Analysis of Physio-Biochemical Responses and Expressional Profiling Antioxidant-Related Genes in Some Neglected Aegilops Species under Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genetic Materials and Experimental Setup
2.2. Determination of Root and Shoot Dry Weight
2.3. Determine of Root and Shoot Na+ and K+ Contents
2.4. Determination of Antioxidant Enzyme Activities
2.5. Estimation of Gene Expression Patterns
2.6. Data Analysis
3. Results
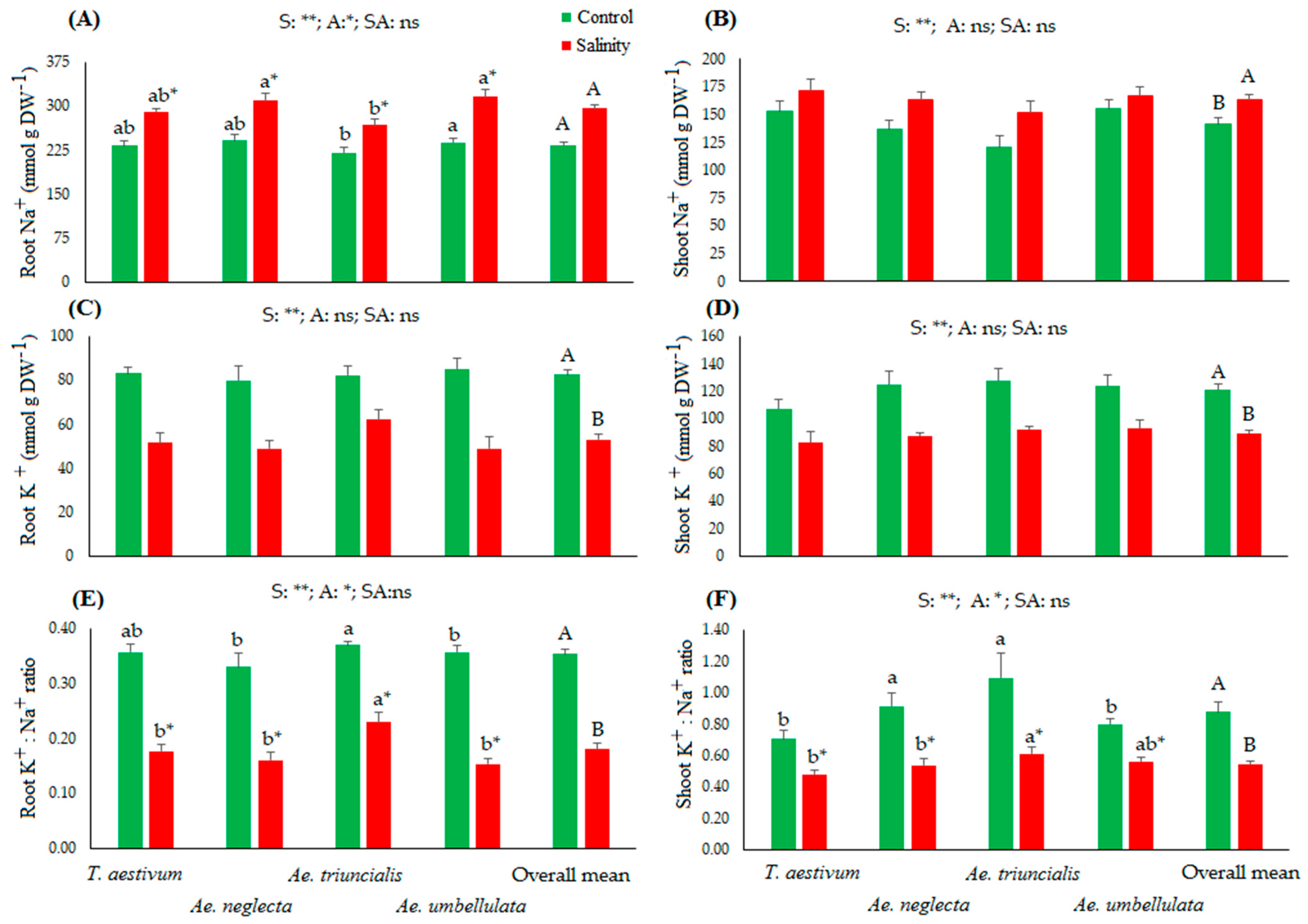
3.1. Root and Shoot Biomass and Their Ion Concentrations
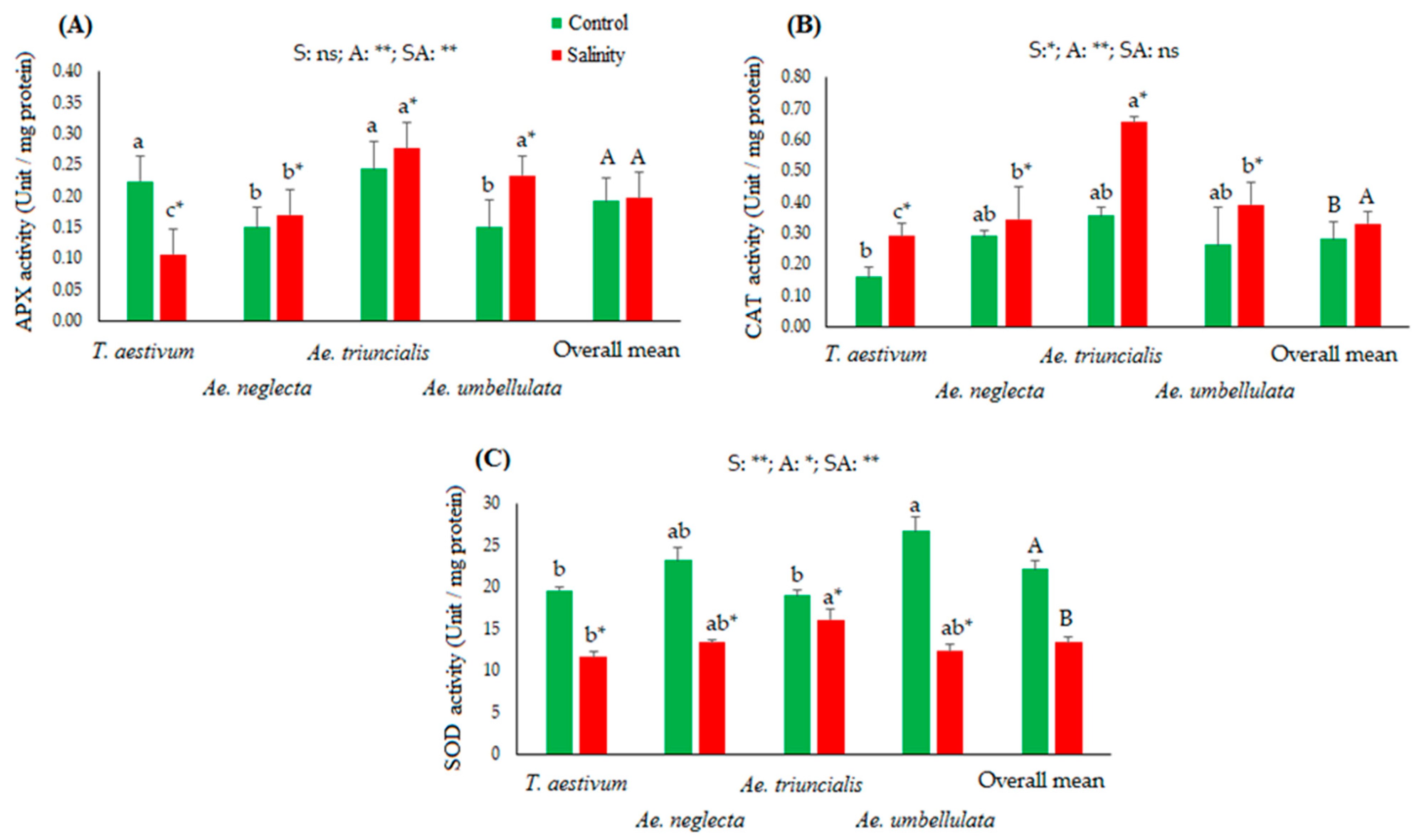
3.2. Biochemical Activities in Studied Species
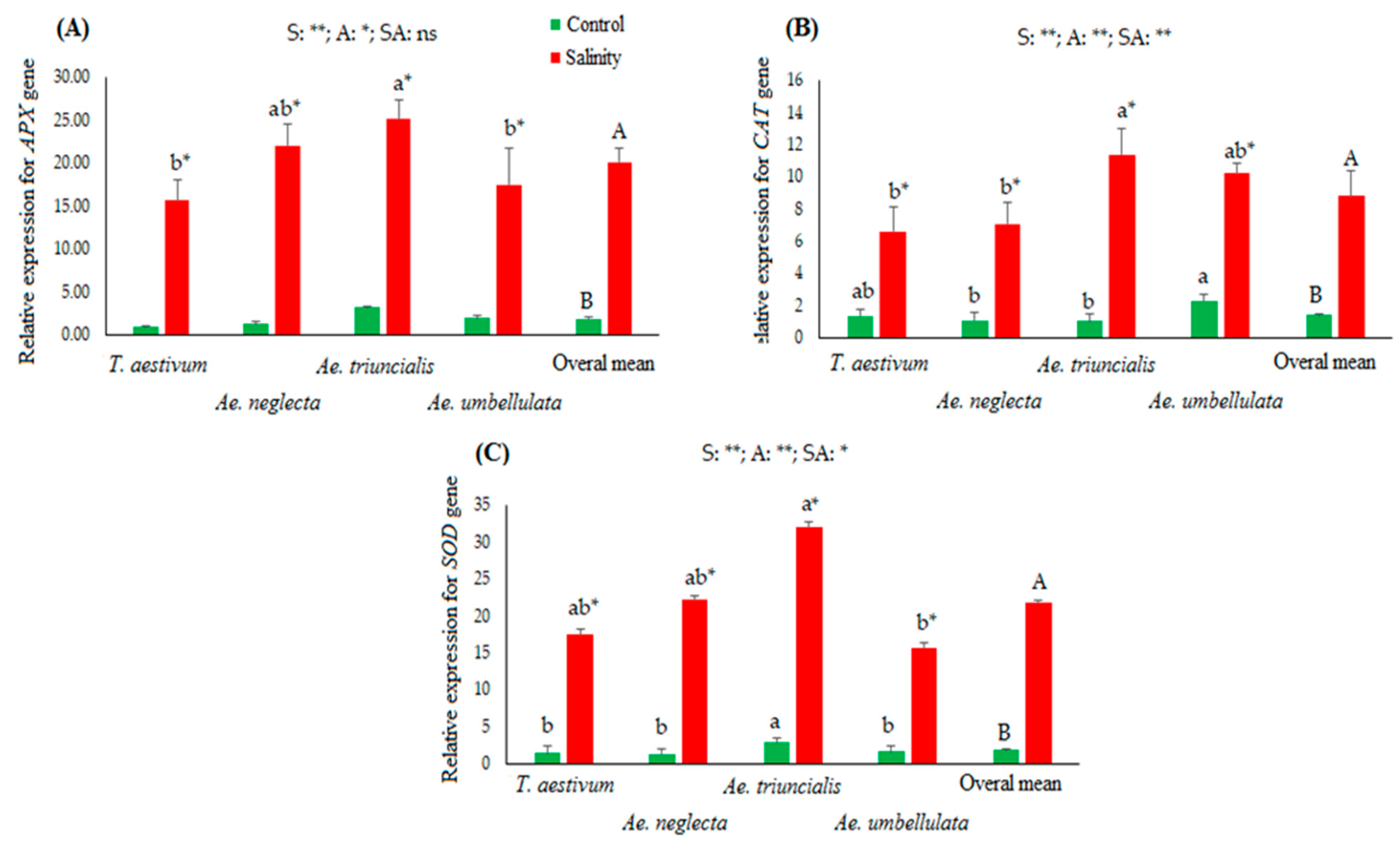
3.3. Gene Expression Evaluation in Studied Accessions
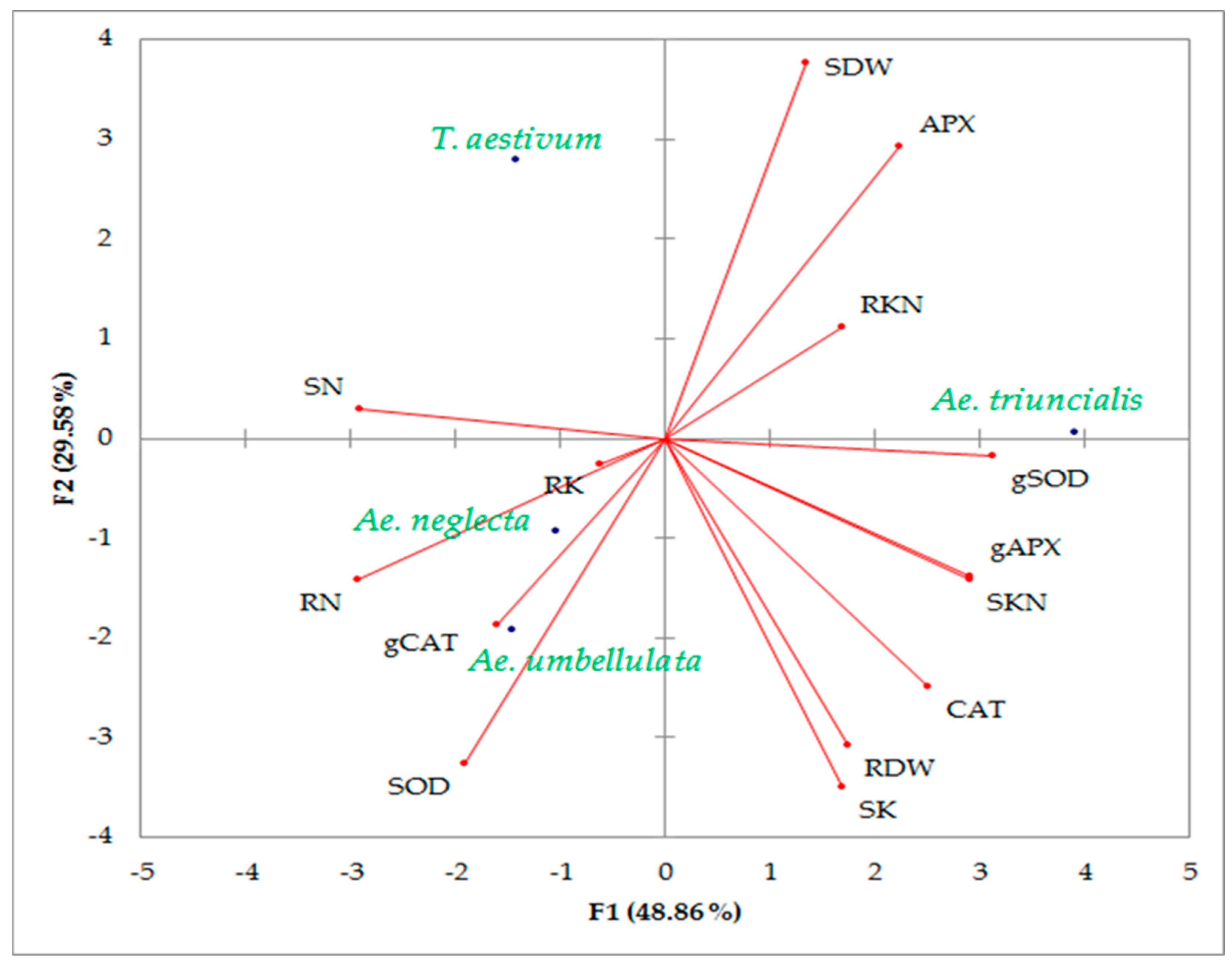
3.4. Association among Measured Trait under Control and Salinity Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity stress in potato: Understanding physiological, biochemical and molecular responses. Life 2021, 11, 545. [Google Scholar] [CrossRef]
- Eliazer Nelson, A.R.L.; Ravichandran, K.; Antony, U. The impact of the green revolution on indigenous crops of India. J. Ethn. Foods 2019, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.K.; Singh, A. Salinity research in India-achievements, challenges and future prospects. Water Energy Int. 2015, 58, 35–45. [Google Scholar]
- FAO. Special Report: 2021 FAO Crop and Food Supply Assessment Mission to the Syrian Arab Republic—December; FAO: Rome, Italy, 2021. [Google Scholar]
- Ghorbani, S.; Etminan, A.; Rashidi, V.; Pour-Aboughadareh, A.; Shooshtari, L. Delineation of physiological and transcriptional responses of different barley genotypes to salt stress. Cereal Res. Commun. 2023, 51, 367–377. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Hagemann, M.; Erdmann, N. Environmental stresses. In Cyanobacterial Nitrogen Metabolism and Environmental Biotechnology; Rai, A.K., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 156–221. [Google Scholar]
- Anil, V.S.; Krishnamurthy, H.; Mathew, M.K. Limiting cytosolic Na+ confers salt tolerance to rice cells in culture: A two-photon microscopy study of SBFI loaded cells. Physiol. Plant 2007, 129, 607–621. [Google Scholar] [CrossRef]
