Inoculation of Triticum Aestivum L. (Poaceae) with Plant-Growth-Promoting Fungi Alleviates Plant Oxidative Stress and Enhances Phenanthrene Dissipation in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Microorganisms
2.3. Greenhouse Experiment
2.4. Mycorrhizal Colonization Parameters
2.5. Analysis of Phenanthrene in Soil
2.6. Phenanthrene in Plant Tissues and Translocation Factor (TF)
2.7. Soil Enzyme Activities
2.7.1. Dehydrogenase Activity
2.7.2. Total Microbial Activity
2.7.3. Manganese Peroxidase
2.7.4. Laccase
2.8. Organic Acid Extraction and Determination
2.9. Antioxidant Activities
2.10. Lipid Peroxidation
2.11. Statistical Analysis
3. Results
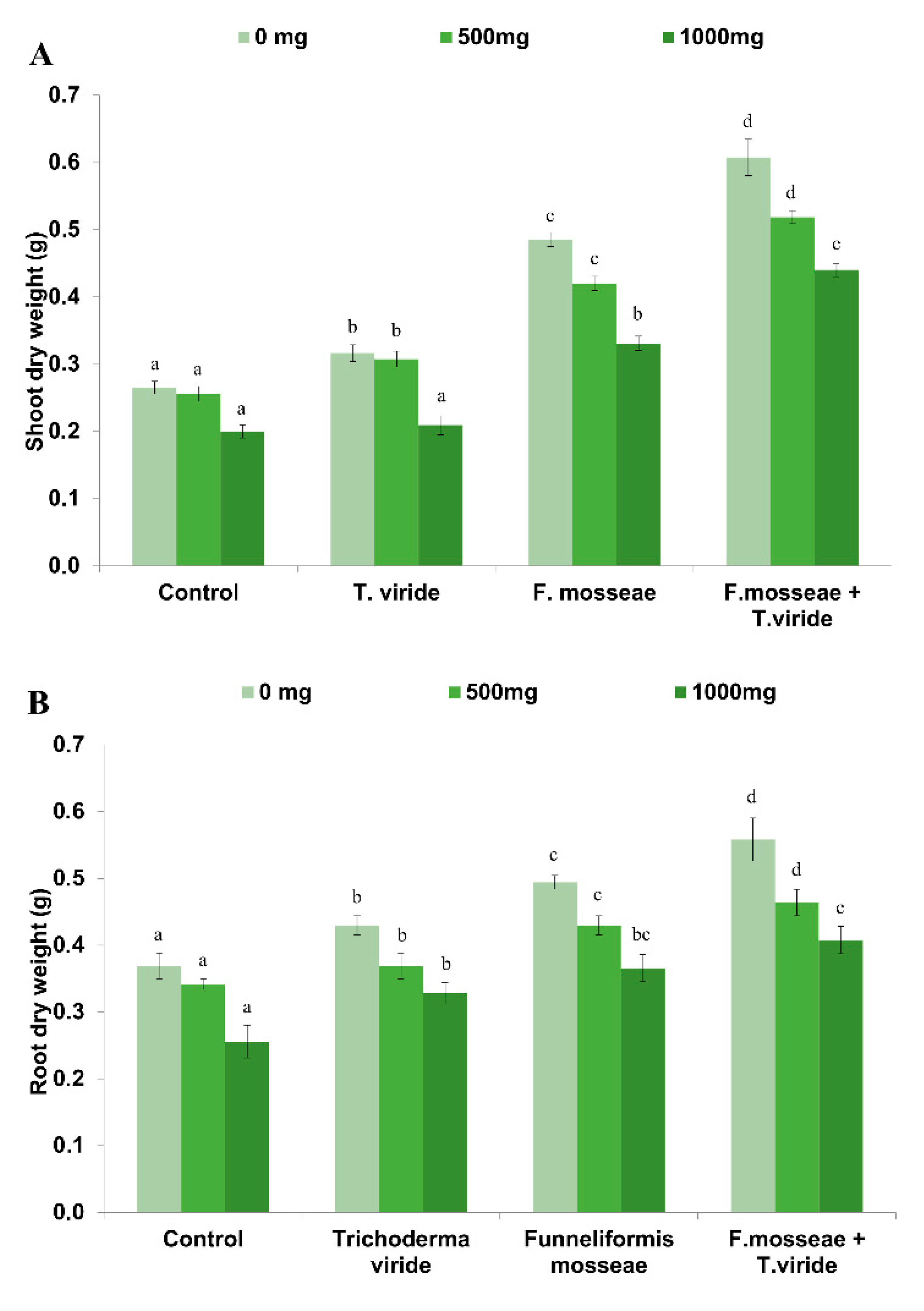
3.1. Dry Biomass
3.2. Arbuscular Mycorrhizal Colonization Parameters
3.3. Phenanthrene in Soil and Plant Tissues
3.4. Phenanthrene Dissipation
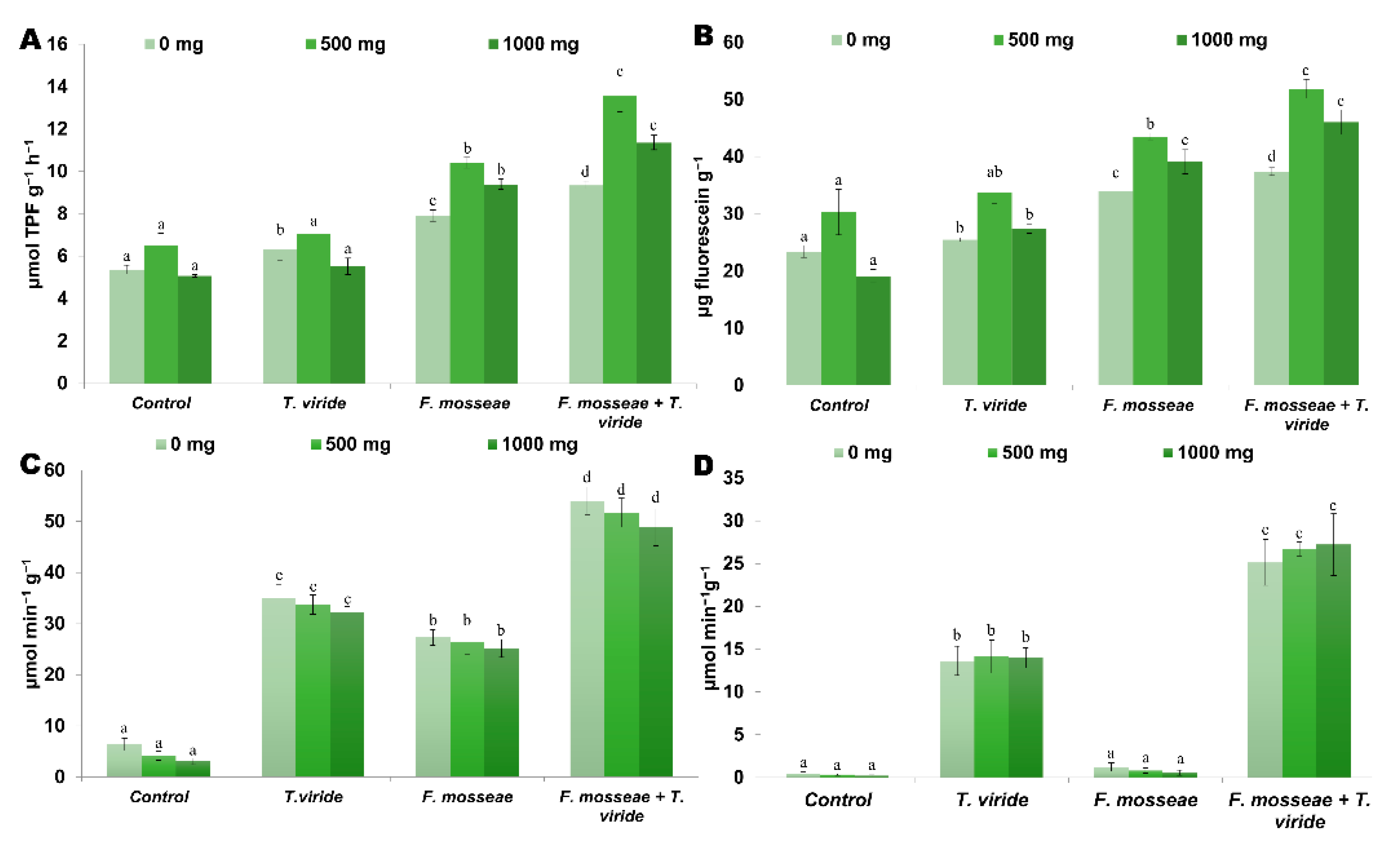
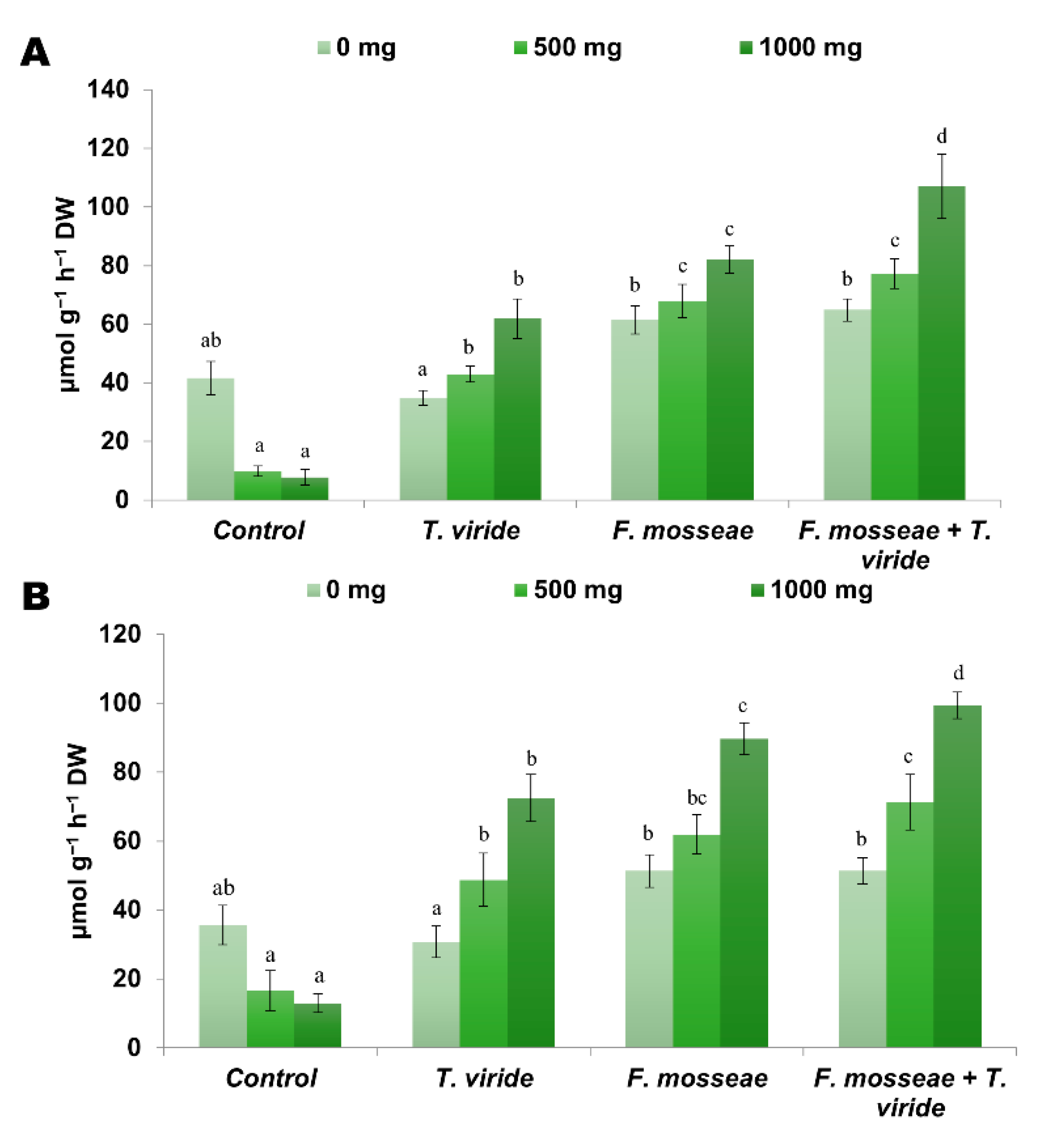
3.5. Soil Enzyme Activities
3.6. Organic Acids
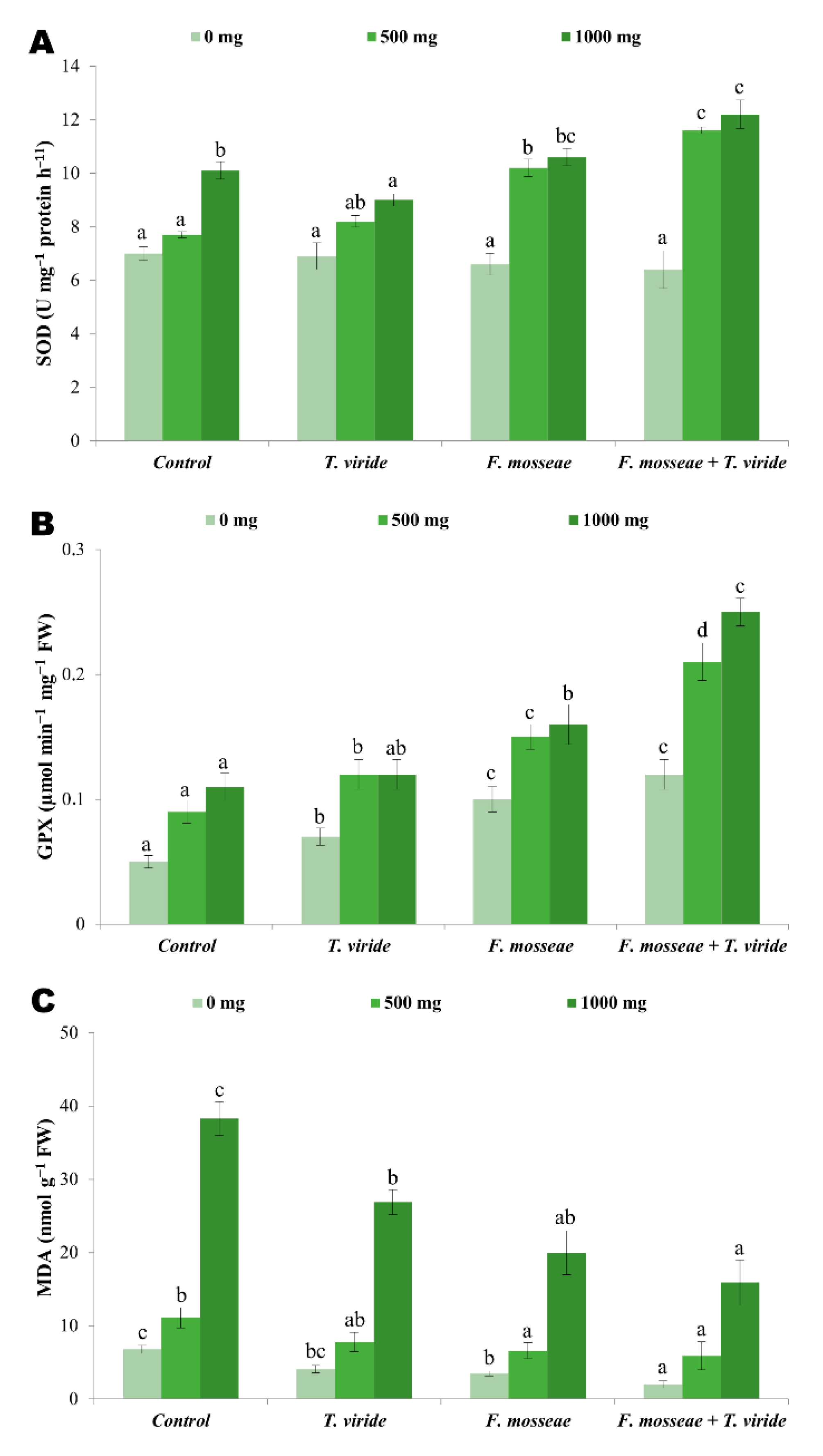
3.7. Antioxidant Enzymes and Lipid Peroxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajendran, P.; Jayakumar, T.; Nishigaki, I.; Ekambaram, G.; Nishigaki, Y.; Vetriselvi, J.; Sakthisekaran, D. Immunomodulatory effect of mangiferin in experimental animals with benzo (a) pyrene-induced lung carcinogenesis. Int. J. Biomed. Sci. 2013, 9, 68. [Google Scholar] [PubMed]
- Sogbanmu, T.O.; Nagy, E.; Phillips, D.H.; Arlt, V.M.; Otitoloju, A.A.; Bury, N.R. Lagos lagoon sediment organic extracts and polycyclic aromatic hydrocarbons induce embryotoxic, teratogenic and genotoxic effects in Danio rerio (zebrafish) embryos. Environ. Sci. Pollut. Res. 2016, 23, 14489–14501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conejo-Saucedo, U.; Olicón-Hernández, D.R.; Robledo-Mahón, T.; Stein, H.P.; Calvo, C.; Aranda, E. Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Soil Through Fungal Communities. In Recent Advancement in White Biotechnology through Fungi; Yadav, N., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 217–236. [Google Scholar]
