef]
- Yu, H.; Ikeda, T. Smart Light-Responsive Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 411–456. [Google Scholar]
- Barzynski, H.; Jun, M.; Saenger, D.; Volkert, O. Lithographic printing plates and photoresists comprising a photosensitive polymer. US. Patent 3,849,137, 19 November 1974. [Google Scholar]
- Bergmann, E.D.; Weizmann, A.; Fischer, E. Structure and Polarity of Some Polycyclic Spirans. J. Am. Chem. Soc. 1950, 72, 5009–5012. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymers—Designer Soft Materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional Block Copolymers: Nanostructured Materials with Emerging Applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, M.; Levy, M.; Milkovich, R. Polymerization initiated by electron transfer to monomer. A new method of formation of block polymers1. J. Am. Chem. Soc. 1956, 78, 2656–2657. [Google Scholar] [CrossRef]
- Webster, O.W. Living Polymerization Methods. Science 1991, 251, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 2009, 1, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, A.; Nikolaou, V.; Nurumbetov, G.; Wilson, P.; Kempe, K.; Quinn, J.F.; Davis, T.P.; Whittaker, M.R.; Haddleton, D.M. Cu(0)-Mediated Living Radical Polymerization: A Versatile Tool for Materials Synthesis. Chem. Rev. 2016, 116, 835–877. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Gregory, A.; Stenzel, M.H. Complex polymer architectures via RAFT polymerization: From fundamental process to extending the scope using click chemistry and nature’s building blocks. Prog. Polym. Sci. 2012, 37, 38–105. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process—A Third Update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Hayward, R.C.; Pochan, D.J. Tailored Assemblies of Block Copolymers in Solution: It Is All about the Process. Macromolecules 2010, 43, 3577–3584. [Google Scholar] [CrossRef]
- Tonhauser, C.; Golriz, A.A.; Moers, C.; Klein, R.; Butt, H.-J.; Frey, H. Stimuli-Responsive Y-Shaped Polymer Brushes Based on Junction-Point-Reactive Block Copolymers. Adv. Mater. 2012, 24, 5559–5563. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.; Barthel, M.J.; Kretschmer, F.; Mansfeld, U.; Hoeppener, S.; Hager, M.D.; Schubert, U.S.; Schacher, F.H. Poly(2-vinyl pyridine)-block-Poly(ethylene oxide) Featuring a Furan Group at the Block Junction—Synthesis and Functionalization. Macromol. Rapid Commun. 2014, 35, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.; Schacher, F.H. Selective crosslinking or addressing of individual domains within block copolymer nanostructures. Eur. Polym. J. 2016, 80, 317–331. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Goldmann, A.S.; Schacher, F.H. Polymer Interfaces: Synthetic Strategies Enabling Functionality, Adaptivity, and Spatial Control. Macromolecules 2016, 49, 5001–5016. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced Motions in Azo-Containing Polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Neckers, D.C. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Assaid, I.; Bosc, D.; Hardy, I. Improvements of the Poly(vinyl cinnamate) Photoresponse in Order to Induce High Refractive Index Variations. J. Phys. Chem. B 2004, 108, 2801–2806. [Google Scholar] [CrossRef]
- Irie, M. Diarylethenes for Memories and Switches. Chem. Rev. 2000, 100, 1685–1716. [Google Scholar] [CrossRef] [PubMed]
- Stobbe, H.; Leuner, K. Farblose Alkylfulgide. (8. Abhandlung über Butadiënverbindungen.). Ber. Dtsch. Chem. Ges. 1905, 38, 3682–3685. [Google Scholar] [CrossRef]
- Irie, M.; Mohri, M. Thermally irreversible photochromic systems. Reversible photocyclization of diarylethene derivatives. J. Org. Chem. 1988, 53, 803–808. [Google Scholar] [CrossRef]
- Tanio, N.; Irie, M. Photooptical Switching of Polymer Film Waveguide Containing Photochromic Diarylethenes. Jpn. J. Appl. Phys. 1994, 33, 1550–1553. [Google Scholar] [CrossRef]
- Fukaminato, T.; Sasaki, T.; Kawai, T.; Tamai, N.; Irie, M. Digital photoswitching of fluorescence based on the photochromism of diarylethene derivatives at a single-molecule level. J. Am. Chem. Soc. 2004, 126, 14843–14849. [Google Scholar] [CrossRef] [PubMed]
- Stellacci, F.; Toscano, F.; Gallazzi, M.C.; Zerbi, G. From a photochromic diarylethene monomer to a dopable photochromic polymer: optical properties. Synth. Met. 1999, 102, 979–980. [Google Scholar] [CrossRef]
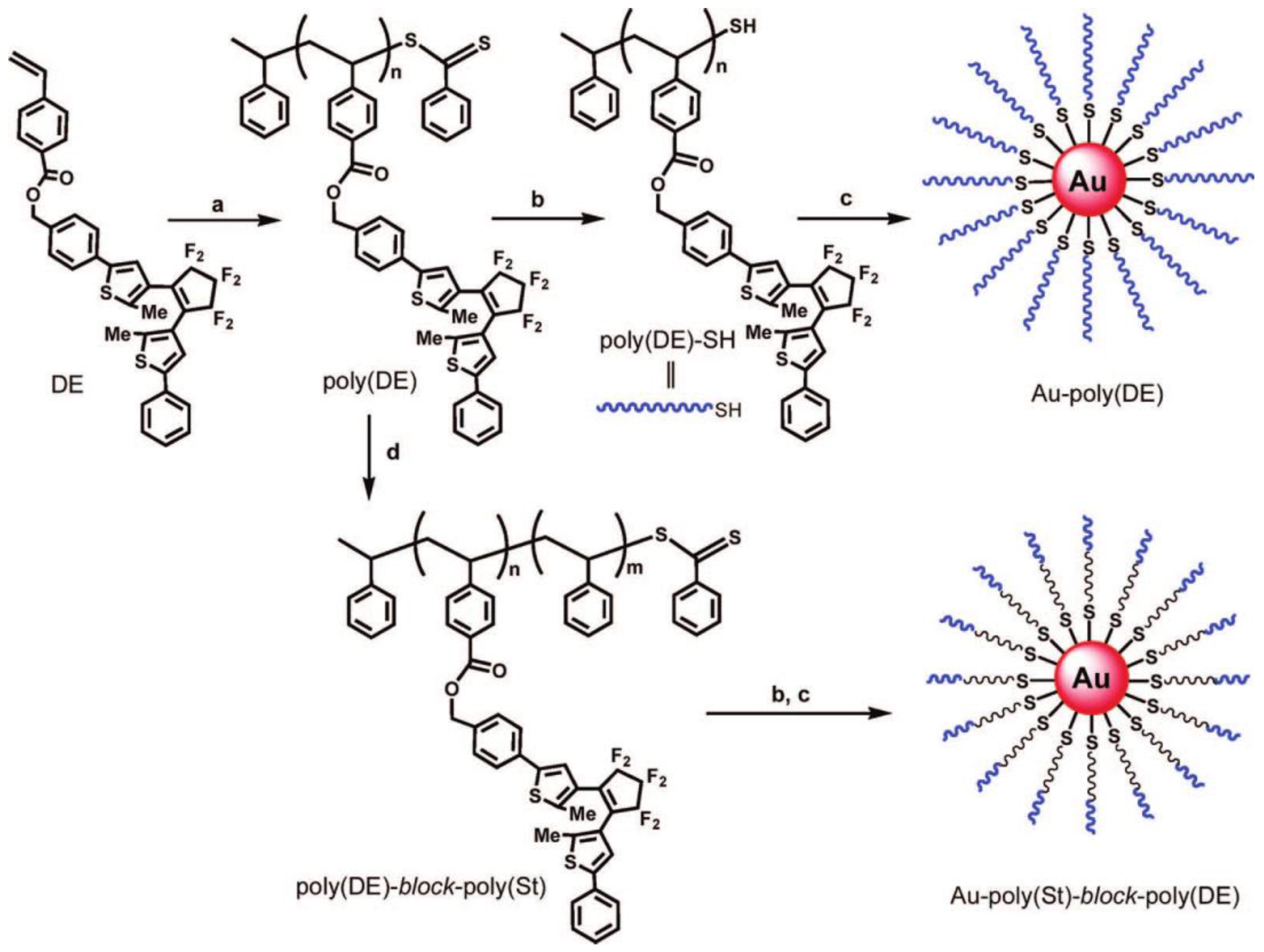
- Nishi, H.; Kobatake, S. Photochromism and Optical Property of Gold Nanoparticles Covered with Low-Polydispersity Diarylethene Polymers. Macromolecules 2008, 41, 3995–4002. [Google Scholar] [CrossRef]