- Munns, R.; Schachtman, D.; Condon, A. The significance of a twophase growth response to salinity in wheat and barley. Funct. Plant Biol. 1995, 22, 561–569. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.J.; Negrao, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Ruelland, E.; Vaultier, M.N.; Zachowski, A.; Hurry, V. Cold signaling and cold acclimation in plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Abedi, T.; Pakniyat, H. Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J. Plant Breed. 2010, 46, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Pour-Aboughadareh, A.; Jadidi, O.; Shooshtari, L.; Poczai, P.; Mehrabi, A.A. Association analysis for some biochemical traits in wild relatives of wheat under drought stress conditions. Genes 2022, 13, 1491. [Google Scholar] [CrossRef]
- Ahmadi, J.; Pour-Aboughadareh, A.; Fabriki-Ourang, S.; Khalili, P.; Poczai, P. Unravelling salinity stress responses in ancestral and neglected wheat species at early growth stage: A baseline for utilization in future wheat improvement programs. Physiol. Mol. Biol. Plants 2020, 26, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Pour-Aboughadareh, A.; Omidi, M.; Naghavi, M.R.; Etminan, A.; Mehrabi, A.A.; Poczai, P. Wild relatives of wheat respond well to water deficit stress: A comparative study of antioxidant enzyme activities and their encoding gene expression. Agriculture 2020, 10, 415. [Google Scholar] [CrossRef]
- Suneja, Y.; Gupta, A.K.; Bains, N.S. Bread wheat progenitors: Aegilops tauschii (DD genome) and Triticum dicoccoides (AABB genome) reveal differential antioxidative response under water stress. Physiol. Mol. Biol. Plants 2017, 23, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Suneja, Y.; Gupta, A.K.; Bains, N.S. Stress adaptive plasticity: Aegilops tauschii and Triticum dicoccoides as potential donors of drought associated morpho-physiological traits in Wheat. Front. Plant Sci. 2019, 10, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousfi, S.; Marquez, A.J.; Betti, M. Gene expression and physiological responses to salinity and water stress of contrasting durum wheat genotypes. J. Integr. Plant Biol. 2016, 58, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.M. Oxidative stress markers and antioxidant potential of wheat treated with phytohormones under salinity stress. J. Stress Physiol. Biochem. 