- Li, Y.; Li, Y.; Yang, H.; Chen, L.; Cao, Y. Sequestration Specificity of Single or Co-existing Benzene, 1, 3, 5-Trimethylbenzene, and Naphthalene in Soil. J. Soil Sci. Plant Nutr. 2019, 19, 299–304. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, H.; Lu, Y.; Yu, X.; Wang, J.; Sun, S.; Liu, M.; Li, D.; Li, Y.-F.; Zhang, D. Uptake and translocation of polycyclic aromatic hydrocarbons (PAHs) and heavy metals by maize from soil irrigated with wastewater. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Sandau, C.D.; Romanoff, L.C.; Caudill, S.P.; Sjodin, A.; Needham, L.L.; Patterson, D.G., Jr. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res. 2008, 107, 320–331. [Google Scholar] [CrossRef]
- Cheema, S.A.; Khan, M.I.; Shen, C.; Tang, X.; Farooq, M.; Chen, L.; Zhang, C.; Chen, Y. Degradation of phenanthrene and pyrene in spiked soils by single and combined plants cultivation. J. Hazard. Mater. 2010, 177, 384–389. [Google Scholar] [CrossRef]
- Wild, E.; Dent, J.; Thomas, G.O.; Jones, K.C. Direct observation of organic contaminant uptake, storage, and metabolism within plant roots. Environ. Sci. Technol. 2005, 39, 3695–3702. [Google Scholar] [CrossRef]
- Arriagada, C.; Aranda, E.; Sampedro, I.; Garcia-Romera, I.; Ocampo, J. Interactions of Trametes versicolor, Coriolopsis rigida and the arbuscular mycorrhizal fungus Glomus deserticola on the copper tolerance of Eucalyptus globulus. Chemosphere 2009, 77, 273–278. [Google Scholar] [CrossRef]
- Herrera, H.; Palma, G.; Almonacid, L.; Campos, R.; Fuentes, A.; Garcia-Romera, I.; Arriagada, C. Improving Soil Simazine Dissipation Through an Organic Amendment Inoculated with Trametes versicolor. J. Soil Sci. Plant Nutr. 2019, 19, 262–269. [Google Scholar]
- Raimondo, E.E.; Aparicio, J.D.; Briceño, G.E.; Fuentes, M.S.; Benimeli, C.S. Lindane Bioremediation in Soils of Different Textural Classes by an Actinobacteria Consortium. J. Soil Sci. Plant Nutr. 2019, 19, 29–41. [Google Scholar] [CrossRef]
- Aranda, E.; Scervino, J.M.; Godoy, P.; Reina, R.; Ocampo, J.A.; Wittich, R.-M.; García-Romera, I. Role of arbuscular mycorrhizal fungus Rhizophagus custos in the dissipation of PAHs under root-organ culture conditions. Environ. Pollut. 2013, 181, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Atagana, H.I. Biodegradation of PAHs by fungi in contaminated-soil containing cadmium and nickel ions. Afr. J. Biotechnol. 2009, 8, 5780–5789. [Google Scholar]
