Melt-Processable Semicrystalline Polyimides Based on 1,4-Bis(3,4-dicarboxyphenoxy)benzene Dianhydride (HQDPA): Synthesis, Crystallization, and Melting Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
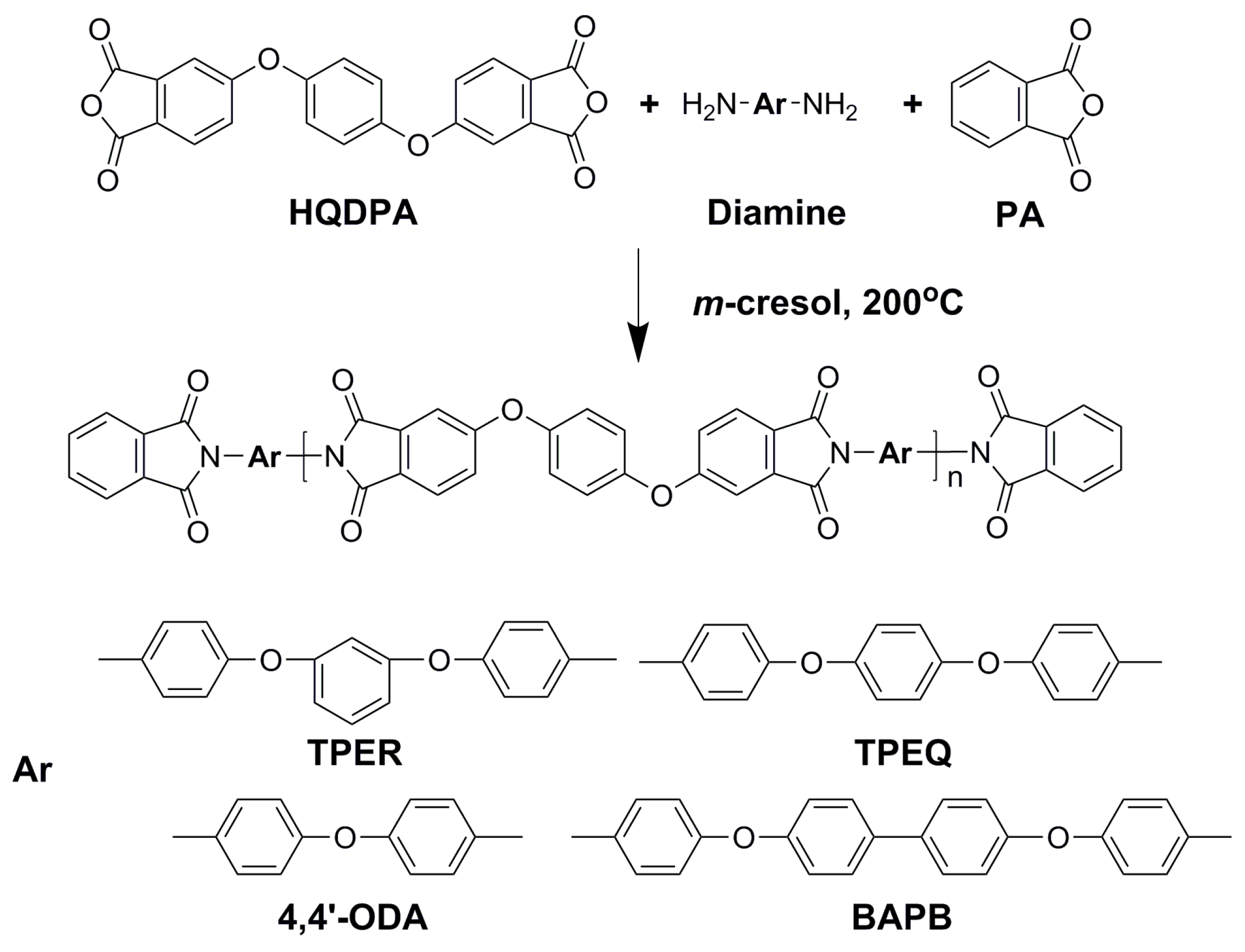
2.2. Synthesis of Polymers
2.3. Polymer Film Preparation
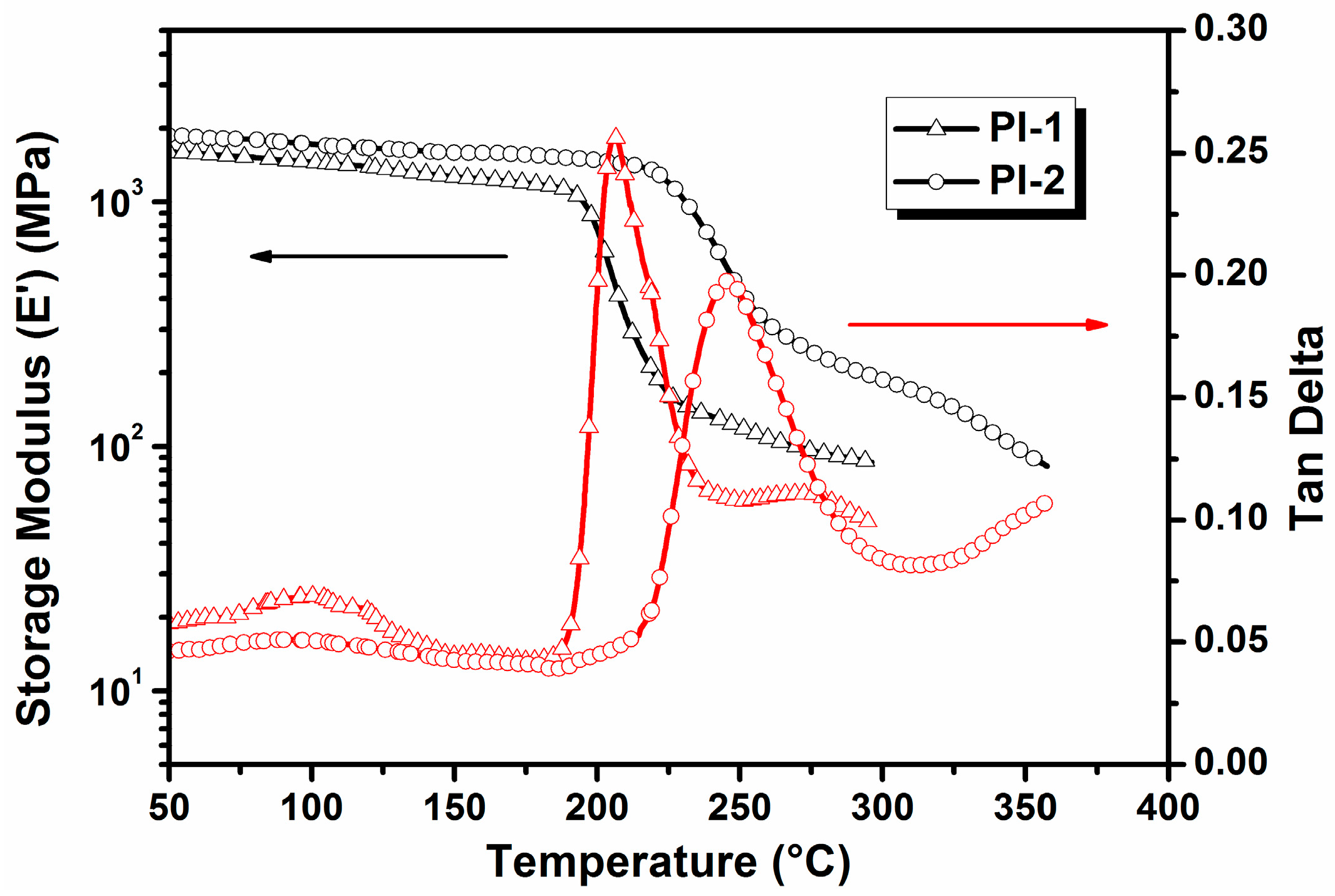
2.4. Measurements
3. Results and Discussion
3.1. Polymer Synthesis
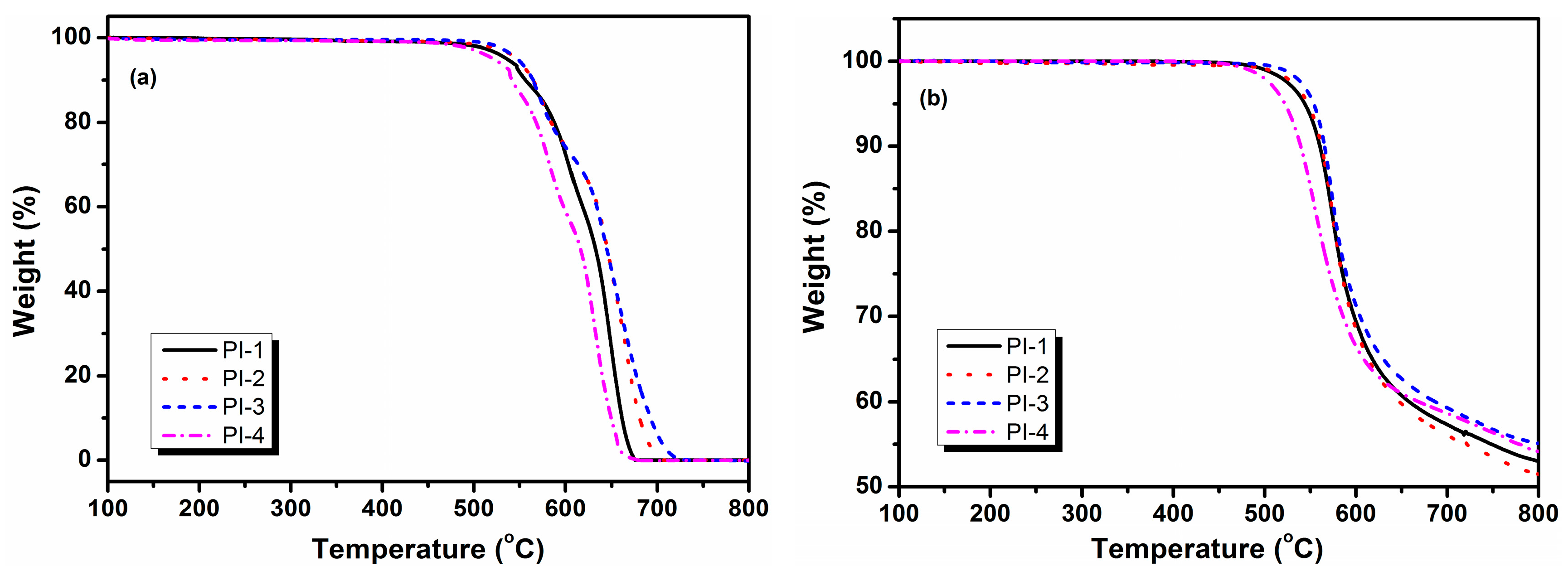
3.2. Thermal Stability
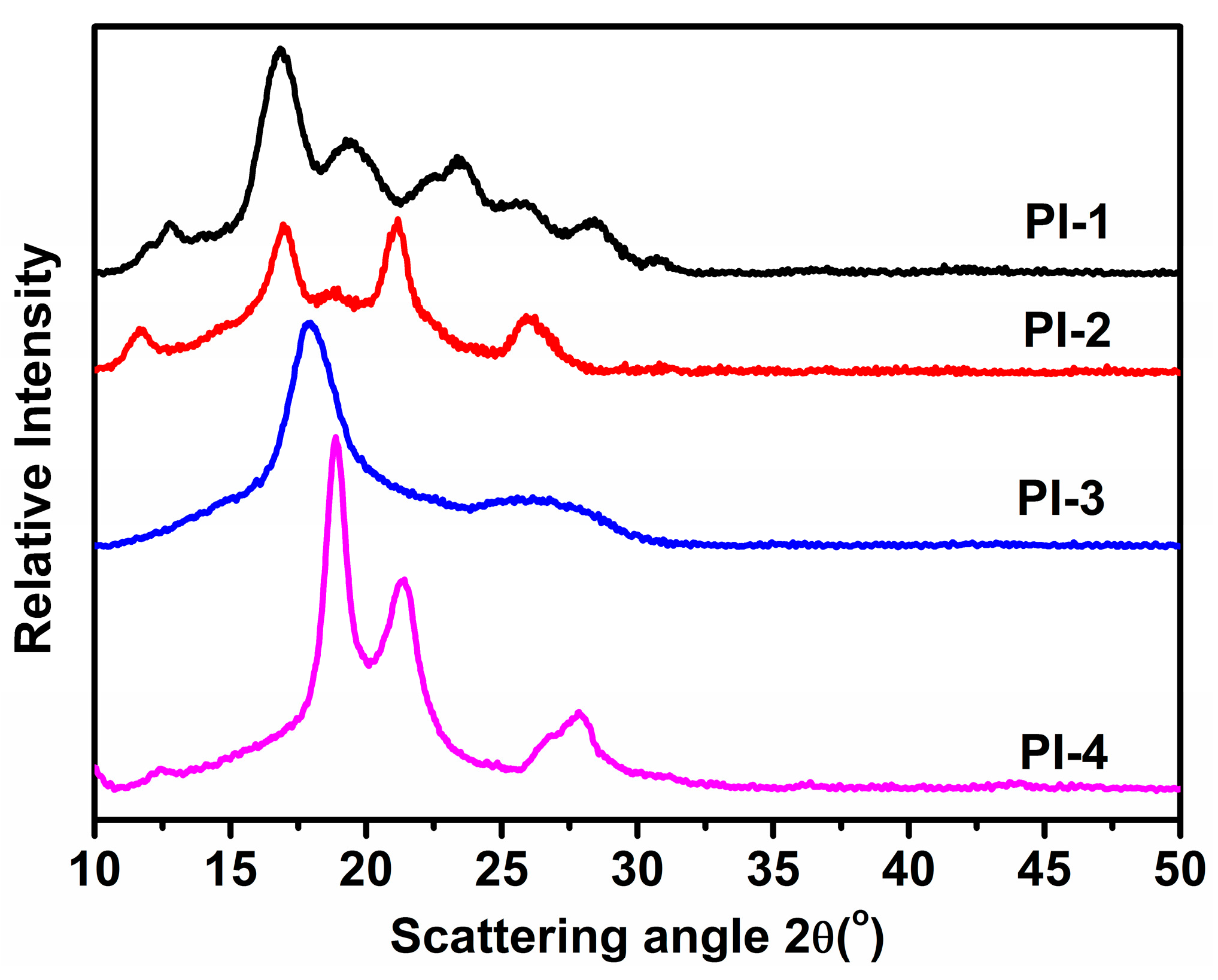
3.3. Wide-Angle X-ray Diffraction
3.4. Melting Behavior
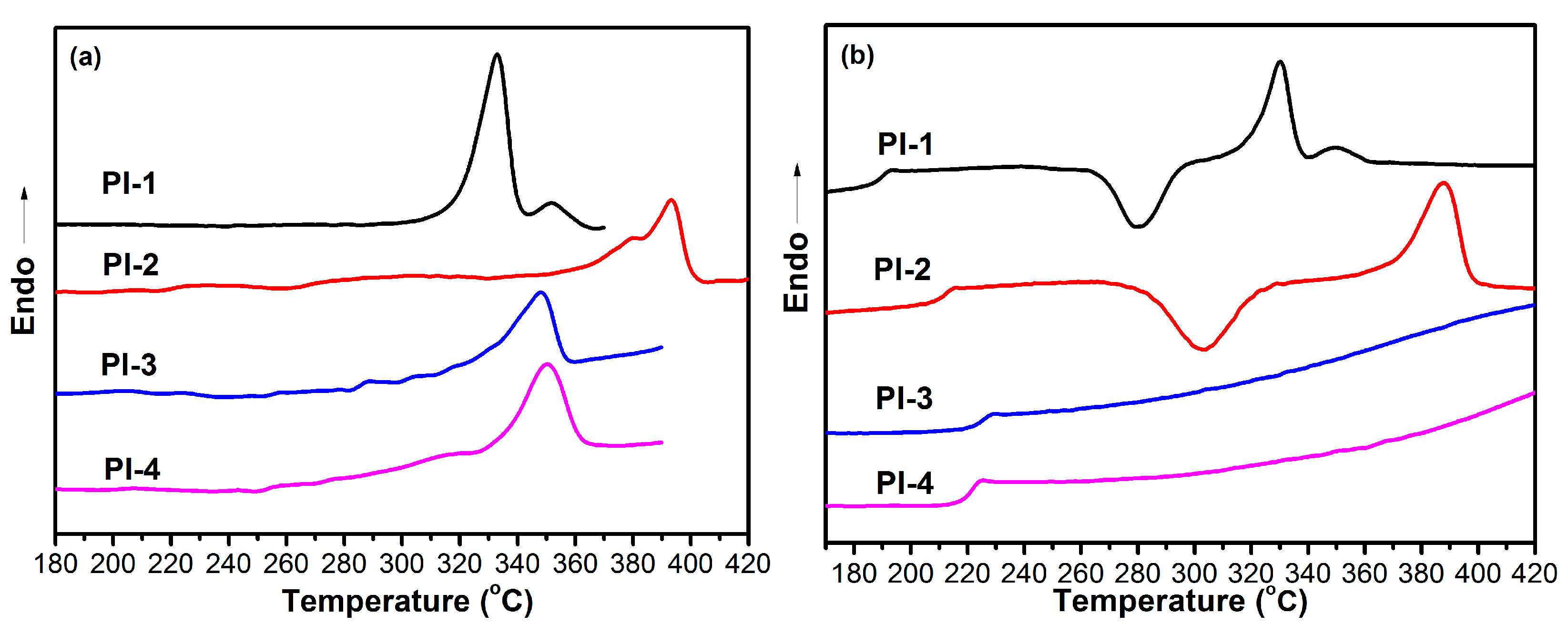
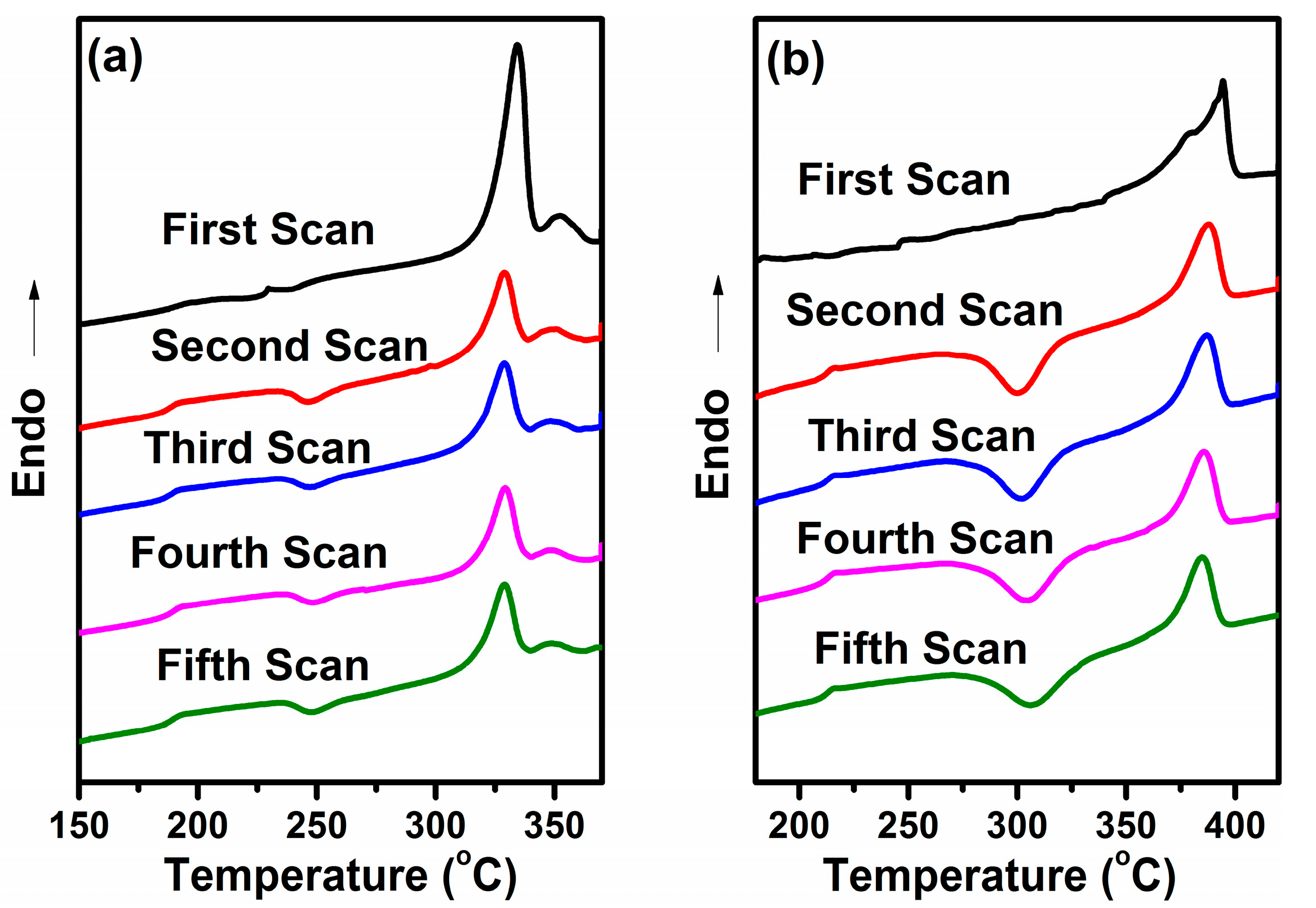
3.4.1. Initial Melting Behavior
3.4.2. Second Melting Behavior
3.4.3. Repeated Melting Behavior
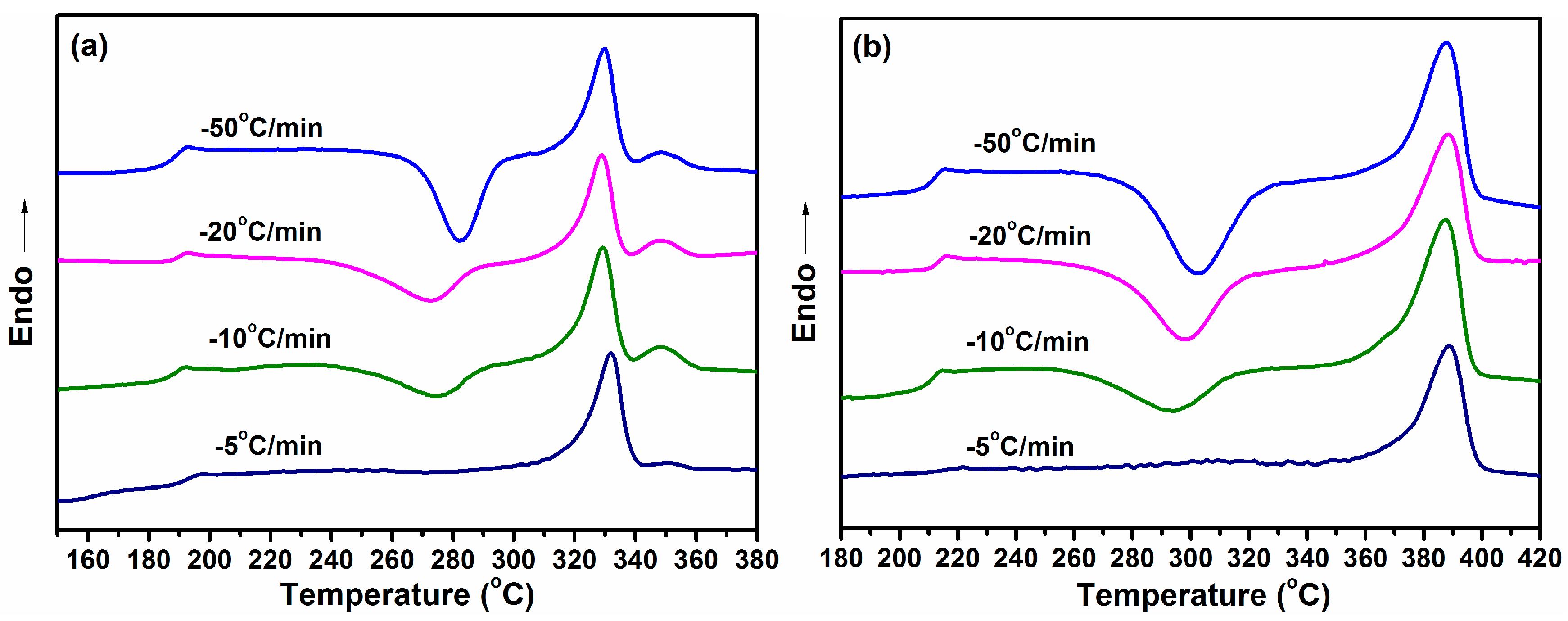
3.5. Nonisothermal Crystallization Behavior
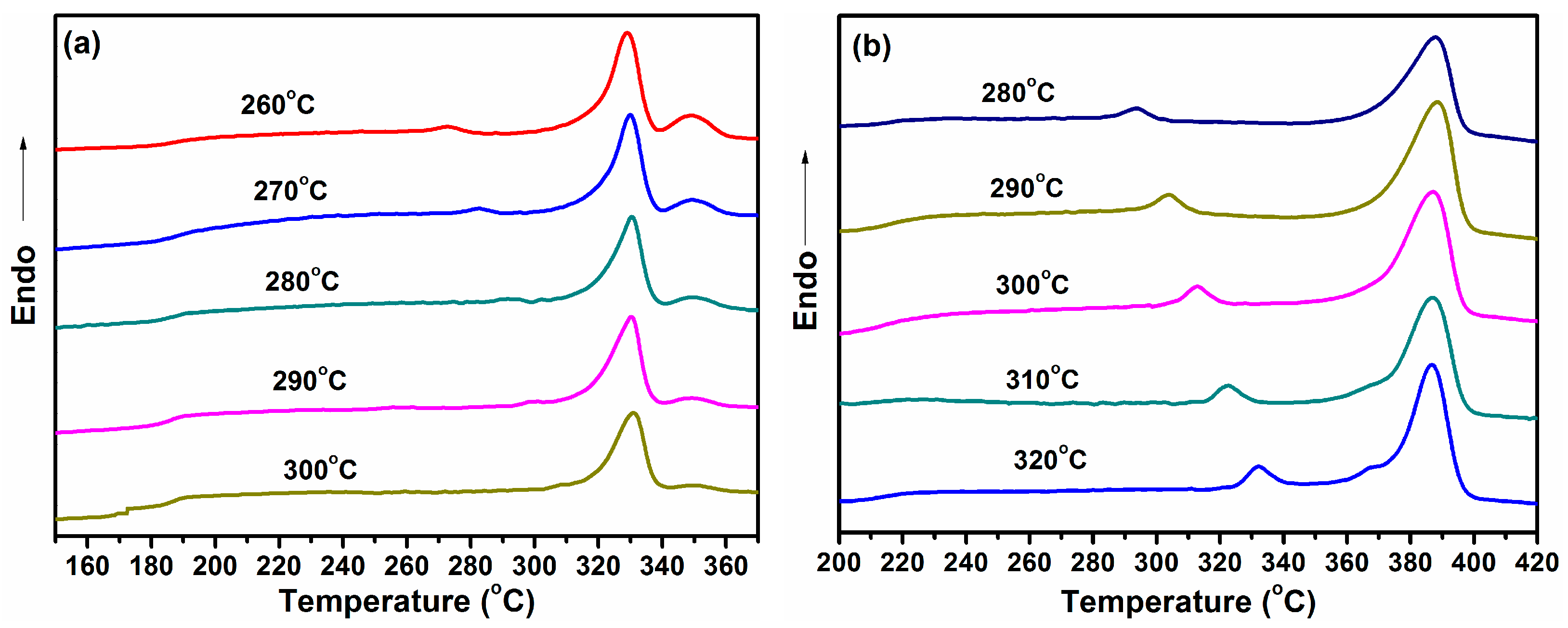
3.6. Isothermal Crystallization Behavior
3.6.1. Equilibrium Melting Temperatures
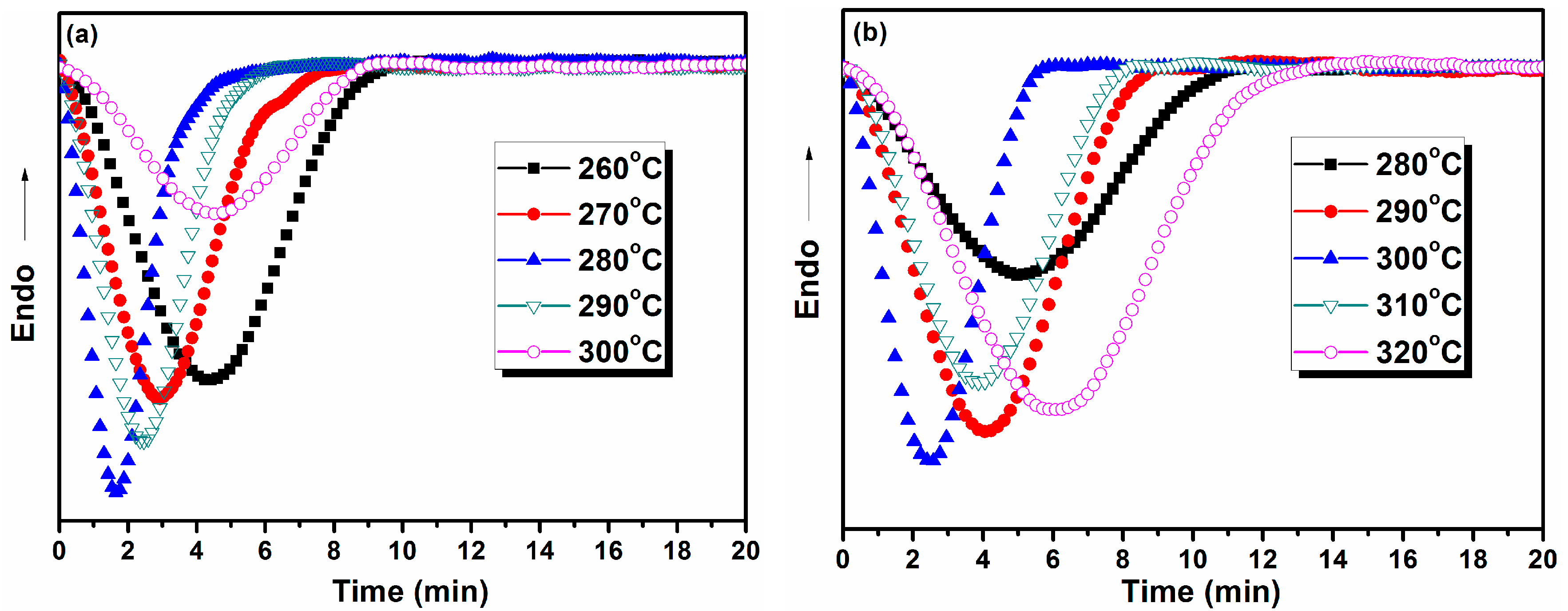
3.6.2. Isothermal Crystallization Kinetics
3.6.3. Comparison with Other Semicrystalline Polymers
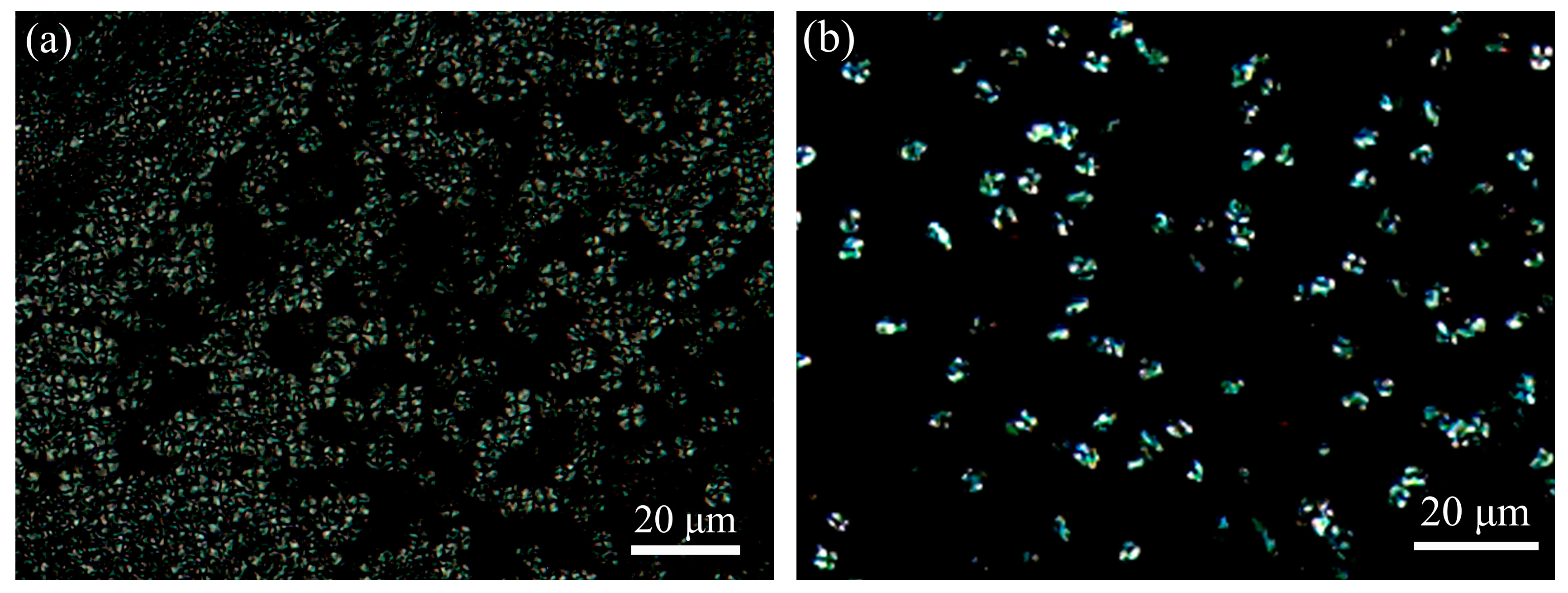
3.7. Crystalline Morphology
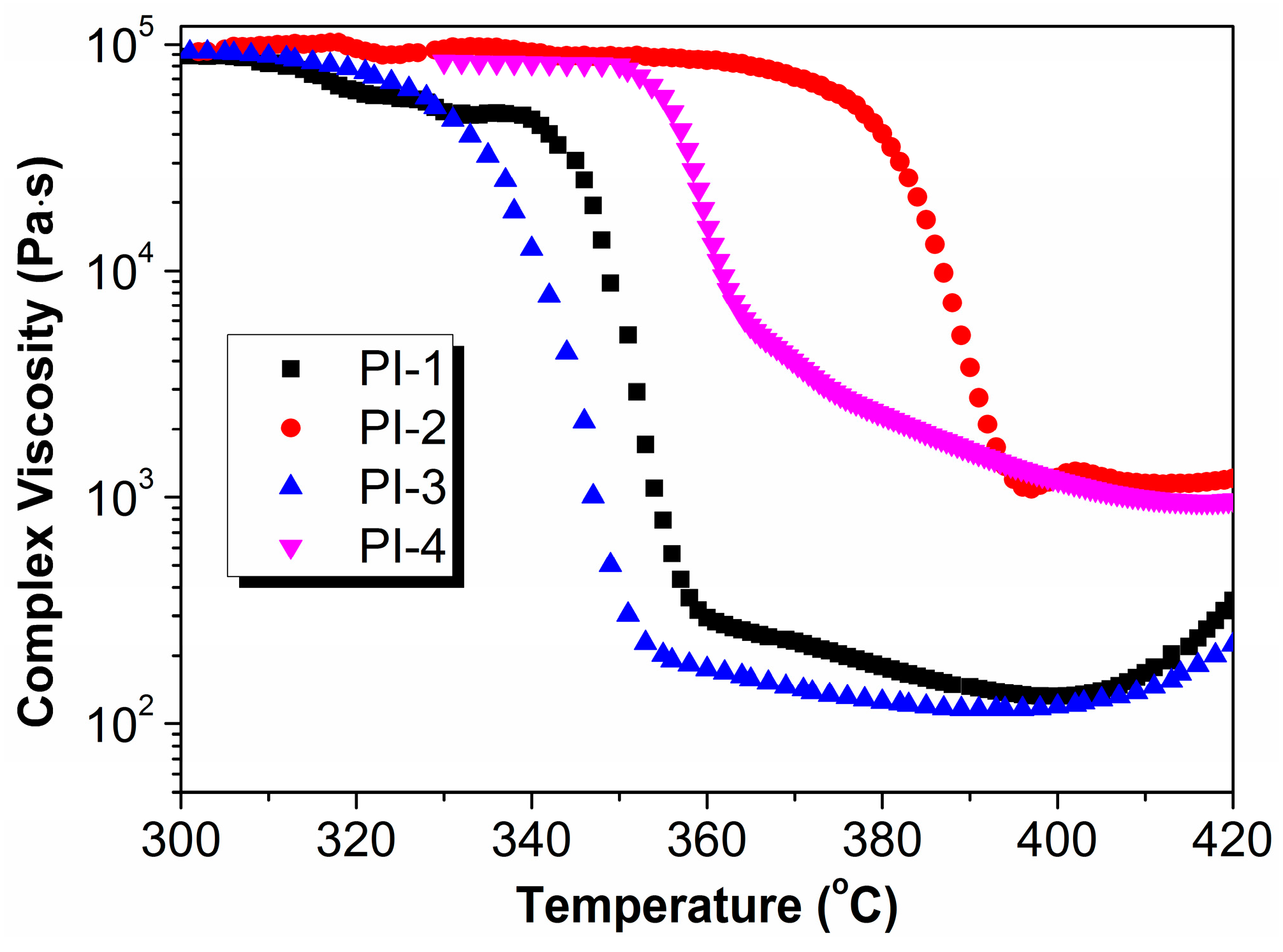
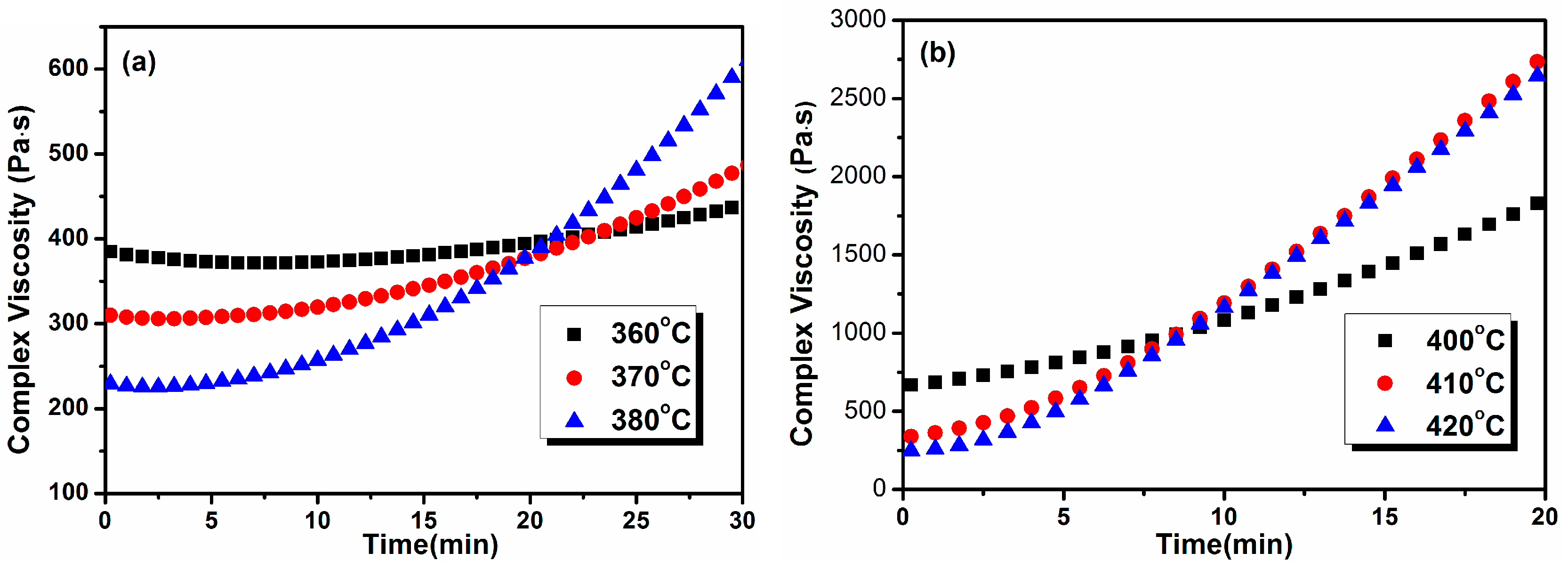
3.8. Melt Processability
3.9. Mechanical Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mittal, K.L. Polyimides: Synthesis, Characterization, and Applications; Plenum: New York, NY, USA, 1984; pp. 149–161. ISBN 978-1-4615-7639-6. [Google Scholar]
- Wilson, D.; Stenzenberger, H.D.; Hergenrother, P.M. Polyimides; Blackie & Son Ltd.: Glasgow, UK, 1990; pp. 58–78. ISBN 978-94-010-9663-8. [Google Scholar]
- DeAbajo, J.; DelaCampa, J.G. Processable aromatic polyimides. In Progress in Polyimide Chemistry I; Kricheldorf, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 140, pp. 23–59. ISBN 978-3-540-64962-5. [Google Scholar]
- Tamai, S.; Yamaguchi, A.; Ohta, M. Melt processible polyimides and their chemical structures. Polymer 1996, 37, 3683–3692. [Google Scholar] [CrossRef]