2011, 7, 250–267. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2013, e217037. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.R.; Sharma, S.K.; Goswami, S.; Singh, K.; Gadpayle, K.A.; Singh, G.P.; Pathak, H.; Rai, R.D. Transcript profiling and biochemical characterization of mitochondrial superoxide dismutase (mtSOD) in wheat (Triticum aestivum) under different exogenous stresses. Aust. J. Crop Sci. 2013, 7, 414–424. [Google Scholar]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef]
- Matsumura, T.; Tabayashi, N.; Kamagata, Y.; Souma, C.; Saruyama, H. Wheat catalase expressed in transgenic rice can improve tolerance against low temperature stress. Physiol. Plantarum. 2002, 116, 317–327. [Google Scholar] [CrossRef]
- Zhai, C.Z.; Zhao, L.; Yin, L.J.; Chen, M.; Wang, Q.Y.; Li, L.C.; Xu, Z.S.; Ma, Y.Z. Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis. PLoS ONE 2013, 8, e73989. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, J.; Pour-Aboughadareh, A.; Fabriki-Ourang, S.; Mehrabi, A.-A.; Siddique, K.H.M. Screening wild progenitors of wheat for salinity stress at early stages of plant growth: Insight into potential sources of variability for salinity adaptation in wheat. Crop Pasture Sci. 2018, 69, 649–658. [Google Scholar] [CrossRef]
- Ahmadi, J.; Pour-Aboughadareh, A.; Ourang, S.F.; Mehrabi, A.A.; Siddique, K.H.M. Wild relatives of wheat: Aegilops—Triticum accessions disclose differential antioxidative and physiological responses to water stress. Acta Physiol. Plant. 2018, 40, 90. [Google Scholar] [CrossRef]
- Arabbeigi, M.; Arzani, A.; Majidi, M.M. Expression Profiles of P5CS and DREB2 Genes under Salt Stress in Aegilops cylindrica. Russ. J. Plant Physiol. 2019, 66, 583–590. [Google Scholar] [CrossRef]
- Arabbeigi, M.; Arzani, A.; Majidi, M.M.; Sayed-Tabatabaei, B.E.; Saha, P. Expression pattern of salt tolerance-related genes in Aegilops cylindrica. Physiol. Mol. Biol. Plants 2018, 24, 61–73. [Google Scholar] [CrossRef]