- Yu, X.; Wu, S.; Wu, F.; Wong, M.H. Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J. Hazard. Mater. 2011, 186, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Crowley, D.E. Biostimulation of PAH degradation with plants containing high concentrations of linoleic acid. Environ. Sci. Technol. 2007, 41, 4382–4388. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wu, Z.; Li, Y.; Cao, H.; Su, C. Effect of various chemical oxidation reagents on soil indigenous microbial diversity in remediation of soil contaminated by PAHs. Chemosphere 2019, 226, 483–491. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, L.; Wang, Y.; Yang, L.; Yin, Y.; Su, X.; Liu, Y.; Pang, H.; Xu, J.; Hu, Y. Effects of polycyclic aromatic hydrocarbons (PAHs) from different sources on soil enzymes and microorganisms of Malus prunifolia var. Ringo. Arch. Agron. Soil Sci. 2020. [Google Scholar] [CrossRef]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar]
- Wagner, D.; Przybyla, D.; op den Camp, R.; Kim, C.; Landgraf, F.; Lee, K.P.; Würsch, M.; Laloi, C.; Nater, M.; Hideg, E. The genetic basis of singlet oxygen–induced stress responses of Arabidopsis thaliana. Science 2004, 306, 1183–1185. [Google Scholar] [CrossRef]
- Liu, H.; Weisman, D.; Tang, L.; Tan, L.; Zhang, W.-K.; Wang, Z.-H.; Huang, Y.-H.; Lin, W.-X.; Liu, X.-M.; Colón-Carmona, A. Stress signaling in response to polycyclic aromatic hydrocarbon exposure in Arabidopsis thaliana involves a nucleoside diphosphate kinase, NDPK-3. Planta 2015, 241, 95–107. [Google Scholar] [CrossRef]
- Pašková, V.; Hilscherová, K.; Feldmannová, M.; Bláha, L. Toxic effects and oxidative stress in higher plants exposed to polycyclic aromatic hydrocarbons and their N-heterocyclic derivatives. Environ. Toxicol. Chem. 2006, 25, 3238–3245. [Google Scholar] [CrossRef]