- Seno, R.; Kobatake, S. Synthesis and characterization of amphiphilic silica nanoparticles covered by block copolymers branching photochromic diarylethene moieties on side chain. Dyes Pigm. 2015, 114, 166–174. [Google Scholar] [CrossRef]
- Griffiths, J., II. Photochemistry of azobenzene and its derivatives. Chem. Soc. Rev. 1972, 1, 481–493. [Google Scholar] [CrossRef]
- Hartley, G.S. The Cis-form of Azobenzene. Nat. Chem. 1937, 140, 281. [Google Scholar] [CrossRef]
- Krollpfeiffer, F.; Mühlhausen, C.; Wolf, G. Zur Kenntnis der Lichtempfindlichkeit von Aryl-β-naphtylamin-azofarbstoffen. Liebigs Ann. Chem. 1934, 508, 39–51. [Google Scholar] [CrossRef]
- Bleger, D.; Schwarz, J.; Brouwer, A.M.; Hecht, S. o-Fluoroazobenzenes as readily synthesized photoswitches offering nearly quantitative two-way isomerization with visible light. J. Am. Chem. Soc. 2012, 134, 20597–20600. [Google Scholar] [CrossRef]
- Ringsdorf, H.; Schmidt, H.-W. Electro-optical effects of azo dye containing liquid crystalline copolymers. Makromol. Chem. 1984, 185, 1327–1334. [Google Scholar] [CrossRef]
- Angeloni, A.S.; Caretti, D.; Carlini, C.; Chiellini, E.; Galli, G.; Altomare, A.; Solaro, R.; Laus, M. Photochromic liquid-crystalline polymers. Main chain and side chain polymers containing azobenzene mesogens. Liq. Cryst. 1989, 4, 513–527. [Google Scholar] [CrossRef]
- Moriya, K.; Seki, T.; Nakagawa, M.; Mao, G.; Ober, C.K. Photochromism of 4-cyanophenylazobenzene in liquid crystalline-coil AB diblock copolymers: the influence of microstructure. Macromol. Rapid Commun. 2000, 21, 1309–1312. [Google Scholar] [CrossRef]
- Frenz, C.; Fuchs, A.; Schmidt, H.-W.; Theissen, U.; Haarer, D. Diblock Copolymers with Azobenzene Side-Groups and Polystyrene Matrix: Synthesis, Characterization and Photoaddressing. Macromol. Chem. Phys. 2004, 205, 1246–1258. [Google Scholar] [CrossRef]
- Wang, G.; Tong, X.; Zhao, Y. Preparation of Azobenzene-Containing Amphiphilic Diblock Copolymers for Light-Responsive Micellar Aggregates. Macromolecules 2004, 37, 8911–8917. [Google Scholar] [CrossRef]
- Hu, J.; Yu, H.; Gan, L.H.; Hu, X. Photo-driven pulsating vesicles from self-assembled lipid-like azopolymers. Soft Matter 2011, 7, 11345–11350. [Google Scholar] [CrossRef]
- Pearson, S.; Vitucci, D.; Khine, Y.Y.; Dag, A.; Lu, H.; Save, M.; Billon, L.; Stenzel, M.H. Light-responsive azobenzene-based glycopolymer micelles for targeted drug delivery to melanoma cells. Eur. Polym. J. 2015, 69, 616–627. [Google Scholar] [CrossRef]
- Se, K.; Kijima, M.; Fujimoto, T. Photochemical isomerization of azobenzene incorporated in poly(N,N-dimethyl-4-vinylphenethylamine-block-styrene) diblock copolymer by cross linkage. Polymer 1997, 38, 5755–5760. [Google Scholar] [CrossRef]
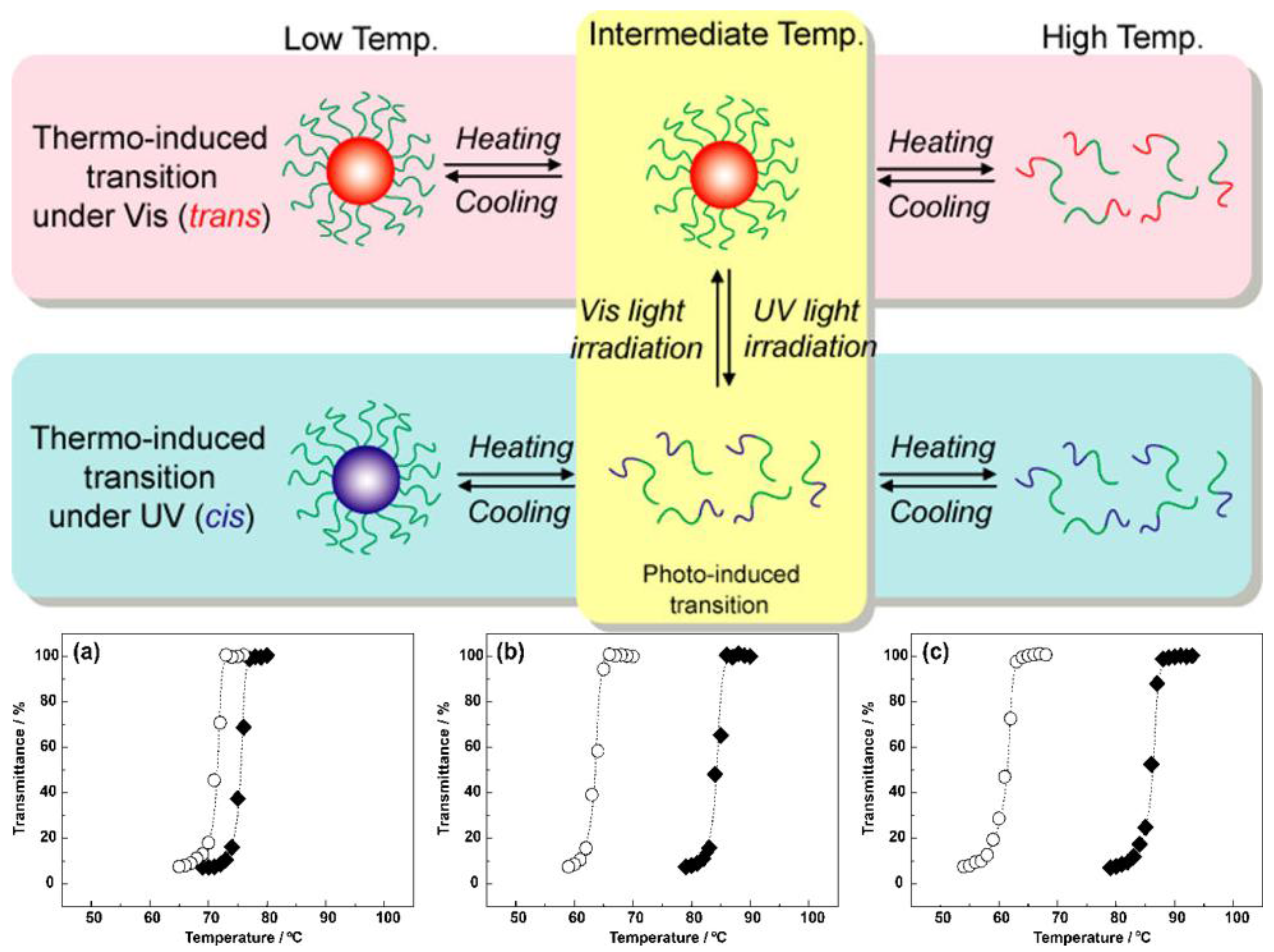
- Ueki, T.; Nakamura, Y.; Lodge, T.P.; Watanabe, M. Light-Controlled Reversible Micellization of a Diblock Copolymer in an Ionic Liquid. Macromolecules 2012, 45, 7566–7573. [Google Scholar] [CrossRef]
- Concellón, A.; Blasco, E.; Martínez-Felipe, A.; Martínez, J.C.; Šics, I.; Ezquerra, T.A.; Nogales, A.; Piñol, M.; Oriol, L. Light-Responsive Self-Assembled Materials by Supramolecular Post-Functionalization via Hydrogen Bonding of Amphiphilic Block Copolymers. Macromolecules 2016, 49, 7825–7836. [Google Scholar] [CrossRef]
- Concellón, A.; Clavería-Gimeno, R.; Velázquez-Campoy, A.; Abian, O.; Piñol, M.; Oriol, L. Polymeric micelles from block copolymers containing 2,6-diacylaminopyridine units for encapsulation of hydrophobic drugs. RSC Adv. 2016, 6, 24066–24075. [Google Scholar] [CrossRef]
- Dilthey, W.; Berres, C. Die Halochromie acylierter Aminochalkone und verwandter Verbindungen. (Heteropolare Kohlenstoffverbindungen. II. J. Prakt. Chem. 1926, 112, 299–313. [Google Scholar] [CrossRef]
- Löwenbein, A.; Katz, W. Über substituiertespiro-Dibenzopyrane. Ber. Dtsch. Chem. Ges. 1926, 59, 1377–1383. [Google Scholar] [CrossRef]
- Koelsch, C.F. Steric Factors in Thermochromism of Spiropyrans and in Reactivities of Certain Methylene Groups. J. Org. Chem. 1951, 16, 1362–1370. [Google Scholar] [CrossRef]
- Hirshberg, Y.; Fischer, E. Photochromism and reversible multiple internal transitions in some spiroPyrans at low temperatures. Part II. J. Chem. Soc. 1954, 3129–3137. [Google Scholar] [CrossRef]
- Raymo, F.M.; Giordani, S. Signal Processing at the Molecular Level. J. Am. Chem. Soc. 2001, 123, 4651–4652. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Neckers, D.C. Benzospiropyrans as photochromic and/or thermochromic photoinitiators. Chem. Mater. 1991, 3, 852–858. [Google Scholar] [CrossRef]