- Darko, E.; Khalil, R.; Dobi, Z.; Kovács, V.; Szalai, G.; Janda, T.; Molnár, I. Addition of Aegilops biuncialis chromosomes 2M or 3M improves the salt tolerance of wheat in different way. Sci. Rep. 2020, 10, 22327. [Google Scholar] [CrossRef]
- Dulai, S. Effects of drought on photosynthetic parameters and heat stability of PSII in wheat and in Aegilops species originating from dry habitats. Acta Biologica Szegediensis 2006, 50, 11–17. [Google Scholar]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef] [PubMed]
- Masoomi-Aladizgeh, F.; Aalami, A.; Esfahani, M.; Aghaei, M.J.; Mozaffari, K. Identification of CBF14 and NAC2 genes in Aegilops tauschii associated with resistance to freezing stress. Appl. Biochem. Biotechnol. 2015, 176, 1059–1070. [Google Scholar] [CrossRef]
- Hairat, S.; Khurana, P. Evaluation of Aegilops tauschii and Aegilops speltoides for acquired thermotolerance: Implications in wheat breeding programmes. Plant Physiol. Biochem. 2015, 95, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pour-Aboughadareh, A.; Kianersi, F.; Poczai, P.; Moradkhani, H. Potential of wild relatives of wheat: Ideal genetic resources for future breeding programs. Agronomy 2021, 11, 1656. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. In California Agricultural Experiment Station, Circular No. 374; The College of Agriculture, University of California: Berkeley, CA, USA, 1950. [Google Scholar]
- Pour-Aboughadareh, A.; Mehrvar, M.R.; Sanjani, S.; Amini, A.; Nikkhah-Chamanabad, H.; Asadi, A. Effects of salinity stress on seeedling biomass, physiochemical properties, and grain yield in different breeding wheat genotypes. Acta Physiol. Plant. 2021, 43, 98. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Antioxidant enzymes and DPPHradical scavenging activity in chilled and heat shocked rice (Oryza sativa L.) seedling radicles. J. Agric. Food Chem. 2002, 50, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, N.K. Hydrogen peroxide is scavenged by ascorbate-specifc peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867. [Google Scholar]