- Oenel, A.; Fekete, A.; Krischke, M.; Faul, S.C.; Gresser, G.; Havaux, M.; Mueller, M.J.; Berger, S. Enzymatic and non-enzymatic mechanisms contribute to lipid oxidation during seed aging. Plant Cell Physiol. 2017, 58, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S. Effect of Nanoparticles on Lipid Peroxidation in Plants. In Advances in Lipid Metabolism; Valenzuela, R., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Alkio, M.; Tabuchi, T.M.; Wang, X.; Colon-Carmona, A. Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. J. Exp. Bot. 2005, 56, 2983–2994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.; Liang, L.; Wei, Y.; Luo, Z.; Yuan, F.; Li, G.; Sang, N. Exposure to Nitro-PAHs interfere with germination and early growth of Hordeum vulgare via oxidative stress. Ecotoxicol. Environ. Saf. 2019, 180, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Herrera, H.; García-Romera, I.; Meneses, C.; Pereira, G.; Arriagada, C. Orchid Mycorrhizal Interactions on the Pacific Side of the Andes from Chile. A Review. J. Soil Sci. Plant Nutr. 2019, 19, 187–202. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Srivastava, A.K.; Zou, Y.-N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hort. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Ren, C.-G.; Kong, C.-C.; Bian, B.; Liu, W.; Li, Y.; Luo, Y.-M.; Xie, Z.-H. Enhanced phytoremediation of soils contaminated with PAHs by arbuscular mycorrhiza and rhizobium. Int. J. Phytoremediat. 2017, 19, 789–797. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, Z.; Ling, W.; Huang, J. Arbuscular mycorrhizal fungal hyphae contribute to the uptake of polycyclic aromatic hydrocarbons by plant roots. Bioresour. Technol. 2010, 101, 6895–6901. [Google Scholar] [CrossRef]
- Fuentes, A.; Almonacid, L.; Ocampo, J.A.; Arriagada, C. Synergistic interactions between a saprophytic fungal consortium and Rhizophagus irregularis alleviate oxidative stress in plants grown in heavy metal contaminated soil. Plant Soil 2016, 407, 355–366. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Q.; Ling, W.; Zhu, X. Arbuscular mycorrhizal phytoremediation of soils contaminated with phenanthrene and pyrene. J. Hazard. Mater. 2011, 185, 703–709. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Chauhan, P.S.; Dwivedi, S.; Bais, R.T.; Tripathi, R.D. Trichoderma: A potential bioremediator for environmental clean up. Clean Technol. Environ. Policy 2013, 15, 541–550. [Google Scholar] [CrossRef]
