Performance and Stability Analysis of Extra-Early Maturing Orange Maize Hybrids under Drought Stress and Well-Watered Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genetic Material
2.2. Generation of Crosses
2.3. Field Evaluation
2.4. Data Collection
2.5. Data Analysis
3. Results
3.1. Analysis of Variance for Grain Yield and Other Traits under Managed Drought and Well-Watered Conditions
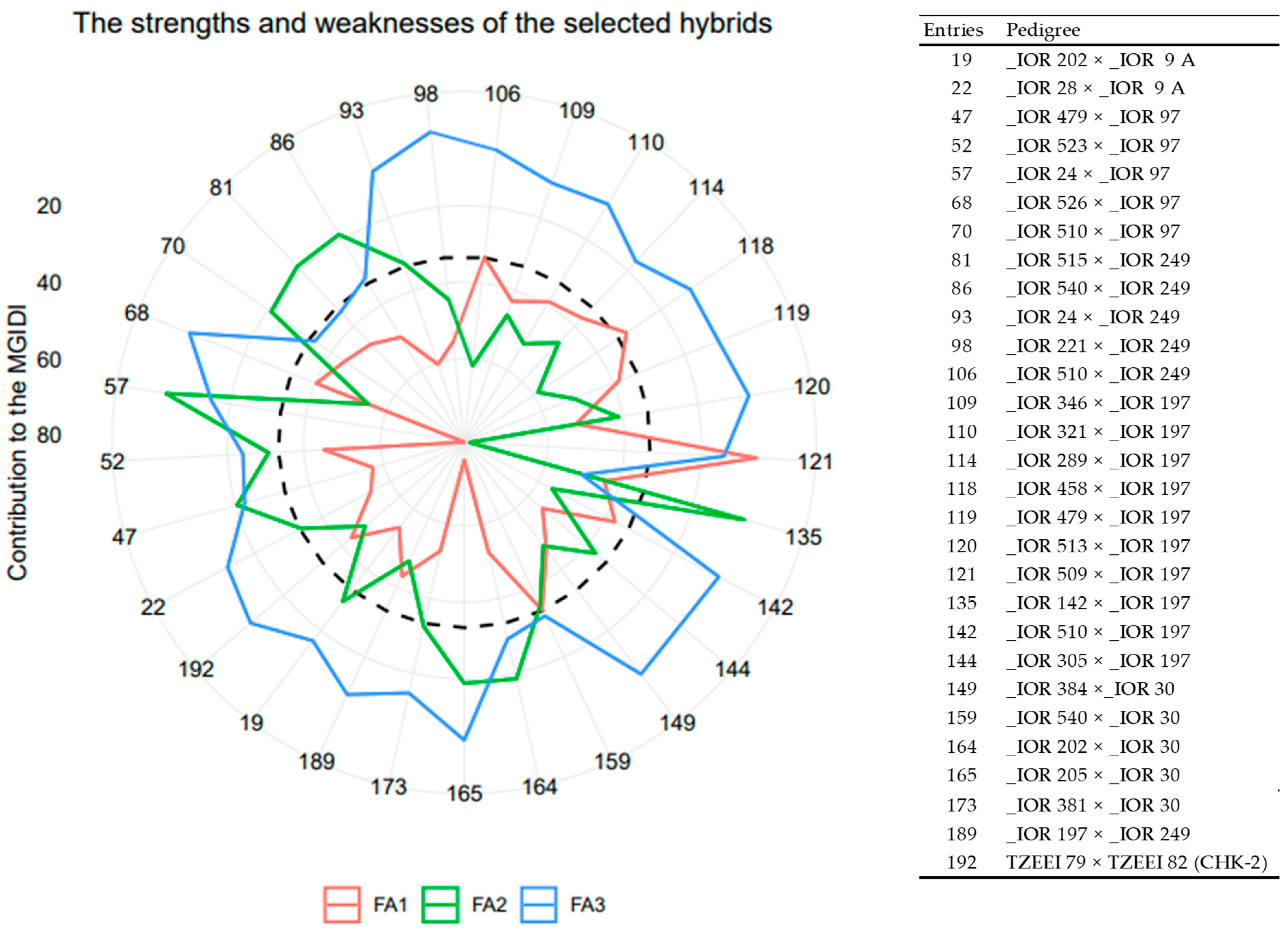
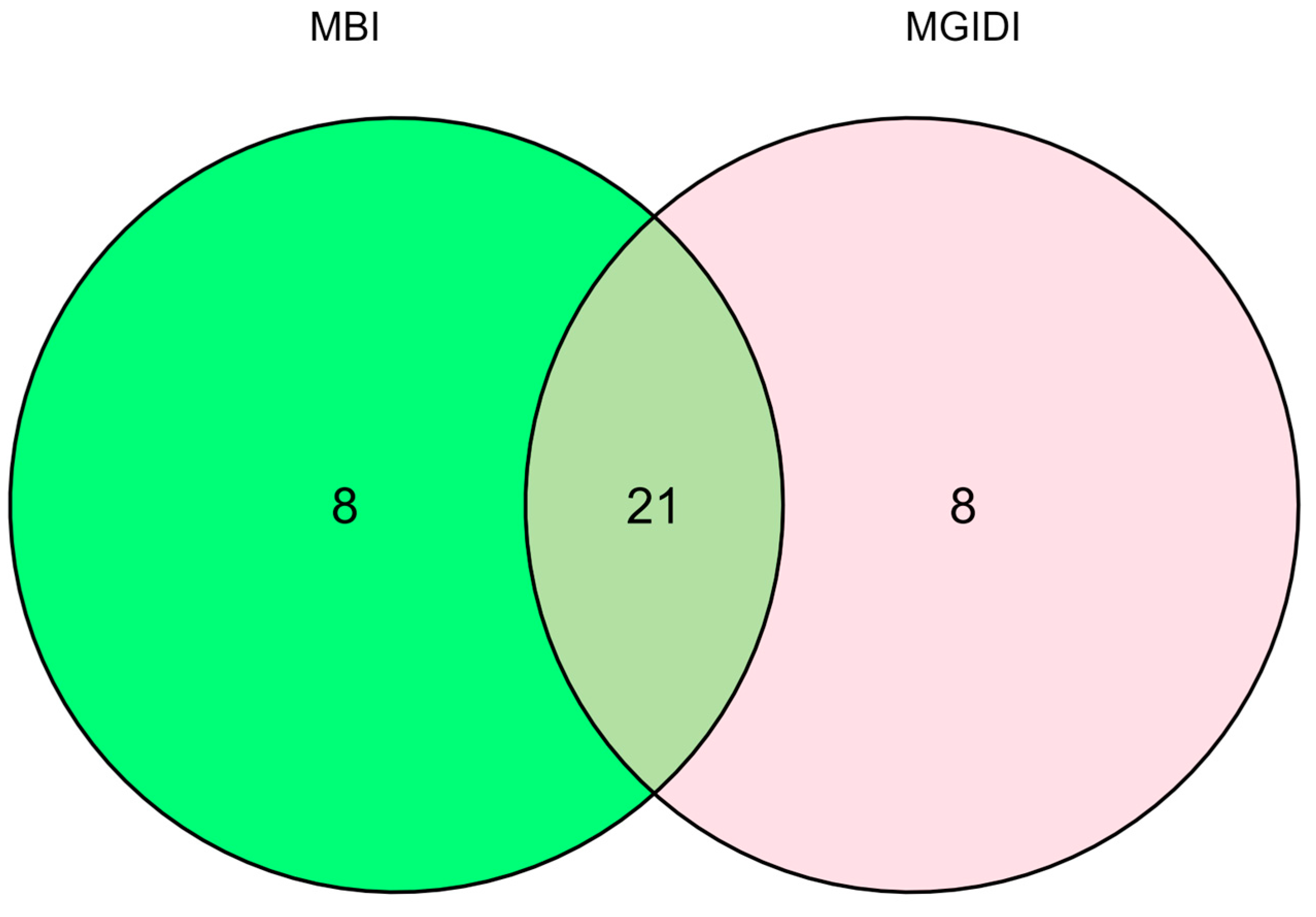
3.2. Selection of Outstanding Orange Hybrids with Drought Tolerance Using the MGIDI Selection Method
3.3. Selection of Drought-Tolerant Hybrids Using the Base Index Derived from Multiple Traits
3.4. Yield Performance and Grain Yield Reduction under Drought
3.5. Stability Analysis of Forty Orange Hybrids Comprising Best, Average, and Worst Performing Genotypes across All Environments
3.6. Phenotypic Correlations among Measured Traits under Managed Drought, Well-Watered, and across Research Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 March 2024).
