Biochar Application Combined with Water-Saving Irrigation Enhances Rice Root Growth and Nitrogen Utilization in Paddy Fields
Abstract
:1. Introduction
2. Materials and Methods
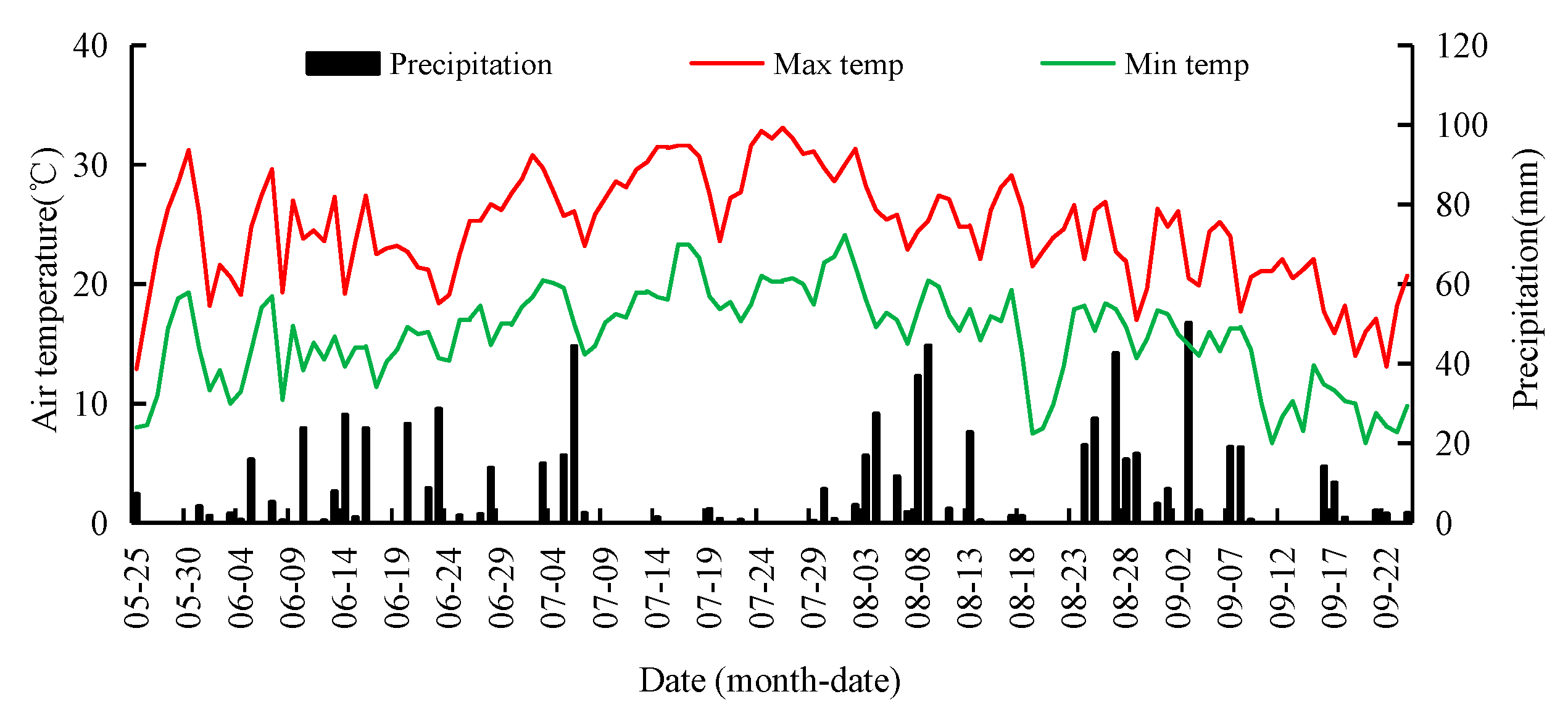
2.1. Site Description
2.2. Experimental Design
2.3. Soil Chemical Indices
2.4. Root Morphological and Physiological Indices
2.5. Plant Nitrogen Utilization