- Balmond, E.I.; Tautges, B.K.; Faulkner, A.L.; Or, V.W.; Hodur, B.M.; Shaw, J.T.; Louie, A.Y. Comparative Evaluation of Substituent Effect on the Photochromic Properties of Spiropyrans and Spirooxazines. J. Org. Chem. 2016, 81, 8744–8758. [Google Scholar] [CrossRef] [PubMed]
- Smets, G. Photochromic behaviour of polymeric systems and related phenomena. Pure Appl. Chem. 1972, 30, 1–24. [Google Scholar] [CrossRef]
- Krongauz, V.A.; Goldburt, E.S. Crystallization of poly(spiropyran methacrylate) with cooperative spiropyran-merocyanine conversion. Macromolecules 1981, 14, 1382–1386. [Google Scholar] [CrossRef]
- Gonzalez-De Los Santos, E.A.; Lozano-Gonzalez, M.J.; Johnson, A.F. Photoresponsive polyurethane-acrylate block copolymers. I. Photochromic effects in copolymers containing 6′-nitro spiropyranes and 6′-nitro-bis-spiropyranes. J. Appl. Polym. Sci. 1999, 71, 259–266. [Google Scholar] [CrossRef]
- Lee, H.I.; Wu, W.; Oh, J.K.; Mueller, L.; Sherwood, G.; Peteanu, L.; Kowalewski, T.; Matyjaszewski, K. Light-induced reversible formation of polymeric micelles. Angew. Chem. Int. Ed. 2007, 46, 2453–2457. [Google Scholar] [CrossRef] [PubMed]
- Kotharangannagari, V.K.; Sánchez-Ferrer, A.; Ruokolainen, J.; Mezzenga, R. Photoresponsive Reversible Aggregation and Dissolution of Rod–Coil Polypeptide Diblock Copolymers. Macromolecules 2011, 44, 4569–4573. [Google Scholar] [CrossRef]
- Berman, E.; Fox, R.E.; Thomson, F.D. Photochromic Spiropyrans. I. The Effect of Substituents on the Rate of Ring Closure. J. Am. Chem. Soc. 1959, 81, 5605–5608. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Pang, M.; Zhang, W. Synthesis and micellization of multi-stimuli responsive block copolymer based on spiropyran. Polym. Chem. 2016. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liu, G.; Tian, J.; Wang, H.; Gong, M.; Liu, S. Reversibly Switching Bilayer Permeability and Release Modules of Photochromic Polymersomes Stabilized by Cooperative Noncovalent Interactions. J. Am. Chem. Soc. 2015, 137, 15262–15275. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, F.; Wu, S.; Chen, Q.; Tong, Z. A core-shell nanoparticle approach to photoreversible fluorescence modulation of a hydrophobic dye in aqueous media. Chem. Eur. J. 2008, 14, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Guragain, S.; Bastakoti, B.P.; Ito, M.; Yusa, S.-I.; Nakashima, K. Aqueous polymeric micelles of poly[N-isopropylacrylamide-b-sodium 2-(acrylamido)-2-methylpropanesulfonate] with a spiropyran dimer pendant: Quadruple stimuli-responsiveness. Soft Matter 2012, 8, 9628. [Google Scholar] [CrossRef]
- Menon, S.; Ongungal, R.M.; Das, S. Photocleavable glycopolymer aggregates. Polym. Chem. 2013, 4, 623–628. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, G.; Ji, J. Micelles and reverse micelles with a photo and thermo double-responsive block copolymer. J. Polym. Sci. A 2010, 48, 2855–2861. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, L.; Guo, A. Diblock Copolymer Nanofibers. Macromolecules 1996, 29, 5508–5510. [Google Scholar] [CrossRef]
- Lendlein, A.; Jiang, H.; Junger, O.; Langer, R. Light-induced shape-memory polymers. Nat. Chem. 2005, 434, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Cheng, Y.; Wei, J. Coumarin-modified fluorescent microcapsules and their photo-switchable release property. Colloids Surf. A 2017, 522, 28–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, Y.; Zhang, P.; Müller, A.H.E.; Zhang, W. Hollow Polymeric Capsules from POSS-Based Block Copolymer for Photodynamic Therapy. Macromolecules 2016, 49, 8440–8448. [Google Scholar] [CrossRef]
- Jensen, A.I.; Binderup, T.; Kumar, E.P.; Kjaer, A.; Rasmussen, P.H.; Andresen, T.L. Positron emission tomography based analysis of long-circulating cross-linked triblock polymeric micelles in a U87MG mouse xenograft model and comparison of DOTA and CB-TE2A as chelators of copper-64. Biomacromolecules 2014, 15, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Jin, C.; Sun, X. Synthesis, properties, and light-induced shape memory effect of multiblock polyesterurethanes containing biodegradable segments and pendant cinnamamide groups. Biomacromolecules 2011, 12, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qi, B.; Lepage, M.; Zhao, Y. Polymer Micelles Stabilization on Demand through Reversible Photo-Cross-Linking. Macromolecules 2007, 40, 790–792. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, G.; Liu, Z.; Liu, Z.; Jiang, J. Amphiphilic Imbalance and Stabilization of Block Copolymer Micelles on-Demand through Combinational Photo-Cleavage and Photo-Crosslinking. Macromol. Rapid Commun. 