- Kurilich, A.C.; Juvik, J.A. Quantification of carotenoid and tocopherol antioxidants in Zea mays. J. Agric. Food Chem. 1999, 47, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Tien, L.D.; Duc, C.H.; Quynh, L.N. Improving nutritional quality of plant proteins through genetic engineering. Curr. Genom. 2016, 17, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Fawzi, W.W. Vitamin A supplementation: Implications for morbidity and mortality in children. J. Infect. Dis. 2000, 182, S122–S133. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A. Vitamin A deficiency and clinical disease: An historical overview. J. Nutr. 2008, 138, 1835–1839. [Google Scholar] [CrossRef]
- Semagn, K.; Beyene, Y.; Babu, R.; Nair, S.; Gowda, M.; Das, B.; Tarekegne, A.; Mugo, S.; Mahuku, G.; Worku, M.; et al. Quantitative trait loci mapping and molecular breeding for developing stress resilient maize for Sub-Saharan Africa. Crop Sci. 2015, 55, 1449–1459. [Google Scholar] [CrossRef]
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Kamara, A.Y.; Ewansiha, S.; Menkir, A. Assessment of nitrogen uptake and utilization in drought tolerant and Striga resistant tropical maize varieties. Arch. Agron. Soil Sci. 2014, 60, 195–207. [Google Scholar] [CrossRef]
- NeSmith, D.; Ritchie, J. Effects of soil water-deficits during tassel emergence on development and yield component of maize (Zea mays). Field Crops Res. 1992, 28, 251–256. [Google Scholar] [CrossRef]
- Menkir, A.; Akintunde, A.O. Evaluation of the performance of maize hybrids, improved open-pollinated and farmers’ local varieties under well-watered and drought stress conditions. Maydica 2001, 46, 227–238. [Google Scholar]
- Badu-Apraku, B.; Fontem, L.A.; Akinwale, R.; Oyekunle, M. Biplot analysis of diallel crosses of early maturing tropical yellow maize inbreds in stress and nonstress environments. Crop Sci. 2011, 51, 173–188. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.M.; Torres, R.V. Genomic and transcriptomic approaches toward plant selection. J. Sci. Res. Rev. Cienc. Investig. 2017, 2, 54–64. [Google Scholar] [CrossRef]
- Campos, H.; Cooper, M.; Edmeades, G.O.; Löffler, C.; Schussler, J.R.; Ibañez, M. Changes in drought tolerance in maize associated with fifty years of breeding for yield in the U.S. corn belt. Maydica 2006, 51, 369–381. [Google Scholar]
- Grant, R.F.; Jackson, B.S.; Kiniry, J.R.; Arkin, G.F. Water deficit timing effects on yield components in maize. Agron. J. 1989, 81, 61–65. [Google Scholar] [CrossRef]
- Monteleone, B.; Borzí, I.; Bonaccorso, B.; Martina, M. Developing stage-specific drought vulnerability curves for maize: The case study of the Po River basin. Agric. Water Manag. 2022, 269, 107713. [Google Scholar] [CrossRef]