- Hou, T.H.; Bai, J.M. Thermal and rheological properties of LARC-TPI 1500 series polymers. High Perform. Polym. 1989, 1, 191–211. [Google Scholar] [CrossRef]
- Sokolova, M.; Smirnov, M.; Geydt, P.; Bugrov, A.; Ovaska, S.S.; Lahderanta, E.; Toikka, A. Structure and transport properties of mixed-matrix membranes based on polyimides with ZrO2 nanostars. Polymers 2016, 8, 403. [Google Scholar] [CrossRef]
- Pei, X.L.; Chen, G.F.; Liu, J.T.; Fang, X.Z. Influence of crystalline polyimide hard block on the properties of poly(imide siloxane) copolymers. Polymer 2015, 56, 229–236. [Google Scholar] [CrossRef]
- Falkovich, S.G.; Larin, S.V.; Lyulin, A.V.; Yudin, V.E.; Kenny, J.M.; Lyulin, S.V. Influence of the carbon nanofiller surface curvature on the initiation of crystallization in thermoplastic polymers. RSC Adv. 2014, 4, 48606–48612. [Google Scholar] [CrossRef]
- Wang, W.; Wang, D.M.; Jia, H.; Liu, C.W.; Zhao, X.G.; Chen, C.H. Effect of copolymerization on crystallization kinetics of semicrystalline polyimides. J. Appl. Polym. Sci. 2013, 127, 4601–4609. [Google Scholar] [CrossRef]
- Wang, W.; Wang, D.M.; Jing, J.; Li, Q.M.; Jia, H.; Zhao, X.G.; Chen, C.H. Effect of monomer composition on properties of copolyimides derived from 3,3′,4,4′-biphenyltetracarboxylic dianhydride/4,4′-oxydianiline/1,3-bis (4-aminophenoxy)benzene. Polym. Int. 2012, 61, 516–523. [Google Scholar] [CrossRef]
- Chao, M.; Kou, K.C.; Wu, G.L.; Zhang, J.Q.; Li, N.; Zhang, D.N. Synthesis and properties of semicrystalline copolyimides based on 4,4′-diaminodiphenylether and 1,3-bis(4-aminophenoxy) benzene. J. Macromol. Sci. B. 2012, 51, 2003–2014. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Zhang, M.; Men, Y.F.; Ding, M.X.; Jiang, W. Crystallization behavior of a thermoplastic polyimide derived from 3,3′,4,4′-oxydiphthalic dianhydride and 4,4′-oxydianiline. J. Appl. Polym. Sci. 2008, 108, 1893–1900. [Google Scholar] [CrossRef]