2016, 38, 1600543. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; He, M.J.; Deng, X.Y.; Du, L.; Fan, C.J.; Yang, K.K.; Wang, Y.Z. Design of Poly(l-lactide)-Poly(ethylene glycol) Copolymer with Light-Induced Shape-Memory Effect Triggered by Pendant Anthracene Groups. ACS Appl. Mater. Interfaces 2016, 8, 9431–9439. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, M.; Ishikawa, T.; Koyama, Y.; Sakuma, K.; Iwamura, H. 1-Pyrenylmethyl esters, photolabile protecting groups for carboxylic acids. Tetrahedron Lett. 1987, 28, 679–682. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Plant, P.J.; Schofield, P. Photosensitive protective groups. Chem. Commun. 1966, 822–823. [Google Scholar] [CrossRef]
- Furuta, T.; Torigai, H.; Sugimoto, M.; Iwamura, M. Photochemical Properties of New Photolabile cAMP Derivatives in a Physiological Saline Solution. J. Org. Chem. 1995, 60, 3953–3956. [Google Scholar] [CrossRef]
- Sheehan, J.C.; Umezawa, K. Phenacyl photosensitive blocking groups. J. Org. Chem. 1973, 38, 3771–3774. [Google Scholar] [CrossRef]
- Wuts, P.G.M. Greene’s Protective Groups in Organic Synthesis; Wuts, P.G.M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1203–1262. [Google Scholar]
- Jiang, J.; Tong, X.; Zhao, Y. A New Design for Light-Breakable Polymer Micelles. J. Am. Chem. Soc. 2005, 127, 8290–8291. [Google Scholar] [CrossRef]
- Jiang, J.; Tong, X.; Morris, D.; Zhao, Y. Toward Photocontrolled Release Using Light-Dissociable Block Copolymer Micelles. Macromolecules 2006, 39, 4633–4640. [Google Scholar] [CrossRef]
- Lee, J.-E.; Ahn, E.; Bak, J.M.; Jung, S.-H.; Park, J.M.; Kim, B.-S.; Lee, H.-I. Polymeric micelles based on photocleavable linkers tethered with a model drug. Polymer 2014, 55, 1436–1442. [Google Scholar] [CrossRef]
- Liu, X.; He, J.; Niu, Y.; Li, Y.; Hu, D.; Xia, X.; Lu, Y.; Xu, W. Photo-responsive amphiphilic poly(α-hydroxy acids) with pendent o-nitrobenzyl ester constructed via copper-catalyzed azide-alkyne cycloaddition reaction. Polym. Adv. Technol. 2015, 26, 449–456. [Google Scholar] [CrossRef]
- Song, Z.; Kim, H.; Ba, X.; Baumgartner, R.; Lee, J.S.; Tang, H.; Leal, C.; Cheng, J. Polypeptide vesicles with densely packed multilayer membranes. Soft Matter 2015, 11, 4091–4098. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bettinger, C.J. Photoreconfigurable Physically Cross-Linked Triblock Copolymer Hydrogels: Photodisintegration Kinetics and Structure–Property Relationships. Macromolecules 2015, 48, 1563–1572. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Hu, J.; Zhang, G.; Liu, S. Concurrent Block Copolymer Polymersome Stabilization and Bilayer Permeabilization by Stimuli-Regulated “Traceless” Crosslinking. Angew. Chem. Int. Ed. 2014, 53, 3138–3142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Y.; Liu, T.; Zhang, G.; Liu, S. Light-Triggered Concomitant Enhancement of Magnetic Resonance Imaging Contrast Performance and Drug Release Rate of Functionalized Amphiphilic Diblock Copolymer Micelles. Biomacromolecules 2012, 13, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hu, X.; Chen, X.; Mo, G.; Sun, J.; Jing, X. A Novel Biodegradable and Light-Breakable Diblock Copolymer Micelle for Drug Delivery. Adv. Eng. Mater. 2009, 11, B7–B11. [Google Scholar] [CrossRef]
- Greco, C.T.; Epps, T.H.; Sullivan, M.O. Mechanistic Design of Polymer Nanocarriers to Spatiotemporally Control Gene Silencing. ACS Biomater. Sci. Eng. 2016, 2, 1582–1594. [Google Scholar] [CrossRef]
- Gupta, M.K.; Balikov, D.A.; Lee, Y.; Ko, E.; Yu, C.; Chun, Y.W.; Sawyer, D.B.; Kim, W.S.; Sung, H.-J. Gradient release of cardiac morphogens by photo-responsive polymer micelles for gradient-mediated variation of embryoid body differentiation. J. Mater. Chem. B 2017, 5, 2019–2033. [Google Scholar] [CrossRef]
- Bertrand, O.; Gohy, J.-F.; Fustin, C.-A. Synthesis of diblock copolymers bearing p-methoxyphenacyl side groups. Polym. Chem. 2011, 2, 2284–2292. [Google Scholar] [CrossRef]
- Schumers, J.-M.; Bertrand, O.; Fustin, C.-A.; Gohy, J.-F. Synthesis and self-assembly of diblock copolymers bearing 2-nitrobenzyl photocleavable side groups. J. Polym. Sci. A 2012, 50, 599–608. [Google Scholar] [CrossRef]
- Song, D.-P.; Wang, X.; Lin, Y.; Watkins, J.J. Synthesis and Controlled Self-Assembly of UV-Responsive Gold Nanoparticles in Block Copolymer Templates. J. Phys. Chem. B 2014, 118, 12788–12795. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.A.; Nouvel, C.; Babin, J.; Six, J.-L. o-Nitrobenzyl acrylate is polymerizable by single electron transfer-living radical polymerization. J. Polym. Sci. A 2014, 52, 2192–2201. [Google Scholar] [CrossRef]