- Hwang, T.; Ndolo, V.U.; Katundu, M.; Nyirenda, B.; Bezner-Kerr, R.; Arntfield, S.; Beta, T. Provitamin A potential of landrace orange maize variety (Zea mays L.) grown in different geographical locations of central Malawi. Food Chem. 2016, 196, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Beta, T.; Arntfield, S.; Noren, N.; Bezner-Kerr, R.; Katundu, M.; Ndolo, V.; Shumba, L. Proximate composition and carotenoid content of white, orange and yellow maize cereals grown in central and northern Malawi—Baseline information. In Proceedings of the 17th IUFoST World Congress of Food Science and Technology and EXPO, Montreal, QC, Canada, 17–21 August 2014. [Google Scholar]
- Badu-Apraku, B.; Fakorede, M. Advances in Genetic Enhancement of Early and Extra-Early Maize for Sub-Saharan Africa; Springer: Berlin/Heidelberg, Germany, 2017; 230p. [Google Scholar]
- Olivoto, T.; Nardino, M. MGIDI: Toward an effective multivariate selection in biological experiments. Bioinformatics 2021, 37, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Bizari, E.H.; Unêda-Trevisoli, S.H.; Val, B.H.P.; Pereira, E.D.M.; Di Mauro, A.O. Selection indices for agronomic traits in segregating populations of soybean. Rev. Cienc. Agron. 2017, 48, 110–117. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B.; Oyekunle, M.; Akinwale, R.O. Selection of extra-early maize inbreds under low N and drought at flowering and grain-filling for hybrid production. Maydica 2011, 56, 29–42. [Google Scholar]
- Khatibi, A.; Omrani, S.; Omrani, A.; Shojaei, S.H.; Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Nagy, J. Response of maize hybrids in drought-stress using drought tolerance indices. Water 2022, 14, 1012. [Google Scholar] [CrossRef]
- Yue, H.; Wei, J.; Xie, J.; Chen, S.; Peng, H.; Cao, H.; Bu, J.; Jiang, X. A study on genotype-by-environment interaction analysis for agronomic traits of maize genotypes across Huang-Huai-Hai region in China. Phyton—Int. J. Exp. Bot. 2022, 91, 57–81. [Google Scholar] [CrossRef]
- Swarup, S.; Cargill, E.J.; Crosby, K.; Flagel, L.; Kniskern, J.; Glenn, K.C. Genetic diversity is indispensable for plant breeding to improve crops. Crop Sci. 2020, 61, 839–852. [Google Scholar] [CrossRef]
- Salgotra, K.R.; Chauhan, S.B. Genetic diversity, conservation, and utilization of plant genetic resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Betrán, F.J.; Rooney, L.W. Combining abilities of quality protein maize inbreds. Crop Sci. 2004, 44, 1997–2005. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Annor, B.; Oyekunle, M.; Akinwale, R.; Fakorede, M.; Talabi, A.; Akaogu, I.; Melaku, G.; Fasanmade, Y. Grouping of early maturing quality protein maize inbreds based on SNP markers and combining ability under multiple environments. Field Crops Res. 2015, 183, 169–183. [Google Scholar] [CrossRef]
- Bhadmus, O.A.; Badu-Apraku, B.; Adeyemo, O.A.; Ogunkanmi, A.L. Genetic analysis of early white quality protein maize inbreds and derived hybrids under low-nitrogen and combined drought and heat stress environments. Plants 2021, 10, 2596. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Olivoto, T.; de Souza, V.Q.; Nardino, M.; Carvalho, I.R.; Ferrari, M.; de Pelegrin, A.J.; Szareski, V.J.; Schmidt, D. Multicollinearity in Path Analysis: A Simple Method to Reduce Its Effects. Agron. J. 2017, 109, 131–142. [Google Scholar] [CrossRef]