- Song, N.H.; Yao, D.J.; Wang, Z.Y.; Sundararajan, P.R. A unique crystallization and double melting behavior of a polyimide derived from 3,3′,4,4′-biphenyltetracarboxylic dianhydride and 1,4-bis(3-aminopropyl)piperazine. Polymer 2005, 46, 3831–3837. [Google Scholar] [CrossRef]
- Tamai, S.; Kuroki, T.; Shibuya, A.; Yamaguchi, A. Synthesis and characterization of thermally stable semicrystalline polyimide based on 3,4′-oxydianiline and 3,3′,4,4′-biphenyltetracarboxylic dianhydride. Polymer 2001, 42, 2373–2378. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jikei, M.; Kakimoto, M. Synthesis and properties of AB-type semicrystalline polyimides prepared from polyamic acid ethyl ester precursors. Macromolecules 2001, 34, 3146–3154. [Google Scholar] [CrossRef]
- Liu, X.Q.; Yamanaka, K.; Jikei, M.; Kakimoto, M. Semicrystalline polyimides starting from AB-type monomers. Chem. Mater. 2000, 12, 3885–3891. [Google Scholar] [CrossRef]
- Hsiao, B.S.; Kreuz, J.A.; Cheng, S.Z.D. Crystalline homopolyimides and copolyimides derived from 3,3′,4,4′-biphenyltetracarboxylic dianhydride/1,3-bis(4-aminophenoxy)benzene/1,12-dodecanediamine. 2. Crystallization, melting, and morphology. Macromolecules 1996, 29, 135–142. [Google Scholar] [CrossRef]
- Kreuz, J.A.; Hsiao, B.S.; Renner, C.A.; Goff, D.L. Crystalline homopolyimides and copolyimides derived from 3,3′,4,4′-biphenyltetracarboxylic dianhydride/1,3-bis(4-aminophenoxy)benzene/ 1,12-dodecanediamine. 1. Materials, preparation, and characterization. Macromolecules 1995, 28, 6926–6930. [Google Scholar] [CrossRef]
- Koning, C.E.; Teuwen, L.; DePlaen, A.; Mercier, J.P. Enhancement of the crystallinity of a thermoplastic polyimide. Polymer 1996, 37, 5619–5625. [Google Scholar] [CrossRef]
- Koning, C.E.; Teuwen, L.; Meijer, E.W.; Moonen, J.P. Synthesis and properties of alpha-omega- diaminoalkane based polyimides. Polymer 1994, 35, 4889–4895. [Google Scholar] [CrossRef]
- Korshak, V.V.; Babchinitser, T.M.; Kazaryan, L.G.; Vasilyev, V.A.; Genin, Y.V.; Azriel, A.Y.; Vygodsky, Y.S.; Churochkina, N.A.; Vinogradova, S.V.; Tsvankin, D.Y. Crystallization of alkyl-aromatic polyimides (polyalkanimides). J. Polym. Sci. Part B Polym. Phys. 1980, 18, 247–263. [Google Scholar] [CrossRef]