- Chance, B.; Maehly, S.K. Assay of catalase and peroxidase. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Dhindsa, R.S.; Plump-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Baek, K.H.; Skinner, D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003, 165, 1221–1227. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, S.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- SAS Institute. Base SAS 9.1 Procedures Guide; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Kiani, R.; Arzani, A.; Habibi, F. Physiology of salinity tolerance in Aegilops cylindrica. Acta Physiol. Plant. 2015, 37, 135–145. [Google Scholar] [CrossRef]
- Itam, M.; Abdelrahman, M.; Yamasaki, Y.; Mega, R.; Gorafi, Y.; Akashi, K.; Tsujimoto, H. Aegilops tauschii introgressions improve physio-biochemical traits and metabolite plasticity in bread wheat under drought stress. Agronomy 2020, 10, 1588. [Google Scholar] [CrossRef]
- Rampino, P.; Pataleo, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006, 29, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Arzani, A.; Ashraf, M. Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Crit. Rev. Plant Sci. 2016, 35, 146–189. [Google Scholar] [CrossRef]
- Arabbeigi, M.; Arzani, A.; Majidi, M.M.; Kiani, R.; Tabatabaei, B.E.S.; Habibi, F. Salinity tolerance of Aegilops cylindrica genotypes collected from hyper-saline shores of Uremia Salt Lake using physiological traits and SSR markers. Acta Physiol. Plant 2014, 36, 2243–2251. [Google Scholar] [CrossRef]
- Mansouri, M.; Naghavi, M.R.; Alizadeh, H.; Mohammadi-Nejad, G.; Mousavi, S.A.; Salekdeh, G.H.; Tada, Y. Transcriptomic analysis of Aegilops tauschii during long-term salinity stress. Funct. Integr. Genom. 2019, 19, 13–28. [Google Scholar] [CrossRef]
- Bocianowski, J.; Prażak, R. Genotype by year interaction for selected quantitative traits in hybrid lines of Triticum aestivum L. with Aegilops kotschyi Boiss. and Ae. variabilis Eig. using the additive main effects and multiplicative interaction model. Euphytica 2022, 218, 11. [Google Scholar] [CrossRef]
- Mguis, K.; Albouchi, A.; Abassi, M.; Khadhri, A.; Ykoubi-Tej, M.; Mahjoub, A.; Brahim, N.B.; Ouerghi, Z. Responses of leaf growth and gas exchanges to salt stress during reproductive stage in wild wheat relative Aegilops geniculata Roth. and wheat (Triticum durum Desf.). Acta Physiol. Plant 2013, 35, 1453–1461. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Schneider, B. Identification of potential allelochemicals from donor plants and their synergistic effects on the metabolome of Aegilops geniculata. Front. Plant Sci. 2020, 11, 1046. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Tewari, R.K.; Sharma, P.N. Cadmium enhances generation of hydrogen peroxide and amplifies activities of catalase, peroxidases and superoxide dismutase in maize. J. Agron. Crop Sci. 2007, 194, 72–80. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Li, S.; Hu, G.; Zhang, L.; Song, X. Tapetal-Delayed Programmed Cell Death (PCD) and Oxidative Stress-Induced Male Sterility of Aegilops uniaristata Cytoplasm in Wheat. Int. J. Mol. Sci. 2018, 19, 1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Türkan, I.; Bor, M.; Özdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Li, L.; Yi, H. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [Green Version]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Jeyasri, R.; Rakkammal, K.; Satish, L.; Shamili, S.; Karthikeyan, A.; Valliammai, A.; Priya, A.; Selvaraj, A.; Gowri, P.; et al. Multi-Omics and integrative approach towards understanding salinity tolerance in rice: A Review. Biology 2022, 11, 1022. [Google Scholar] [CrossRef]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M.; Rahimmalek, M.; Razavi, K. Morpho-physiological and gene expression responses of wheat by Aegilops cylindrica amphidiploids to salt stress. Plant Cell Tiss. Organ. Cult. 2021, 144, 619–639. [Google Scholar] [CrossRef]
- Saisho, D.; Takumi, S.; Matsuoka, Y. Salt tolerance during germination and seedling growth of wild wheat Aegilops tauschii and its impact on the species range expansion. Sci. Rep. 2016, 6, 38554. [Google Scholar] [CrossRef] [Green Version]
- Samtani, H.; Sharma, A.; Khurana, J.P.; Khurana, P. The heat stress transcription factor family in Aegilops tauschii: Genome-wide identification and expression analysis under various abiotic stresses and light conditions. Mol. Genet. Genom. 2022, 297, 1689–1709. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, A.; Yang, C.; Miller, M.E.; Kianian, S.F.; Marchetto, P.M. A novel approach to assess salt stress tolerance in wheat using hyperspectral imaging. Front. Plant Sci. 2018, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Yang, X.E.; Yang, M.J.; Tian, S.K. Responses of antioxidant enzyme system to copper toxicity and copper detoxification in the leaves of Elsholtzia splendens. J. Plant Nutr. 2006, 29, 1619–1635. [Google Scholar] [CrossRef]