- Singamsetti, A.; Zaidi, P.H.; Seetharam, K.; Vinayan, M.T.; Olivoto, T.; Mahato, A.; Madankar, K.; Kumar, M.; Shikha, K. Genetic gains in tropical maize hybrids across moisture regimes with multi-trait-based index selection. Front. Plant Sci. 2023, 14, 1147424. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Sanjani, S.; Nikkhah-Chamanabad, H.; Mehrvar, M.R.; Asadi, A.; Amini, A. Identification of salt-tolerant barley genotypes using multiple-traits index and yield performance at the early growth and maturity stages. Bull. Natl. Res. Cent. 2021, 45, 117. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.; Talabi, A.; Oyekunle, M.; Aderounmu, M.; Lum, A.; Ribeiro, P.; Adu, G.; Toyinbo, J. Genetic studies of extra-early provitamin-A maize inbred lines and their hybrids in multiple environments. Crop Sci. 2020, 60, 1325–1345. [Google Scholar] [CrossRef] [PubMed]
- Edmeades, G.O.; Bolaños, J.; Hernàndez, M.; Bello, S. Causes for silk delay in a lowland tropical maize population. Crop Sci. 1993, 33, 1029–1035. [Google Scholar] [CrossRef]
- Lima, D.C.; de Leon, N.; Kaeppler, S.M. Utility of anthesis–silking interval information to predict grain yield under water and nitrogen limited conditions. Crop Sci. 2023, 63, 151–163. [Google Scholar] [CrossRef]
- Oyekunle, M. Genetic Analysis and Molecular Characterisation of Early Maturing Maize (Zea mays L.) Inbred Lines for Drought Tolerance; University of Ibadan: Ibadan, Nigeria, 2014. [Google Scholar]
- Badu-Apraku, B.; Menkir, A.; Ajala, S.; Akinwale, R.; Oyekunle, M.; Obeng-Antwi, K. Performance of tropical early-maturing maize cultivars in multiple stress environments. Can. J. Plant Sci. 2010, 90, 831–852. [Google Scholar] [CrossRef]
- Bonea, D. Grain yield and drought tolerance indices of maize hybrids. Not. Sci. Biol. 2020, 12, 376–386. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordoñez, R.; Araus, J.L. Dissecting maize productivity: Ideotypes associated with grain yield under drought stress and well-watered conditions. J. Integr. Plant Biol. 2012, 54, 1007–1020. [Google Scholar] [CrossRef]
- Széles, A.; Horváth, É.; Simon, K.; Zagyi, P.; Huzsvai, L. Maize production under drought stress: Nutrient supply, yield prediction. Plants 2023, 12, 3301. [Google Scholar] [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Meseka, S.; Menkir, A.; Bossey, B.; Mengesha, W. Performance assessment of drought tolerant maize hybrids under combined drought and heat stress. Agronomy 2018, 8, 274. [Google Scholar] [CrossRef]
- Cairns, J.E.; Crossa, J.; Zaidi, P.H.; Grudloyma, P.; Sanchez, C.; Araus, J.L.; Thaitad, S.; Makumbi, D.; Magorokosho, C.; Bänziger, M.; et al. Identification of drought, heat, and combined drought and heat tolerant donors in maize. Crop Sci. 2013, 53, 1335–1346. [Google Scholar] [CrossRef]