- Stclair, T.L.; Stclair, A.K. Crystalline polyimides containing 4,4′-bis(3,4-dicarboxyphenoxy)-diphenyl sulfide dianhydride. J. Polym. Sci. Part A Polym. Chem. 1977, 15, 1529–1533. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Wakelyn, N.T.; Havens, S.J. Polyimides containing carbonyl and ether connecting groups. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 1093–1103. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Havens, S.J. Polyimides containing carbonyl and ether connecting groups. II. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 1161–1174. [Google Scholar] [CrossRef]
- Brillhart, M.V.; Cheng, Y.Y.; Nagarkar, P.; Cebe, P. Molecular modelling and structure studies of LARC-CPI semicrystalline polyimide. Polymer 1997, 38, 3059–3068. [Google Scholar] [CrossRef]
- Brandom, D.K.; Wilkes, G.L. Influence of thermal imidization on the crystallization and melting behavior of the aromatic polyimide, LARC CPI-2. Polymer 1995, 36, 4083–4089. [Google Scholar] [CrossRef]
- Brandom, D.K.; Wilkes, G.L. Study of the multiple melting behavior of the aromatic polyimide LARC CPI-2. Polymer 1994, 35, 5672–5677. [Google Scholar] [CrossRef]
- Muellerleile, J.T.; Risch, B.G.; Rodrigues, D.E.; Wilkes, G.L.; Jones, D.M. Crystallization behavior and morphological features of LARC-CPI. Polymer 1993, 34, 789–806. [Google Scholar] [CrossRef]
- Tsai, R.S.; Lee, D.K.; Liu, Y.C.; Tsai, H.B. Crystallization of a thermoplastic polyimide. J. Appl. Polym. Sci. 2003, 90, 2604–2608. [Google Scholar] [CrossRef]
- Srinivas, S.; Wilkes, G.L. Structural and relaxation studies during crystallization of New TPI polyimide. Polymer 1998, 39, 5839–5851. [Google Scholar] [CrossRef]
- Hsiao, B.S.; Sauer, B.B.; Biswas, A. Crystallization study of a thermoplastic polyimide (New-TPI). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 737–747. [Google Scholar] [CrossRef]