- Li, X.; Peng, D.; Zhang, Y.; Ju, D.; Guan, C. Klebsiella sp. PD3, a phenanthrene (PHE)-degrading strain with plant growth promoting properties enhances the PHE degradation and stress tolerance in rice plants. Ecotoxicol. Environ. Saf. 2020, 201, 110804. [Google Scholar] [CrossRef]
- Urana, R.; Dahiya, A.; Sharma, P.; Singh, N. Effects of Plant Growth Promoting Rhizobacteria on Phytoremediation of Phenanthrene Contaminated Sodic Soil. Polycycl. Aromat. Compd. 2019. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Lin, X.; Zhang, J.; Zeng, J. Dissipation of polycyclic aromatic hydrocarbons (PAHs) in soil microcosms amended with mushroom cultivation substrate. Soil Biol. Biochem. 2012, 47, 191–197. [Google Scholar] [CrossRef]
- Zafra, G.; Cortés-Espinosa, D.V. Biodegradation of polycyclic aromatic hydrocarbons by Trichoderma species: A mini review. Environ. Sci. Pollut. Res. 2015, 22, 19426–19433. [Google Scholar] [CrossRef]
- Arriagada, C.; Manquel, D.; Cornejo, P.; Soto, J.; Sampedro, I.; Ocampo, J. Effects of the co-inoculation with saprobe and mycorrhizal fungi on Vaccinium corymbosum growth and some soil enzymatic activities. J. Soil Sci. Plant Nutr. 2012, 12, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Lagos, C.; Larsen, J.; Correa, E.S.; Almonacid, L.; Herrera, H.; Fuentes, A.; Arriagada, C. Dual inoculation with mycorrhizal and saprotrophic fungi suppress the maize growth and development under phenanthrene exposure. J. Soil Sci. Plant Nutr. 2018, 18, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Bouranis, D.; Chorianopoulou, S.; Nocito, F.; Sacchi, G.; Serelis, K. The crucial role of sulfur in a phytoremediation process: Lessons from the Poaceae species as phytoremediants: A review. In Proceedings of an International Conference; Protection and Restoration of the Environment XI; Katsifarakis, K., Theodossiou, N., Christodoulatos, C., Koutsospyros, A., Mallios, Z., Eds.; Natural Treatment Systems: Thessaloniki, Greece; pp. 634–643.