- Bankole, F.; Menkir, A.; Olaoye, G.; Olakojo, O.; Melaku, G. Association studies between grain yield and agronomic traits of a MARS maize (Zea mays L.) population under drought and non-stress condition. Acta Agric. Slov. 2019, 114, 75–84. [Google Scholar] [CrossRef]
- Nasser, L.M.; Badu-Apraku, B.; Gracen, V.E.; Mafouasson, H.N.A. Combining ability of early-maturing yellow maize inbreds under combined drought and heat stress and well-watered environments. Agronomy 2020, 10, 1585. [Google Scholar] [CrossRef]
- Ziyomo, C.; Bernardo, R. Drought tolerance in maize: Indirect selection through secondary traits versus genomewide selection. Crop Sci. 2013, 53, 1269–1275. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crop Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Kumar, P.; Hazarika, M. Application of correlation analysis in conventional plant breeding and genome wide association mapping. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3372–3375. [Google Scholar] [CrossRef]



| Traits | Stage | Description |
|---|---|---|
| DA | Flowering | Number of days from sowing 50% of the plants in a row had pollen shed |
| DS | Flowering | Number of days from sowing 50% of the plants in a row had silk emergence |
| ASI | Flowering | The interval between 50% anthesis and silking |
| PHT | Post flowering | Distance in centimeters from the base of the plant and the first tassel branch |
| EHT | Post flowering | Distance in centimeters between the base of the plant and the uppermost ear |
| PASP | Post-flowering | The general phenotypic appearance of the plants in a plot at 70 days after sowing scored on a scale of 1–9, where 1 = excellent and 9 = very poor |
| HCV | Post-flowering | The protective outer covering of the cob is scored on a scale of 1 to 9 at 70 days after sowing, where 1 = husks tightly arranged and extended beyond the ear tip and 9 = ear tips exposed |
| STGC | Post-flowering | The ability of the plant to retain the greenness of the leaves at 70 days after sowing is scored on a scale of 1 to 9, where 1 = almost all leaves green and 9 = virtually all leaves dead |
| EASP | Harvest | The phenotypic appearance of the cob after harvest scored on a scale of 1–9, where 1 = clean, uniform, large, and well-filled ears and 9 = ears with undesirable features, such as diseases, small ears, ears rot and ears with poorly filled grains |
| EPP | Harvest | Calculated by dividing the number of ears harvested by the number of plants from where the ears were harvested. |
| GY | Harvest | Computed from the weight of the shelled grain adjusted to 80% shelling percentage and corrected for 15% moisture content |
| Source of Variation | DF | GY | ASI | PHT | EHT | PASP | EASP | EPP | HCV |
|---|---|---|---|---|---|---|---|---|---|
| Environment (Env) | 1 | 37,593,481 * | 46.76 * | 6.65 ns | 50.26 ns | 37.12 * | 3.46 ns | 1.25 * | 3.71 ns |
| Genotype (Gen) | 195 | 4,330,349 *** | 0.71 ** | 422.55 *** | 192.61 *** | 2.07 *** | 0.85 ns | 0.02 *** | 7.19 *** |
| Env × Gen | 195 | 1,174,838 ns | 0.42 ns | 67.95 ns | 54.75 ns | 0.65 ns | 1.00 ns | 0.01 ns | 0.82 ns |