- Friler, J.B.; Cebe, P. Development of crystallinity in New-TPI polyimide. Polym. Eng. Sci. 1993, 33, 587–597. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M.; Shumakov, A.N.; Letenko, D.G.; Feldman, A.Y.; Marom, G. The nucleating effect of carbon nanotubes on crystallinity in R-BAPB-type thermoplastic polyimide. Macromol. Rapid Commun. 2005, 26, 885–888. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M.; Gubanova, G.N.; Didenko, A.L.; Sukhanova, T.E.; Kudryavtsev, V.V.; Ratner, S.; Marom, G. Semicrystalline polyimide matrices for composites: Crystallization and properties. J. Appl. Polym. Sci. 2002, 83, 2873–2882. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M.; Gubanova, G.N.; Didenko, A.L.; Popova, E.N.; Sukhanova, T.E.; Grigoriev, A.I.; Kostereva, T.A.; Arbel, I.; Marom, G. Influence of crystallinity of R-BARB type polyimide matrix on thermal and mechanical properties of carbon fiber reinforced composites. In Polyimides and Other High Temperature Polymers: Synthesis, Characterization and Applications; Mittal, K.L., Ed.; CRC Press: Boca Raton, FL, USA, 2005; Volume 3, pp. 299–316. ISBN 978-9-04-741514-5. [Google Scholar]
- Yudin, V.E.; Svetlichnyi, V.M.; Gubanova, G.N.; Grigoriev, A.I.; Didenko, A.L.; Sukhanova, T.E.; Kudryavtsev, V.V.; Ratner, S.; Marom, G. Semicrystalline polyimides for advanced composites. In Polyimides and Other High Temperature Polymers: Synthesis, Characterization and Applications; Mittal, K.L., Ed.; Brill Academic Publishers: Zeist, The Netherlands, 2003; Volume 2, pp. 523–532. ISBN 90-6764-378-5. [Google Scholar]
- Heberer, D.P.; Cheng, S.Z.D.; Barley, J.S.; Lien, S.H.S.; Bryant, R.G.; Harris, F.W. Crystallization and morphology of semicrystalline polyimides. Macromolecules 1991, 24, 1890–1898. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Mittleman, M.L.; Janimak, J.J.; Shen, D.X.; Chalmers, T.M.; Lien, H.S.; Tso, C.C.; Gabori, P.A.; Harris, F.W. Crystal-structure, crystallization kinetics and morphology of a new polyimide. Polym. Int. 1992, 29, 201–208. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Heberer, D.P.; Janimak, J.J.; Lien, S.H.S.; Harris, F.W. Structure and thermal history dependent enthalpy relaxation at the glass-transition of semicrystalline polyimides. Polymer 1991, 32, 2053–2059. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Heberer, D.P.; Lien, H.S.; Harris, F.W. Glass-transition and crystal melting in semicrystalline polyimides. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 655–674. [Google Scholar] [CrossRef]
- Ratta, V.; Ayambem, A.; McGrath, J.E.; Wilkes, G.L. Crystallization and multiple melting behavior of a new semicrystalline polyimide based on 1,3-bis(4-aminophenoxy)benzene (TPER) and 3,3′,4,4′-biphenonetetracarboxylic dianhydride (BTDA). Polymer 2001, 42, 6173–6186. [Google Scholar] [CrossRef]
- Srinivas, S.; Caputo, F.E.; Graham, M.; Gardner, S.; Davis, R.M.; McGrath, J.E.; Wilkes, G.L. Semicrystalline polyimides based on controlled molecular weight phthalimide end-capped 1,3-bis(4-aminophenoxy)benzene and 3,3′,4,4′-biphenyltetracarboxylic dianhydride: Synthesis, crystallization, melting, and thermal stability. Macromolecules 1997, 30, 1012–1022. [Google Scholar] [CrossRef]
- Ratta, V.; Ayambem, A.; Young, R.; McGrath, J.E.; Wilkes, G.L. Thermal stability, crystallization kinetics and morphology of a new semicrystalline polyimide based on 1,3-bis(4-aminophenoxy) benzene and 3,3′,4,4′-biphenyltetracarboxylic dianhydride. Polymer 2000, 41, 8121–8138. [Google Scholar] [CrossRef]
- Ratta, V.; Stancik, E.J.; Ayambem, A.; Pavatareddy, H.; McGrath, J.E.; Wilkes, G.L. A melt-processable semicrystalline polyimide structural adhesive based on 1,3-bis(4-aminophenoxy)benzene and 3,3′,4,4′-biphenyltetracarboxylic dianhydride. Polymer 1999, 40, 1889–1902. [Google Scholar] [CrossRef]
- Srinivas, S.; Graham, M.; Brink, M.H.; Gardner, S.; Davis, R.M.; McGrath, J.E.; Wilkes, G.L. Influence of melt stability on the crystallization of bis(4-aminophenoxy)benzene-oxydiphthalic anhydride based polyimides. Polym. Eng. Sci. 1996, 36, 1928–1940. [Google Scholar] [CrossRef]