- Jana, S.; Saha, A.; Paira, T.K.; Mandal, T.K. Synthesis and Self-Aggregation of Poly(2-ethyl-2-oxazoline)-Based Photocleavable Block Copolymer: Micelle, Compound Micelle, Reverse Micelle, and Dye Encapsulation/Release. J. Phys. Chem. B 2016, 120, 813–824. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, B.; Riordon, J.; Zhao, Y.; Sinton, D.; Moffitt, M.G. Microfluidic Synthesis of Photoresponsive Spool-Like Block Copolymer Nanoparticles: Flow-Directed Formation and Light-Triggered Dissociation. Chem. Mater. 2015, 27, 8094–8104. [Google Scholar] [CrossRef]
- Liu, G.; Dong, C.-M. Photoresponsive Poly(S-(o-nitrobenzyl)-l-cysteine)-b-PEO from a l-Cysteine N-Carboxyanhydride Monomer: Synthesis, Self-Assembly, and Phototriggered Drug Release. Biomacromolecules 2012, 13, 1573–1583. [Google Scholar] [CrossRef]
- Jiang, X.; Lavender, C.A.; Woodcock, J.W.; Zhao, B. Multiple Micellization and Dissociation Transitions of Thermo- and Light-Sensitive Poly(ethylene oxide)-b-poly(ethoxytri(ethylene glycol) acrylate-co-o-nitrobenzyl acrylate) in Water. Macromolecules 2008, 41, 2632–2643. [Google Scholar] [CrossRef]
- Yuan, W.; Guo, W. Ultraviolet light-breakable and tunable thermoresponsive amphiphilic block copolymer: From self-assembly, disassembly to re-self-assembly. Polym. Chem. 2014, 5, 4259–4267. [Google Scholar] [CrossRef]
- Yang, F.; Cao, Z.; Wang, G. Micellar assembly of a photo- and temperature-responsive amphiphilic block copolymer for controlled release. Polym. Chem. 2015, 6, 7995–8002. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, S.; Zhong, Q.; Dadmun, M.D.; Zhao, B. Stimuli-Induced Multiple Sol−Gel−Sol Transitions of Aqueous Solution of a Thermo- and Light-Sensitive Hydrophilic Block Copolymer. Macromolecules 2009, 42, 8468–8476. [Google Scholar] [CrossRef]
- Yao, C.; Wang, X.; Liu, G.; Hu, J.; Liu, S. Distinct Morphological Transitions of Photoreactive and Thermoresponsive Vesicles for Controlled Release and Nanoreactors. Macromolecules 2016. [Google Scholar] [CrossRef]
- Shrivastava, S.; Matsuoka, H. Photocleavable amphiphilic diblock copolymer micelles bearing a nitrobenzene block. Colloid Polym. Sci. 2016, 294, 879–887. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Lin, Y.-K.; Wang, S.-W.; Li, Y.-C.; Lee, R.-S. Synthesis and characterization of dual-stimuli-responsive micelles based on poly(N-isopropylacrylamide) and polycarbonate with photocleavable moieties. React. Funct. Polym. 2015, 95, 46–54. [Google Scholar] [CrossRef]
- Sun, T.; Li, P.; Oh, J.K. Dual Location Dual Reduction/Photoresponsive Block Copolymer Micelles: Disassembly and Synergistic Release. Macromol. Rapid Commun. 2015, 36, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.; Kuo, Y.-S.; Cheng, C.-H. Dual-stimuli responsive polymeric micelles: Preparation, characterization, and controlled drug release. J. Polym. Res. 2015, 22, 80. [Google Scholar] [CrossRef]
- Jin, Q.; Cai, T.; Wang, Y.; Wang, H.; Ji, J. Light-Responsive Polyion Complex Micelles with Switchable Surface Charge for Efficient Protein Delivery. ACS Macro Lett. 2014, 3, 679–683. [Google Scholar] [CrossRef]
- Kalva, N.; Parekh, N.; Ambade, A.V. Controlled micellar disassembly of photo- and pH-cleavable linear-dendritic block copolymers. Polym. Chem. 2015, 6, 6826–6835. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, H.; Hu, J.; Liu, T.; Zhang, G.; Liu, S. Thermo- and Light-Regulated Formation and Disintegration of Double Hydrophilic Block Copolymer Assemblies with Tunable Fluorescence Emissions. Langmuir 2013, 29, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Ma, X.; Dong, Y.; Qu, F. Light/temperature dual-responsive ABC miktoarm star terpolymer micelles for controlled release. Eur. Polym. J. 2017, 87, 331–343. [Google Scholar] [CrossRef]
- He, L.; Hu, B.; Henn, D.M.; Zhao, B. Influence of cleavage of photosensitive group on thermally induced micellization and gelation of a doubly responsive diblock copolymer in aqueous solutions: A SANS study. Polymer 2016, 105, 25–34. [Google Scholar] [CrossRef]
- Tao, Z.; Peng, K.; Fan, Y.; Liu, Y.; Yang, H. Multi-stimuli responsive supramolecular hydrogels based on Fe3+ and diblock copolymer micelle complexation. Polym. Chem. 2016, 7, 1405–1412. [Google Scholar] [CrossRef]
- Kumar, S.; Dory, Y.L.; Lepage, M.; Zhao, Y. Surface-Grafted Stimuli-Responsive Block Copolymer Brushes for the Thermo-, Photo- and pH-Sensitive Release of Dye Molecules. Macromolecules 2011, 44, 7385–7393. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, G.; Li, X.; Tang, B.; Wei, Z.; Mai, C. Synthesis of multi-responsive polymeric nanocarriers for controlled release of bioactive agents. Polym. Chem. 2013, 4, 4574–4577. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, H.; Dong, J.; Wang, G. Quadruple-Stimuli-Sensitive Polymeric Nanocarriers for Controlled Release under Combined Stimulation. Macromolecules 2014, 47, 8777–8783. [Google Scholar] [CrossRef]