- Rakszegi, M.; Darkó, É.; Alison Lovegrove, A.; Molnár, I.; Láng, L.; Bedő, Z.; Molnár-Láng, M.; Shewry, P. Drought stress affects the protein and dietary fiber content of wholemeal wheat flour in wheat/Aegilops addition lines. PLoS ONE 2019, 14, e0211892. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Begum, M.C.; Kabir, A.H.; Alam, M.F. Molecular and biochemical mechanisms associated with differential responses to drought tolerance in wheat (Triticum aestivum L.). J. Plant Interact. 2015, 10, 195–201. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Al Mahmud, J.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Yu, J.; Cang, J.; Lu, Q.; Fan, B.; Xu, Q.; Li, W.; Wang, X. ABA enhanced cold tolerance of wheat ‘dn1′ via increasing ROS scavenging system. Plant Signal. Behav. 2020, 15, 1780403. [Google Scholar] [CrossRef]
- Li, C.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Effects of exogenous spermidine on antioxidants and glyoxalase system of lettuce seedlings under high temperature. Plant Signal. Behav. 2020, 15, 12. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.; Wang, Y.; He, G.; Wang, J.; Guo, D.; Li, T.; Sun, G.; Zhang, H. The role of antioxidant mechanism in photosynthesis under heavy metals Cd or Zn exposure in tobacco leaves. J. Plant Interact. 2021, 16, 354–366. [Google Scholar] [CrossRef]
- Joshi, B.; Chaudhary, A.; Singh, H.; Kumar, P.A. Prospective evaluation of individual and consortia plant growth promoting rhizobacteria for drought stress amelioration in rice (Oryza sativa L.). Plant Soil 2020, 457, 225–240. [Google Scholar] [CrossRef]
- Demirevska-Kepova, K.; Simova-Stoilova, L.; Stoyanova, Z.P.; Feller, U. Cadmium stress in barley: Growth, leaf pigment, and protein composition and detoxification of reactive oxygen species. J. Plant Nutr. 2006, 29, 451–468. [Google Scholar] [CrossRef]