- Sazanov, Y.N.; Florinsky, F.S.; Koton, M.M. Investigation of thermal and thermooxidative degradation of some polyimides containing oxyphenylene groups in the main chain. Eur. Polym. J. 1979, 15, 781–786. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Dai, L.R. Synthesis and properties of poly(ether imide)s based on the bis(ether anhydride)s from hydroquinone and its derivatives. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 665–675. [Google Scholar] [CrossRef]
- Takekoshi, T.; Kochanowski, J.E.; Manello, J.S.; Webber, M.J. Polyetherimides. II. High-temperature solution polymerization. J. Polym. Sci. Polym. Symp. 1986, 74, 93–108. [Google Scholar] [CrossRef]
- Han, Y.; Fang, X.Z.; Zuo, X.X. The influence of molecular weight on properties of melt-processable copolyimides derived from thioetherdiphthalic anhydride isomers. J. Mater. Sci. 2010, 45, 1921–1929. [Google Scholar] [CrossRef]
- Wunderlich, B. Chapter IX—Irreversible melting. In Macromolecular Physics: Crystal Melting; Academic Press: San Diego, CA, USA, 1980; Volume 3, pp. 128–253. ISBN 978-0-08-092664-3. [Google Scholar]
- Avrami, M. Kinetics of phase change I—General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Wunderlich, B. Chapter VI—The growth of crystals. In Macromolecular Physics: Crystal Nucleation, Growth, Annealing; Academic Press: New York, NY, USA, 1976; Volume 2, pp. 115–347. ISBN 978-0-12-765602-1. [Google Scholar]
- Cebe, P.; Hong, C.H. Crystallization behaviour of poly(ether-ether-ketone). Polymer 1986, 27, 1183–1192. [Google Scholar] [CrossRef]
- Carroccio, S.; Puglisi, C.; Montaudo, G. New vistas in polymer degradation. Thermal oxidation processes in poly(ether imide). Macromolecules 2005, 38, 6849–6862. [Google Scholar] [CrossRef]
| Sample | Diamine | ηinh a (dL/g) | T5% (°C) b | R800 c (%) | Tg d (°C) | Tm e (°C) | Tm * f (°C) | |
|---|---|---|---|---|---|---|---|---|
| N2 | Air | |||||||
| PI-1 | TPER | 0.43 | 540 | 536 | 52.6 | 190 | 333,351 | 331,350 |
| PI-2 | TPEQ | 0.58 | 548 | 545 | 51.5 | 214 | 393 | 388 |
| PI-3 | 4,4′-ODA | 0.46 | 553 | 547 | 56.3 | 223 | 349 | - |