- Han, D.; Tong, X.; Zhao, Y. Fast Photodegradable Block Copolymer Micelles for Burst Release. Macromolecules 2011, 44, 437–439. [Google Scholar] [CrossRef]
- Han, D.; Tong, X.; Zhao, Y. Block Copolymer Micelles with a Dual-Stimuli-Responsive Core for Fast or Slow Degradation. Langmuir 2012, 28, 2327–2331. [Google Scholar] [CrossRef] [PubMed]
- Cabane, E.; Malinova, V.; Meier, W. Synthesis of Photocleavable Amphiphilic Block Copolymers: Toward the Design of Photosensitive Nanocarriers. Macromol. Chem. Phys. 2010, 211, 1847–1856. [Google Scholar] [CrossRef]
- Zhao, H.; Sterner, E.S.; Coughlin, E.B.; Theato, P. o-Nitrobenzyl Alcohol Derivatives: Opportunities in Polymer and Materials Science. Macromolecules 2012, 45, 1723–1736. [Google Scholar] [CrossRef]
- Zhao, H.; Gu, W.; Thielke, M.W.; Sterner, E.; Tsai, T.; Russell, T.P.; Coughlin, E.B.; Theato, P. Functionalized Nanoporous Thin Films and Fibers from Photocleavable Block Copolymers Featuring Activated Esters. Macromolecules 2013, 46, 5195–5201. [Google Scholar] [CrossRef]
- Xuan, J.; Han, D.; Xia, H.; Zhao, Y. Dual-Stimuli-Responsive Micelle of an ABC Triblock Copolymer Bearing a Redox-Cleavable Unit and a Photocleavable Unit at Two Block Junctions. Langmuir 2014, 30, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lv, A.; Deng, X.-X.; Du, F.-S.; Li, Z.-C. Facile synthesis of photo-cleavable polymers via Passerini reaction. Chem. Commun. 2013, 49, 8549–8551. [Google Scholar] [CrossRef] [PubMed]
- Schumers, J.-M.; Gohy, J.-F.; Fustin, C.-A. A versatile strategy for the synthesis of block copolymers bearing a photocleavable junction. Polym. Chem. 2010, 1, 161–163. [Google Scholar] [CrossRef]
- Gungor, E.; Armani, A.M. Photocleavage of Covalently Immobilized Amphiphilic Block Copolymer: From Bilayer to Monolayer. Macromolecules 2016, 49, 5773–5781. [Google Scholar] [CrossRef]
- Lee, R.-S.; Li, Y.-C.; Wang, S.-W. Synthesis and characterization of amphiphilic photocleavable polymers based on dextran and substituted-ε-caprolactone. Carbohyd. Polym. 2015, 117, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Shota, Y.; Hidemi, T.; Shuya, Y.; Seiichi, N.; Kazuo, Y. Synthesis of Amphiphilic Diblock Copolymer Using Heterobifunctional Linkers, Connected by a Photodegradable N-(2-Nitrobenzyl)imide Structure and Available for Two Different Click Chemistries. Bull. Chem. Soc. Jpn. 2016, 89, 481–489. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, H.; Zhou, H.; Li, X.; Harniman, R.; Winnik, M.A.; Manners, I. Crystallization-Driven Solution Self-Assembly of Block Copolymers with a Photocleavable Junction. J. Am. Chem. Soc. 2015, 137, 2203–2206. [Google Scholar] [CrossRef] [PubMed]
- Coumes, F.; Malfait, A.; Bria, M.; Lyskawa, J.; Woisel, P.; Fournier, D. Catechol/boronic acid chemistry for the creation of block copolymers with a multi-stimuli responsive junction. Polym. Chem. 2016, 7, 4682–4692. [Google Scholar] [CrossRef]
- Katz, J.S.; Zhong, S.; Ricart, B.G.; Pochan, D.J.; Hammer, D.A.; Burdick, J.A. Modular Synthesis of Biodegradable Diblock Copolymers for Designing Functional Polymersomes. J. Am. Chem. Soc. 2010, 132, 3654–3655. [Google Scholar] [CrossRef] [PubMed]
- Gamys, C.G.; Schumers, J.-M.; Vlad, A.; Fustin, C.-A.; Gohy, J.-F. Amine-functionalized nanoporous thin films from a poly(ethylene oxide)-block-polystyrene diblock copolymer bearing a photocleavable o-nitrobenzyl carbamate junction. Soft Matter 2012, 8, 4486–4493. [Google Scholar] [CrossRef]
- Zhao, H.; Gu, W.; Sterner, E.; Russell, T.P.; Coughlin, E.B.; Theato, P. Highly Ordered Nanoporous Thin Films from Photocleavable Block Copolymers. Macromolecules 2011, 44, 6433–6440. [Google Scholar] [CrossRef]
- Yang, L.; Lei, M.; Zhao, M.; Yang, H.; Zhang, H.; Li, Y.; Zhang, K.; Lei, Z. Synthesis of the light/pH responsive polymer for immobilization of α-amylase. Mater. Sci. Eng. C 2017, 71, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Babin, J.; Pelletier, M.; Lepage, M.; Allard, J.-F.; Morris, D.; Zhao, Y. A New Two-Photon-Sensitive Block Copolymer Nanocarrier. Angew. Chem. Int. Ed. 2009, 48, 3329–3332. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Mitschang, F.; Agarwal, S. Biocompatible Drug Delivery System for Photo-Triggered Controlled Release of 5-Fluorouracil. Biomacromolecules 2011, 12, 3684–3691. [Google Scholar] [CrossRef]