- Gama, P.B.S.; Tanaka, K.; Eneji, A.E.; Eltayeb, A.E.; El Siddig, K. Salt-Induced stress effects on biomass, photosynthetic rate, and reactive oxygen species-scavenging enzyme accumulation in common bean. J. Plant Nutr. 2009, 32, 837–854. [Google Scholar] [CrossRef]
- Filiz, E.; Ozyigit, I.I.; Saracoglu, I.A.; Uras, M.E.; Sen, U.; Yalcin, B. Abiotic stress-induced regulation of antioxidant genes in different Arabidopsis ecotypes: Microarray data evaluation. Biotechnol. Biotechnol. Equip. 2019, 33, 128–143. [Google Scholar] [CrossRef] [Green Version]
- Nasibi, F.; Yaghoobi, M.M.; Kalantari, K.M. Effect of exogenous arginine on alleviation of oxidative damage in tomato plant underwater stress. J. Plant Interact. 2011, 6, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Mandal, C.; Ghosh, N.; Maiti, S.; Das, K.; Gupta, S.; Dey, N.; Adak, M.K. Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it’s modulation by polyamine. Physiol. Mol. Biol. Plants 2013, 19, 91–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, R.; Thukral, A.K.; Narang, U. Interactive effects of binary combinations of manganese with other heavy metals on metal uptake and antioxidative enzymes in Brassica juncea L. seedlings. J. Plant Interact. 2011, 6, 25–34. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamshidi, B.; Pour-Aboughadareh, A.; Bocianowski, J.; Shooshtari, L.; Bujak, H.; Türkoğlu, A.; Nowosad, K. Analysis of Physio-Biochemical Responses and Expressional Profiling Antioxidant-Related Genes in Some Neglected Aegilops Species under Salinity Stress. Agronomy 2023, 13, 1981. https://doi.org/10.3390/agronomy13081981
Jamshidi B, Pour-Aboughadareh A, Bocianowski J, Shooshtari L, Bujak H, Türkoğlu A, Nowosad K. Analysis of Physio-Biochemical Responses and Expressional Profiling Antioxidant-Related Genes in Some Neglected Aegilops Species under Salinity Stress. Agronomy. 2023; 13(8):1981. https://doi.org/10.3390/agronomy13081981
Chicago/Turabian StyleJamshidi, Bita, Alireza Pour-Aboughadareh, Jan Bocianowski, Lia Shooshtari, Henryk Bujak, Aras Türkoğlu, and Kamila Nowosad. 2023. "Analysis of Physio-Biochemical Responses and Expressional Profiling Antioxidant-Related Genes in Some Neglected Aegilops Species under Salinity Stress" Agronomy 13, no. 8: 1981. https://doi.org/10.3390/agronomy13081981