| PI-4 | BAPB | 0.56 | 525 | 522 | 54.1 | 223 | 354 | - |
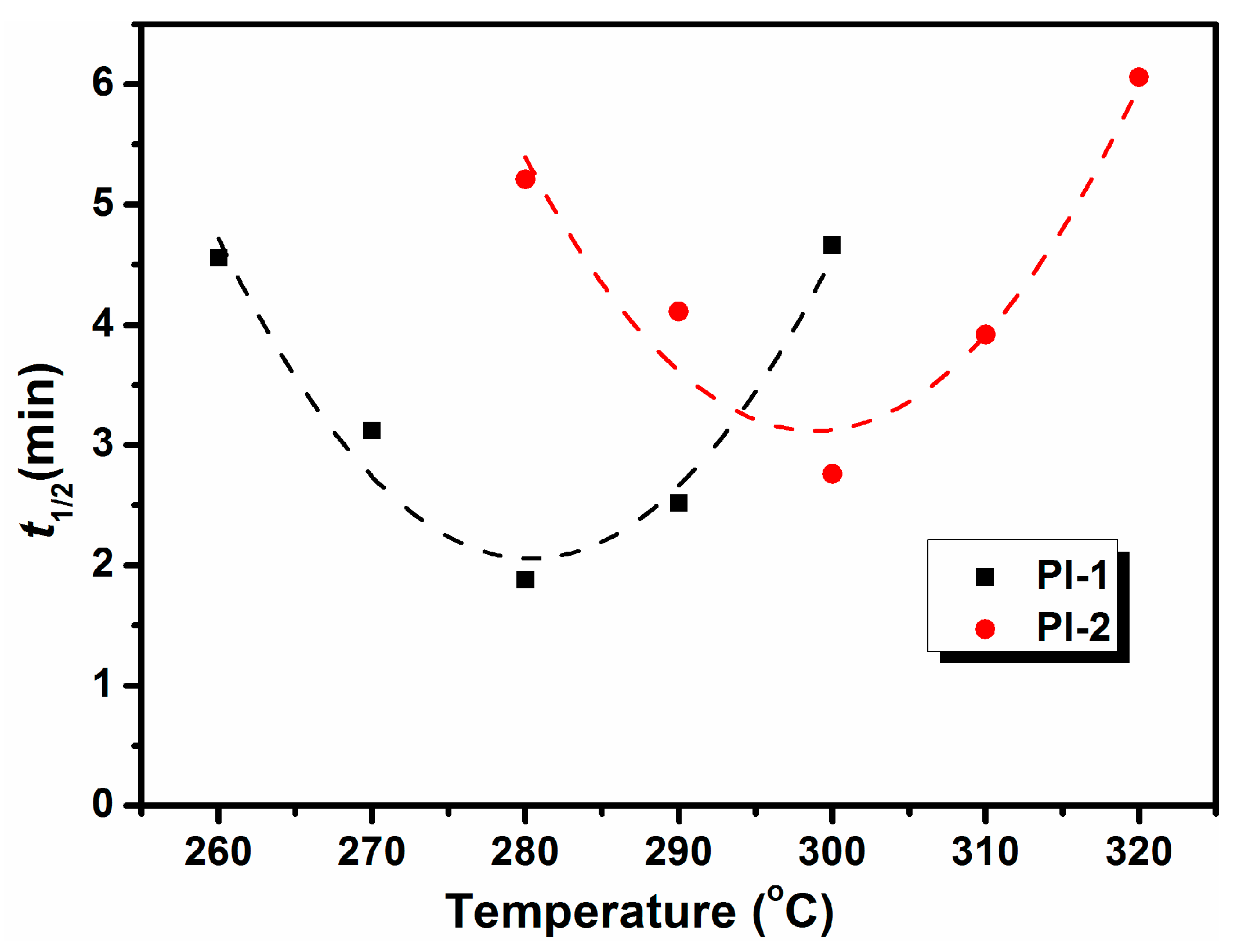
| Sample | Tc a (°C) | n | K (min−n) | K1/n | t1/2 (min) | τ1/2 (min−1) |
|---|---|---|---|---|---|---|
| PI-1 | 260 | 2.5 | 0.0149 | 0.19 | 4.56 | 0.22 |
| 270 | 2.4 | 0.0464 | 0.27 | 3.12 | 0.32 | |
| 280 | 2.3 | 0.1649 | 0.45 | 1.88 | 0.53 | |
| 290 | 2.5 | 0.0694 | 0.34 | 2.52 | 0.40 | |
| 300 | 2.6 | 0.0123 | 0.19 | 4.66 | 0.21 | |
| PI-2 | 280 | 2.5 | 0.0115 | 0.17 | 5.21 | 0.19 |
| 290 | 2.7 | 0.0163 | 0.21 | 4.11 | 0.24 | |
| 300 | 2.3 | 0.0658 | 0.31 | 2.76 | 0.36 | |
| 310 | 2.8 | 0.0147 | 0.22 | 3.92 | 0.26 | |
| 320 | 2.9 | 0.0039 | 0.15 | 6.06 | 0.17 |
| Polymer | Melt Temperature (°C) | Undercooling, ΔT (°C) | K1/n | Ref. |
|---|---|---|---|---|
| PEEK | 410 | 80 | 0.06 | [53] |
| New-TPI | 410 | 121 | 0.04 | [31] |
| BPDA/TPER | 430 | 55 | 0.61 | [43] |
| PI-1 | 370 | 76 | 0.45 | This work |
| PI-2 | 420 | 90 | 0.31 | This work |
| Sample | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|
| PI-1 | 97 | 3.1 | 5 |
| PI-2 | 105 | 3.3 | 5 |
| PI-3 | 103 | 2.5 | 18 |
| PI-4 | 85 | 2.0 | 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, W.; Chen, G.; Zhang, A.; Fang, X. Melt-Processable Semicrystalline Polyimides Based on 1,4-Bis(3,4-dicarboxyphenoxy)benzene Dianhydride (HQDPA): Synthesis, Crystallization, and Melting Behavior. Polymers 2017, 9, 420. https://doi.org/10.3390/polym9090420
Zhang H, Wang W, Chen G, Zhang A, Fang X. Melt-Processable Semicrystalline Polyimides Based on 1,4-Bis(3,4-dicarboxyphenoxy)benzene Dianhydride (HQDPA): Synthesis, Crystallization, and Melting Behavior. Polymers. 2017; 9(9):420. https://doi.org/10.3390/polym9090420
Chicago/Turabian StyleZhang, Hongfei, Wei Wang, Guofei Chen, Anjiang Zhang, and Xingzhong Fang. 2017. "Melt-Processable Semicrystalline Polyimides Based on 1,4-Bis(3,4-dicarboxyphenoxy)benzene Dianhydride (HQDPA): Synthesis, Crystallization, and Melting Behavior" Polymers 9, no. 9: 420. https://doi.org/10.3390/polym9090420