- Bertrand, O.; Fustin, C.-A.; Gohy, J.-F. Multiresponsive Micellar Systems from Photocleavable Block Copolymers. ACS Macro Lett. 2012, 1, 949–953. [Google Scholar] [CrossRef]
- Guo, A.; Liu, G.; Tao, J. Star Polymers and Nanospheres from Cross-Linkable Diblock Copolymers. Macromolecules 1996, 29, 2487–2493. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Liu, G. Preparation and Application of a Dual Light-Responsive Triblock Terpolymer. Macromolecules 2012, 45, 5586–5595. [Google Scholar] [CrossRef]
- Ding, J.; Liu, G. Hairy, Semi-shaved, and Fully Shaved Hollow Nanospheres from Polyisoprene-block-poly(2-cinnamoylethyl methacrylate). Chem. Mater. 1998, 10, 537–542. [Google Scholar] [CrossRef]
- Yang, H.; Jia, L.; Wang, Z.; Di-Cicco, A.; Lévy, D.; Keller, P. Novel Photolabile Diblock Copolymers Bearing Truxillic Acid Derivative Junctions. Macromolecules 2011, 44, 159–165. [Google Scholar] [CrossRef]
- Chen, C.-J.; Liu, G.-Y.; Shi, Y.-T.; Zhu, C.-S.; Pang, S.-P.; Liu, X.-S.; Ji, J. Biocompatible Micelles Based on Comb-like PEG Derivates: Formation, Characterization, and Photo-responsiveness. Macromol. Rapid Commun. 2011, 32, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Kuwayama, S. Micelle formation induced by photo-Claisen rearrangement of poly(4-allyloxystyrene)-block-polystyrene. Colloid Polym. Sci. 2009, 287, 789–793. [Google Scholar] [CrossRef]
- Ishida, Y.; Takeda, Y.; Kameyama, A. Synthesis of block copolymer with photo-decomposable polyurethane and its photo-initiated domino decomposition. React. Funct. Polym. 2016, 107, 20–27. [Google Scholar] [CrossRef]
- Tian, M.; Cheng, R.; Zhang, J.; Liu, Z.; Liu, Z.; Jiang, J. Amphiphilic Polymer Micellar Disruption Based on Main-Chain Photodegradation. Langmuir 2016, 32, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kang, H.; Leolukman, M.; Nealey, P.F.; Gopalan, P. Synthesis of Photoacid Generator-Containing Patternable Diblock Copolymers by Reversible Addition−Fragmentation Transfer Polymerization. Chem. Mater. 2009, 21, 3030–3032. [Google Scholar] [CrossRef]
- Helmy, S.; Leibfarth, F.A.; Oh, S.; Poelma, J.E.; Hawker, C.J.; Read de Alaniz, J. Photoswitching using visible light: A new class of organic photochromic molecules. J. Am. Chem. Soc. 2014, 136, 8169–8172. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, J. Ueber die Oele, die bei der Einwirkung der Schwefelsäure auf verschiedene Vegetabilien entstehen. Liebigs Ann. Chem. 1850, 74, 278–297. [Google Scholar] [CrossRef]
- Balamurugan, A.; Lee, H.-I. A Visible Light Responsive On–Off Polymeric Photoswitch for the Colorimetric Detection of Nerve Agent Mimics in Solution and in the Vapor Phase. Macromolecules 2016, 49, 2568–2574. [Google Scholar] [CrossRef]
- Sinawang, G.; Wu, B.; Wang, J.; Li, S.; He, Y. Polystyrene Based Visible Light Responsive Polymer with Donor-Acceptor Stenhouse Adduct Pendants. Macromol. Chem. Phys. 2016, 217, 2409–2414. [Google Scholar] [CrossRef]
- Bleger, D.; Hecht, S. Visible-Light-Activated Molecular Switches. Angew. Chem. Int. Ed. 2015, 54, 11338–11349. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Iyoda, T.; Ikeda, T. Photoinduced alignment of nanocylinders by supramolecular cooperative motions. J. Am. Chem. Soc. 2006, 128, 11010–11011. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Parowatkin, M.; Steffen, W.; Butt, H.-J.; Mailänder, V.; Wu, S. Ruthenium-Containing Block Copolymer Assemblies: Red-Light-Responsive Metallopolymers with Tunable Nanostructures for Enhanced Cellular Uptake and Anticancer Phototherapy. Adv. Healthc. Mater. 2016, 5, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Guo, J.; Kim, G.B.; Li, J.; Chen, X.; Yang, J.; Huang, Y. Simultaneously Photo-Cleavable and Activatable Prodrug-Backboned Block Copolymer Micelles for Precise Anticancer Drug Delivery. Adv. Healthc. Mater. 2016, 5, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Hu, J.; Zhou, R.; Ju, Y.; Yin, Y.; Yuan, J. Visible light-responsive micelles formed from dialkoxyanthracene-containing block copolymers. Chem. Commun. 2012, 48, 1913–1915. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, S.; Cao, W.; Li, Y.; Sun, Z.; Wang, Z.; Xu, H. Red light responsive diselenide-containing block copolymer micelles. J. Mater. Chem. B 2013, 1, 740–743. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Lee, J.; Kim, J.; Kim, W.J. Visible light-induced singlet oxygen-mediated intracellular disassembly of polymeric micelles co-loaded with a photosensitizer and an anticancer drug for enhanced photodynamic therapy. Chem. Commun. 2015, 51, 9995–9998. [Google Scholar] [CrossRef]