- Kuang, S.; Yu, W.; Song, Y.; Su, Y.; Wang, H.; Wang, L. Dissipation of polycyclic aromatic hydrocarbons in crop soils amended with oily sludge. Acta Geochim. 2016, 35, 437–444. [Google Scholar] [CrossRef]
- Yu, W.; Kuang, S.; Zhao, L. Uptake, accumulation and translocation of polycyclic aromatic hydrocarbons by winter wheat cultured on oily sludge-amended soil. Chin. J. Geochem. 2013, 32, 295–302. [Google Scholar] [CrossRef]
- Herrera, H.; Sanhueza, T.; Martiarena, R.; Valadares, R.; Fuentes, A.; Arriagada, C. Mycorrhizal Fungi Isolated from Native Terrestrial Orchids from Region of La Araucanía, Southern Chile. Microorganisms 2020, 8, 1120. [Google Scholar] [CrossRef] [PubMed]
- Northcott, G.L.; Jones, K.C. Spiking hydrophobic organic compounds into soil and sediment: A review and critique of adopted procedures. Environ. Toxicol. Chem. Int. J. 2000, 19, 2418–2430. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Mycocalc. Available online: https://www2.dijon.inra.fr/mychintec/Mycocalc-prg/download.html (accessed on 20 March 2020).
- Acevedo, F.; Pizzul, L.; del Pilar, M.C.; Cuevas, R.; Diez, M.C. Degradation of polycyclic aromatic hydrocarbons by the Chilean white-rot fungus Anthracophyllum discolor. J. Hazard. Mater. 2011, 185, 212–219. [Google Scholar] [CrossRef]
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium macrocarpon (Cranberry)-Based Dietary Supplements: Variation in Mass Uniformity, Proanthocyanidin Dosage and Anthocyanin Profile Demonstrates Quality Control Standard Needed. Nutrients 2020, 12, 992. [Google Scholar] [CrossRef] [Green Version]
- Zitka, O.; Babula, P.; Sochor, J.; Kummerova, M.; Krystofova, O.; Adam, V.; Havel, L.; Beklova, M.; Hubalek, J.; Kizek, R. Determination of eight polycyclic aromatic hydrocarbons and in pea plants (Pisum sativum L.) extracts by high performance liquid chromatography with electrochemical detection. Int. J. Electrochem. Sci. 2012, 7, 908–927. [Google Scholar]
- Casida, L.; Klein, D.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Elgueta, S.; Santos, C.; Lima, N.; Diez, M. Immobilization of the white-rot fungus Anthracophyllum discolor to degrade the herbicide atrazine. AMB Express 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Herrera, H.; Valadares, R.; Oliveira, G.; Fuentes, A.; Almonacid, L.; do Nascimento, S.V.; Bashan, Y.; Arriagada, C. Adaptation and tolerance mechanisms developed by mycorrhizal Bipinnula fimbriata plantlets (Orchidaceae) in a heavy metal-polluted ecosystem. Mycorrhiza 2018, 28, 651–663. [Google Scholar]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of antioxidants to paraquat in pea leaves (relationships to resistance). Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinhero, R.G.; Rao, M.V.; Paliyath, G.; Murr, D.P.; Fletcher, R.A. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol. 1997, 114, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Jajoo, A.; Mekala, N.R.; Tomar, R.S.; Grieco, M.; Tikkanen, M.; Aro, E.-M. Inhibitory effects of polycyclic aromatic hydrocarbons (PAHs) on photosynthetic performance are not related to their aromaticity. J. Photochem. Photobiol. B Biol. 2014, 137, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Weisman, D.; Alkio, M.; Colón-Carmona, A. Transcriptional responses to polycyclic aromatic hydrocarbon-induced stress in Arabidopsis thalianareveal the involvement of hormone and defense signaling pathways. BMC Plant Biol. 2010, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwoko, C.O. Effect of Arbuscular Mycorrhizal (AM) Fungi on the physiological performance of phaseolus vulgaris grown under crude oil contaminated soil. J. Geosci. Environ. Prot. 2014, 2, 9–14. [Google Scholar] [CrossRef]
- Franco-Ramírez, A.; Ferrera-Cerrato, R.; Varela-Fregoso, L.; Pérez-Moreno, J.; Alarcón, A. Arbuscular mycorrhizal fungi in chronically petroleum-contaminated soils in Mexico and the effects of petroleum hydrocarbons on spore germination. J. Basic Microbiol. 2007, 47, 378–383. [Google Scholar] [CrossRef]
- Gaspar, M.; Cabello, M.; Cazau, M.; Pollero, R. Effect of phenanthrene and Rhodotorula glutinis on arbuscular mycorrhizal fungus colonization of maize roots. Mycorrhiza 2002, 12, 55–59. [Google Scholar]