| Residual | 1,108,155 | 0.40 | 69.28 | 52.12 | 0.89 | 0.75 | 0.01 | 0.84 | |
| Min | 590.5 | 0.00 | 129.2 | 57 | 2 | 2 | 0.38 | 1 | |
| Max | 10,064 | 4.00 | 213.8 | 180 | 8 | 8 | 1.27 | 8 | |
| Mean | 5568 | 0.62 | 179.3 | 83.56 | 4.44 | 4.68 | 0.92 | 3.92 |
| Source of Variation | DF | GY | ASI | PHT | EHT | PASP | EASP | EPP | HCV | STGC |
|---|---|---|---|---|---|---|---|---|---|---|
| Environment (Env) | 1 | 1,560,132 ns | 35.57 * | 673.19 ns | 2.05 ns | 8.51 ns | 2.45 ns | 0.19 ns | 81.67 *** | 0.10 ns |
| Genotype (Gen) | 195 | 1,464,750 *** | 3.085 *** | 485.84 *** | 193.81 *** | 1.55 *** | 1.61 *** | 0.05 *** | 3.90 *** | 1.93 *** |
| Env × Gen | 195 | 979,359.6 ns | 2.16 * | 258.2 ** | 70.25 ns | 1.08 ns | 1.15 ns | 0.04 ** | 0.95 ns | 1.07 *** |
| Residual | 835,346 | 1.57 ns | 191.5 | 65.2 | 0.98 | 1 | 0.03 | 0.78 | 0.99 | |
| Min | 0 | 0 | 65 | 34 | 2 | 1 | 0 | 1 | 2 | |
| Max | 8407 | 9 | 205.8 | 128.4 | 9 | 9 | 1.17 | 8 | 9 | |
| Mean | 2138.74 | 1.5 | 147.9 | 76.27 | 5.42 | 5.13 | 0.71 | 3.97 | 5.02 |
| Traits | FA1 | FA2 | FA3 | Xo | Xs | Predicted Gain | Sense | Goal |
|---|---|---|---|---|---|---|---|---|
| ASI | −0.62 | 0.49 | −0.13 | 1.51 | 1.49 | −1.03 | decrease | 100 |
| PHT | −0.28 | −0.33 | 0.80 | 147.87 | 148.24 | 0.25 | increase | 100 |
| EHT | −0.11 | 0.13 | 0.93 | 76.36 | 78.69 | 3.04 | increase | 100 |
| HCV | 0.00 | −0.90 | −0.02 | 3.97 | 3.57 | −10.13 | decrease | 100 |
| PASP | −0.73 | −0.20 | 0.41 | 5.42 | 5.41 | −0.17 | decrease | 100 |
| EASP | −0.89 | −0.08 | 0.19 | 5.13 | 5.06 | −1.24 | decrease | 100 |
| EPP | −0.85 | 0.02 | 0.11 | 0.71 | 0.73 | 2.63 | increase | 100 |
| GY | −0.91 | −0.02 | 0.19 | 2138.74 | 2267.90 | 6.04 | increase | 100 |
| STGC | −0.48 | −0.47 | 0.27 | 5.02 | 4.82 | −4.08 | decrease | 100 |
| Traits | FA1 | FA2 | FA3 | Communality | Uniqueness |
|---|---|---|---|---|---|
| ASI | 0.13 | 0.26 | 0.87 | 0.84 | 0.16 |
| PHT | 0.15 | −0.84 | −0.05 | 0.73 | 0.27 |
| EHT | −0.27 | −0.71 | 0.10 | 0.59 | 0.41 |
| HCV | −0.02 | 0.36 | −0.74 | 0.69 | 0.31 |
| PASP | 0.72 | 0.21 | 0.01 | 0.57 | 0.43 |
| EASP | 0.91 | −0.06 | 0.03 | 0.82 | 0.18 |
| EPP | 0.74 | 0.00 | 0.14 | 0.57 | 0.43 |
| GY | 0.81 | −0.08 | 0.19 | 0.69 | 0.31 |
| STGC | 0.38 | 0.09 | −0.18 | 0.18 | 0.82 |
| Entries | Genotypes | GY (kg/ha) under MD | GY (kg/ha) under WW | YR% | MBI | MGIDI | DSI |
|---|---|---|---|---|---|---|---|
| 19 | _IOR 202 × _IOR 9 A | 2098 | 6714 | 68.75 | 5.39 * | 2.65 | 1.12 |
| 22 | _IOR 28 × _IOR 9 A | 2501 | 5341 | 53.17 | 7.79 | 2.06 | 0.86 |
| 39 | _IOR 314 × _IOR 97 | 3604 | 6993 | 48.46 | 8.38 | 3.37 * | 0.79 |
| 47 | _IOR 479 × _IOR 97 | 2858 | 5248 | 45.54 | 7.7 | 2.69 | 0.74 |
| 52 | _IOR 523 × _IOR 97 | 3054 | 6386 | 52.17 | 10.5 | 2.12 | 0.85 |
| 57 | _IOR 24 × _IOR 97 | 2503 | 4392 | 43.02 | 6.41 | 2.66 | 0.70 |
| 68 | _IOR 526 × _IOR 97 | 3319 | 6436 | 48.43 | 9.69 | 2.07 | 0.79 |
| 70 | _IOR 510 × _IOR 97 | 3569 | 7380 | 51.64 | 15.07 | 1.53 | 0.84 |
| 74 | _IOR 321 × _IOR 249 | 3208 | 4918 | 34.78 | 9.31 | 2.89 * | 0.56 |