- Zhai, S.; Hu, X.; Hu, Y.; Wu, B.; Xing, D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials 2017, 121, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Chung, M.; Shim, M.S. Engineered photo-responsive materials for near-infrared-triggered drug delivery. J. Ind. Eng. Chem. 2015, 31, 15–25. [Google Scholar] [CrossRef]
- Bort, G.; Gallavardin, T.; Ogden, D.; Dalko, P.I. From One-Photon to Two-Photon Probes: “Caged” Compounds, Actuators, and Photoswitches. Angew. Chem. Int. Ed. 2013, 52, 4526–4537. [Google Scholar] [CrossRef] [PubMed]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, S.; Chen, Y.; Li, S.; Li, X.; Deng, D.; Qian, Z.; Tang, L.; Gu, Y. Near-infrared light-triggered micelles for fast controlled drug release in deep tissue. Biomaterials 2013, 34, 6272–6283. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, D.; Huang, S.; Deng, D.; Gu, Y.; Tang, L. Multifunctional near-infrared light-triggered biodegradable micelles for chemo- and photo-thermal combination therapy. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Aujard, I.; Benbrahim, C.; Gouget, M.; Ruel, O.; Baudin, J.-B.; Neveu, P.; Jullien, L. o-Nitrobenzyl Photolabile Protecting Groups with Red-Shifted Absorption: Syntheses and Uncaging Cross-Sections for One- and Two-Photon Excitation. Chem. Eur. J. 2006, 12, 6865–6879. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Allard, J.-F.; Morris, D.; Dory, Y.L.; Lepage, M.; Zhao, Y. Near-infrared light sensitive polypeptide block copolymer micelles for drug delivery. J. Mater. Chem. 2012, 22, 7252–7257. [Google Scholar] [CrossRef]
- Ji, W.; Li, N.; Chen, D.; Qi, X.; Sha, W.; Jiao, Y.; Xu, Q.; Lu, J. Coumarin-containing photo-responsive nanocomposites for NIR light-triggered controlled drug release via a two-photon process. J. Mater. Chem. B 2013, 1, 5942. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Cao, W.; Wang, L.; Xu, H. Near-infrared light stimuli-responsive synergistic therapy nanoplatforms based on the coordination of tellurium-containing block polymer and cisplatin for cancer treatment. Biomaterials 2017, 133, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Carling, C.-J.; Boyer, J.-C.; Branda, N.R. Remote-Control Photoswitching Using NIR Light. J. Am. Chem. Soc. 2009, 131, 10838–10839. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Boyer, J.-C.; Branda, N.R.; Zhao, Y. Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. J. Am. Chem. Soc. 2011, 133, 19714–19717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yao, R.; Ding, B.; Shen, Y.; Shui, S.; Wang, L.; Li, Y.; Yang, X.; Tao, W. Fabrication of Upconverting Hybrid Nanoparticles for Near-Infrared Light Triggered Drug Release. Adv. Mater. Sci. Eng. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Li, S.; He, Y. NIR light and enzyme dual stimuli-responsive amphiphilic diblock copolymer assemblies. J. Polym. Sci. A Polym. 2017, 55, 2450–2457. [Google Scholar] [CrossRef]
- Wu, S.; Butt, H.-J. Near-Infrared-Sensitive Materials Based on Upconverting Nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, N.; Xu, T.; Wang, D.; Ren, L.; Guo, X. Core-shell hybrid upconversion nanoparticles carrying stable nitroxide radicals as potential multifunctional nanoprobes for upconversion luminescence and magnetic resonance dual-modality imaging. Nanoscale 2015, 7, 5249–5261. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, Y.; Cao, Z.; Wu, B.; Wang, L.; Wang, H.; Dang, Z.; Wang, G. Nanocomposites of Spiropyran-Functionalized Polymers and Upconversion Nanoparticles for Controlled Release Stimulated by Near-Infrared Light and pH. Macromolecules 2016, 49, 7490–7496. [Google Scholar] [CrossRef]
- Xing, Q.; Li, N.; Jiao, Y.; Chen, D.; Xu, J.; Xu, Q.; Lu, J. Near-infrared light-controlled drug release and cancer therapy with polymer-caged upconversion nanoparticles. RSC Adv. 2015, 5, 5269–5276. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimm, O.; Wendler, F.; Schacher, F.H. Micellization of Photo-Responsive Block Copolymers. Polymers 2017, 9, 396. https://doi.org/10.3390/polym9090396
Grimm O, Wendler F, Schacher FH. Micellization of Photo-Responsive Block Copolymers. Polymers. 2017; 9(9):396. https://doi.org/10.3390/polym9090396
Chicago/Turabian StyleGrimm, Oliver, Felix Wendler, and Felix H. Schacher. 2017. "Micellization of Photo-Responsive Block Copolymers" Polymers 9, no. 9: 396. https://doi.org/10.3390/polym9090396
APA StyleGrimm, O., Wendler, F., & Schacher, F. H. (2017). Micellization of Photo-Responsive Block Copolymers. Polymers, 9(9), 396. https://doi.org/10.3390/polym9090396