- Zafra, G.; Moreno-Montaño, A.; Absalón, Á.E.; Cortés-Espinosa, D.V. Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environ. Sci. Pollut. Res. 2015, 22, 1034–1042. [Google Scholar] [CrossRef]
- Korolev, N.; David, D.R.; Elad, Y. The role of phytohormones in basal resistance and Trichoderma-induced systemic resistance to Botrytis cinerea in Arabidopsis thaliana. BioControl 2008, 53, 667–683. [Google Scholar] [CrossRef]
- Fracchia, S.; Mujica, M.; García-Romera, I.; Garcia-Garrido, J.; Martin, J.; Ocampo, J.; Godeas, A. Interactions between Glomus mosseae and arbuscular mycorrhizal sporocarp-associated saprophytic fungi. Plant Soil 1998, 200, 131–137. [Google Scholar] [CrossRef]
- Saldajeno, M.; Chandanie, W.; Kubota, M.; Hyakumachi, M. Effects of interactions of arbuscular mycorrhizal fungi and beneficial saprophytic mycoflora on plant growth and disease protection. In Mycorrhizae: Sustainable Agriculture and Forestry; Siddiqui, Z., Akhtar, M., Futai, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 211–226. [Google Scholar]
- Almonacid, L.; Fuentes, A.; Ortiz, J.; Salas, C.; Garcia-Romera, I.; Ocampo, J.; Arriagada, C. Effect of mixing soil saprophytic fungi with organic residues on the response of S olanum lycopersicum to arbuscular mycorrhizal fungi. Soil Use Manag. 2015, 31, 155–164. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Hartley, I.P.; Ineson, P.; Fitter, A.H. Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Glob. Chang. Biol. 2008, 14, 1181–1190. [Google Scholar] [CrossRef]
- Cabello, M.N. Hydrocarbon pollution: Its effect on native arbuscular mycorrhizal fungi (AMF). FEMS Microbiol. Ecol. 1997, 22, 233–236. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Lu, M. Remediation of PAH-contaminated soil by the combination of tall fescue, arbuscular mycorrhizal fungus and epigeic earthworms. J. Hazard. Mater. 2015, 285, 535–541. [Google Scholar] [CrossRef]
- Wu, F.; Yu, X.; Wu, S.; Lin, X.; Wong, M.H. Phenanthrene and pyrene uptake by arbuscular mycorrhizal maize and their dissipation in soil. J. Hazard. Mater. 2011, 187, 341–347. [Google Scholar] [CrossRef]
- Sadhana, B. Arbuscular Mycorrhizal Fungi (AMF) as a biofertilizer-a review. Int. J. Curr. Microbiol. App. Sci 2014, 3, 384–400. [Google Scholar]
- Gerhardt, K.E.; Huang, X.-D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Li, Y.-T.; Rouland, C.; Benedetti, M.; Li, F.-B.; Pando, A.; Lavelle, P.; Dai, J. Microbial biomass, enzyme and mineralization activity in relation to soil organic C, N and P turnover influenced by acid metal stress. Soil Biol. Biochem. 2009, 41, 969–977. [Google Scholar] [CrossRef]
- Kumar, S.; Chaudhuri, S.; Maiti, S. Soil dehydrogenase enzyme activity in natural and mine soil-a review. Middle East J. Sci. Res. 2013, 13, 898–906. [Google Scholar]
- Zhang, N.; Xing-Dong, H.; Yu-Bao, G.; Yong-Hong, L.; Hai-Tao, W.; Di, M.; Zhang, R.; Yang, S. Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in Artemisia ordosica community. Pedosphere 2010, 20, 229–235. [Google Scholar] [CrossRef]
- Rabie, G.H. Role of arbuscular mycorrhizal fungi in phytoremediation of soil rhizosphere spiked with poly aromatic hydrocarbons. Mycobiology 2005, 33, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, J.; Soto, J.; Fuentes, A.; Herrera, H.; Meneses, C.; Arriagada, C. The endophytic fungus Chaetomium cupreum regulates expression of genes involved in the tolerance to metals and plant growth promotion in Eucalyptus globulus roots. Microorganisms 2019, 7, 490. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.S.; Singh, A.; Singh, H.B.; Sarma, B.K. Plant genotype, microbial recruitment and nutritional security. Front. Plant Sci. 2015, 6, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Chen, C.; Xu, Z. Citric acid enhances the mobilization of organic phosphorus in subtropical and tropical forest soils. Biol. Fertil. Soils 2010, 46, 765–769. [Google Scholar] [CrossRef]
- Ling, W.; Sun, R.; Gao, X.; Xu, R.; Li, H. Low-molecular-weight organic acids enhance desorption of polycyclic aromatic hydrocarbons from soil. Eur. J. Soil Sci. 2015, 66, 339–347. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.-S.; Sun, C.-C.; Wu, M.-L.; Peng, Y.-L.; Deng, C.; Li, Q.P. Effects of polycyclic aromatic hydrocarbons exposure on antioxidant system activities and proline content in Kandelia candel. Oceanol. Hydrobiol. Stud. 2011, 40, 9. [Google Scholar] [CrossRef]