| 81 | _IOR 515 × _IOR 249 | 2917 | 6722 | 56.6 | 9.81 | 2.36 | 0.92 |
| 86 | _IOR 540 × _IOR 249 | 2923 | 5818 | 49.76 | 9.02 | 2.6 | 0.81 |
| 92 | _IOR 205 × _IOR 249 | 2559 | 5240 | 51.17 | 8.46 | 2.91 * | 0.83 |
| 93 | _IOR 24 × _IOR 249 | 1989 | 4367 | 54.46 | 2.29 * | 2.83 | 0.88 |
| 98 | _IOR 221 × _IOR 249 | 2157 | 5186 | 58.41 | 4.19 * | 2.68 | 0.95 |
| 106 | _IOR 510 × _IOR 249 | 3295 | 5846 | 43.64 | 12.15 | 1.85 | 0.71 |
| 109 | _IOR 346 × _IOR 197 | 2438 | 6398 | 61.89 | 6.58 | 2.59 | 1.00 |
| 110 | _IOR 321 × _IOR 197 | 3116 | 5714 | 45.46 | 11.24 | 1.9 | 0.74 |
| 114 | _IOR 289 × _IOR 197 | 2873 | 7341 | 60.86 | 7.61 | 2.85 | 0.99 |
| 118 | _IOR 458 × _IOR 197 | 3643 | 7062 | 48.42 | 15.91 | 2.06 | 0.79 |
| 119 | _IOR 479 × _IOR 197 | 3074 | 6428 | 52.18 | 10.41 | 2.24 | 0.85 |
| 120 | _IOR 513 × _IOR 197 | 2799 | 6953 | 59.75 | 6.03 * | 2.85 | 0.97 |
| 121 | _IOR 509 × _IOR 197 | 4044 | 7455 | 45.75 | 15.94 | 2.17 | 0.74 |
| 128 | _IOR 205 × _IOR 197 | 2308 | 5990 | 61.46 | 6.71 | 2.95 * | 1.00 |
| 135 | _IOR 142 × _IOR 197 | 2578 | 5954 | 56.69 | 6.03 * | 2.66 | 0.92 |
| 142 | _IOR 510 × _IOR 197 | 2629 | 7051 | 62.72 | 8.9 | 2.42 | 1.02 |
| 144 | _IOR 305 × _IOR 197 | 2648 | 5885 | 55 | 6.08 * | 2.64 | 0.89 |
| 149 | _IOR 384 ×_IOR 30 | 3415 | 6753 | 49.43 | 10.71 | 1.96 | 0.80 |
| 158 | _IOR 509 × _IOR 30 | 3097 | 5469 | 43.37 | 6.84 | 3.01 * | 0.70 |
| 159 | _IOR 540 × _IOR 30 | 3261 | 7086 | 53.98 | 10.69 | 2.36 | 0.88 |
| 164 | _IOR 202 × _IOR 30 | 2297 | 4918 | 53.29 | 8.97 | 2.08 | 0.87 |
| 165 | _IOR 205 × _IOR 30 | 2360 | 6280 | 62.42 | 4.80 * | 2.76 | 1.01 |
| 173 | _IOR 381 × _IOR 30 | 3000 | 5898 | 49.13 | 6.97 | 2.67 | 0.80 |
| 175 | _IOR 536 × _IOR 30 | 3498 | 7390 | 52.67 | 6.34 | 3.13 * | 0.86 |
| 178 | _IOR 525 × _IOR 30 | 3217 | 5618 | 42.73 | 8.85 | 3.17 * | 0.69 |
| 179 | _IOR 510 × _IOR 30 | 3805 | 6392 | 40.47 | 15.18 | 3.63 * | 0.66 |
| 189 | _IOR 197 × _IOR 249 | 2300 | 5464 | 57.91 | 4.53 * | 2.78 | 0.94 |
| 192 | TZEEI 79 × TZEEI 82 (CHK-2) | 3015 | 4720 | 36.13 | 7.91 | 2.26 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonkoungou, T.O.; Badu-Apraku, B.; Adetimirin, V.O.; Nanema, K.R.; Adejumobi, I.I. Performance and Stability Analysis of Extra-Early Maturing Orange Maize Hybrids under Drought Stress and Well-Watered Conditions. Agronomy 2024, 14, 847. https://doi.org/10.3390/agronomy14040847
Bonkoungou TO, Badu-Apraku B, Adetimirin VO, Nanema KR, Adejumobi II. Performance and Stability Analysis of Extra-Early Maturing Orange Maize Hybrids under Drought Stress and Well-Watered Conditions. Agronomy. 2024; 14(4):847. https://doi.org/10.3390/agronomy14040847
Chicago/Turabian StyleBonkoungou, Tégawendé Odette, Baffour Badu-Apraku, Victor Olawale Adetimirin, Kiswendsida Romaric Nanema, and Idris Ishola Adejumobi. 2024. "Performance and Stability Analysis of Extra-Early Maturing Orange Maize Hybrids under Drought Stress and Well-Watered Conditions" Agronomy 14, no. 4: 847. https://doi.org/10.3390/agronomy14040847