- Wei, H.; Song, S.; Tian, H.; Liu, T. Effects of phenanthrene on seed germination and some physiological activities of wheat seedling. C. R. Biol. 2014, 337, 95–100. [Google Scholar] [CrossRef]
- Carrasco-Ríos, L.; Pinto, M. Effect of salt stress on antioxidant enzymes and lipid peroxidation in leaves in two contrasting corn, ‘Lluteno’ and ‘Jubilee’. Chil. J. Agric. Res. 2014, 74, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Kusvuran, S.; Kiran, S.; Ellialtioglu, S.S. Antioxidant enzyme activities and abiotic stress tolerance relationship in vegetable crops. In Abiotic and Biotic Stress in Plants-Recent Advances and Future Perspectives; InTechOpen: London, UK, 2016; pp. 481–503. [Google Scholar]
- Chang, W.; Sui, X.; Fan, X.-X.; Jia, T.-T.; Song, F.-Q. Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef] [Green Version]
| Parameter. | Phenanthrene Dose (mg kg−1) | F. Mosseae | F. Mosseae + T. Viride |
|---|---|---|---|
| F% | 0 | 73.1 ± 8.5 c | 80.3 ± 6.3 c |
| 500 | 51.3 ± 4.9 b | 60.5 ± 3.8 b | |
| 1000 | 39.8 ± 4.7 a | 49.4 ± 4.4 a | |
| I% | 0 | 46.1 ± 8.6 b | 51.2 ± 5.5 c |
| 500 | 36.1 ± 4.1 b | 42.8 ± 4.9 b | |
| 1000 | 28.5 ± 2.1 a | 34.9 ± 1.4 a | |
| A% | 0 | 53.3 ± 3.9 c | 56.5 ± 3.1 c |
| 500 | 36.8 ± 5.3 b | 44.1 ± 6.7 b | |
| 1000 | 27.0 ± 1.6 a | 32.6 ± 2.1 a | |
| Vesicles per 100 cm root | 0 | 18.0 ± 1.9 a | 28.4 ± 3.2 a |
| 500 | 52.0 ± 2.5 b | 60.2 ± 1.6 b | |
| 1000 | 165.2 ± 14.1 c | 178.6 ± 11.7 c |
| Parameter | Phenanthrene Dose (mg kg−1) | Control | T. Viride | F. Mosseae | F. Mosseae + T. Viride |
|---|---|---|---|---|---|
| Phenanthrene residual in soil (ppm) | 500 | 422.8 ± 37.9 d | 351.7 ± 28.9 c | 211.8 ± 21.6 b | 164.3 ± 22.7 a |
| 1000 | 753.1 ± 55.6 c | 696.3 ± 66.8 c | 426.6 ± 38.4 b | 339.6 ± 29.4 a | |
| Phenanthrene content in shoots (ppm) | 500 | 11.1 ± 2.8 b | 10.4 ± 2.5 b | 5.0 ± 1.7 a | 4.7 ± 1.1 a |
| 1000 | 19.1 ± 5.7 c | 17.4 ± 5.4 c | 9.3 ± 2.7 b | 3.7 ± 0.9 a | |
| Phenanthrene content in roots (ppm) | 500 | 18.8 ± 4.9 ab | 12.8 ± 6.1 a | 23.1 ± 2.9 b | 26.3 ± 3.9 b |
| 1000 | 25.2 ± 2.7 a | 28.2 ± 3.9 a | 34.0 ± 1.8 b | 40.4 ± 3.8 c | |
| Translocation factor | 500 | 0.81 ± 0.17 c | 0.49 ± 0.11 b | 0.21 ± 0.05 a | 0.18 ± 0.06 a |
| 1000 | 0.76 ± 0.14 d | 0.61 ± 0.05 c | 0.27 ± 0.03 b | 0.09 ± 0.03 a |
| Parameter | Phenanthrene Dose (mg kg−1) | Control | T. Viride | F. Mosseae | F. Mosseae + T. Viride |
|---|---|---|---|---|---|
| Soil (%) | 500 | 15.4 ± 4.4 a | 29.7 ± 3.8 b | 57.6 ± 5.8 c | 74.5 ± 3.6 d |
| 1000 | 24.7 ± 5.4 a | 30.3 ± 8.8 a | 56.3 ± 5.2 b | 66.0 ± 4.1 c | |
| Shoots (%) | 500 | 2.2 ± 0.3 b | 2.1 ± 0.6 b | 1.0 ± 0.2 a | 0.9 ± 0.2 a |
| 1000 | 1.9 ± 0.6 b | 1.7 ± 0.6 b | 0.9 ± 0.7 a | 0.4 ± 0.3 a | |
| Roots (%) | 500 | 4.6 ± 0.8 b | 2.6 ± 0.5 a | 4.6 ± 1.1 b | 5.3 ± 1.5 b |
| 1000 | 2.5 ± 0.7 a | 2.8 ± 0.6 a | 3.4 ± 1.0 ab | 4.0 ± 1.1 b | |
| ND (%) | 500 | 8.6 ± 1.2 a | 25.0 ± 2.8 b | 52.0 ± 6.2 c | 68.3 ± 10.1 d |
| 1000 | 20.3 ± 3.5 a | 25.8 ± 7.7 a | 53.0 ± 7.1 b | 61.6 ± 12.5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagos, C.; Larsen, J.; Fuentes, A.; Herrera, H.; García-Romera, I.; Campos-Vargas, R.; Arriagada, C. Inoculation of Triticum Aestivum L. (Poaceae) with Plant-Growth-Promoting Fungi Alleviates Plant Oxidative Stress and Enhances Phenanthrene Dissipation in Soil. Agronomy 2021, 11, 411. https://doi.org/10.3390/agronomy11030411
Lagos C, Larsen J, Fuentes A, Herrera H, García-Romera I, Campos-Vargas R, Arriagada C. Inoculation of Triticum Aestivum L. (Poaceae) with Plant-Growth-Promoting Fungi Alleviates Plant Oxidative Stress and Enhances Phenanthrene Dissipation in Soil. Agronomy. 2021; 11(3):411. https://doi.org/10.3390/agronomy11030411
Chicago/Turabian StyleLagos, Claudio, John Larsen, Alejandra Fuentes, Hector Herrera, Inmaculada García-Romera, Reinaldo Campos-Vargas, and Cesar Arriagada. 2021. "Inoculation of Triticum Aestivum L. (Poaceae) with Plant-Growth-Promoting Fungi Alleviates Plant Oxidative Stress and Enhances Phenanthrene Dissipation in Soil" Agronomy 11, no. 3: 411. https://doi.org/10.3390/agronomy11030411