2.6. Nitrogen Agronomic Efficiency and Nitrogen Partial Factor Productivity
2.7. Rice Yields
2.8. Statistical Analyses
3. Results
3.1. Soil Chemical Properties
3.2. Root Morphological Characteristics
3.2.1. Root Length, Volume, and Fresh Weight
3.2.2. Root–Shoot Ratios
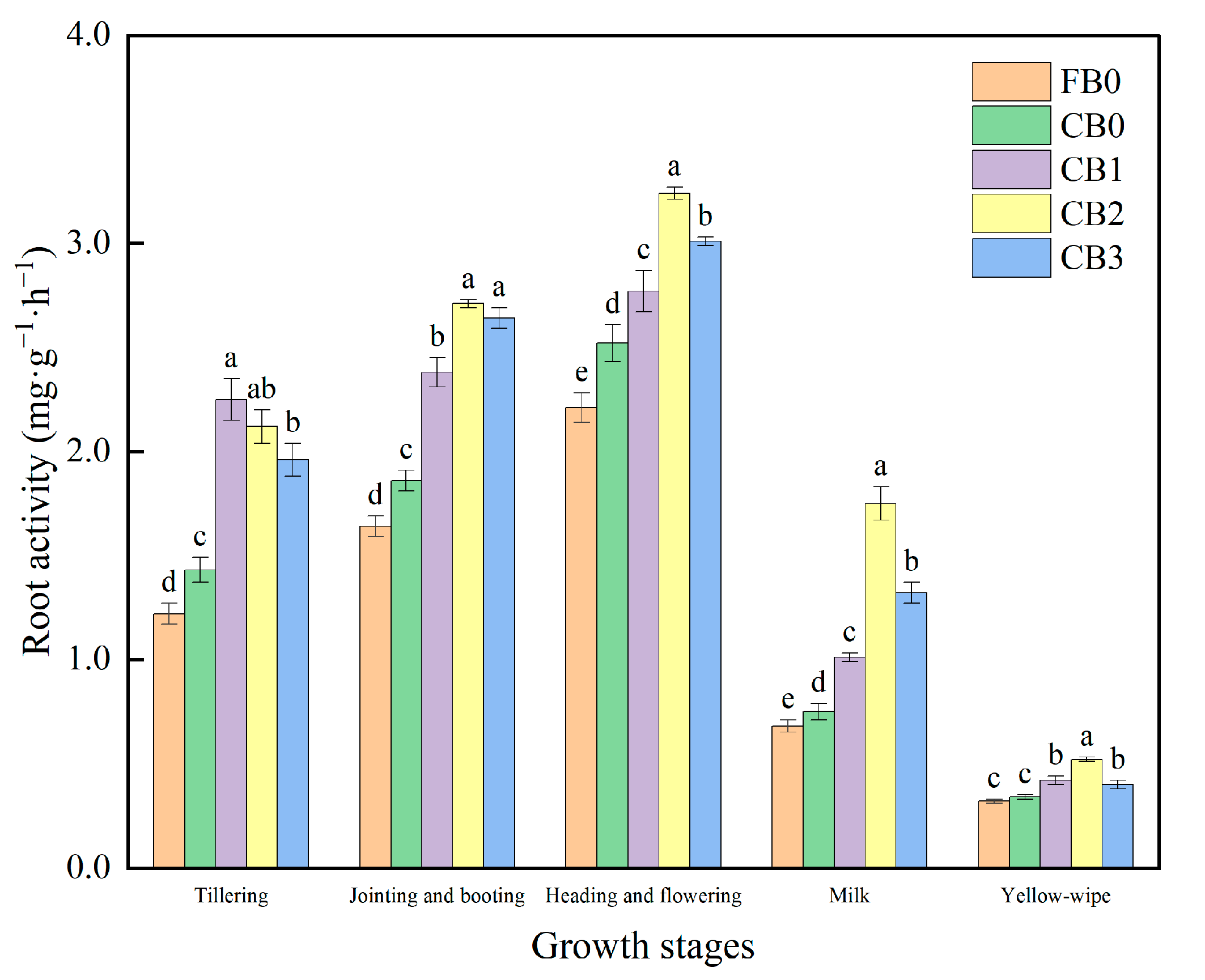
3.3. Root Physiological Characteristics
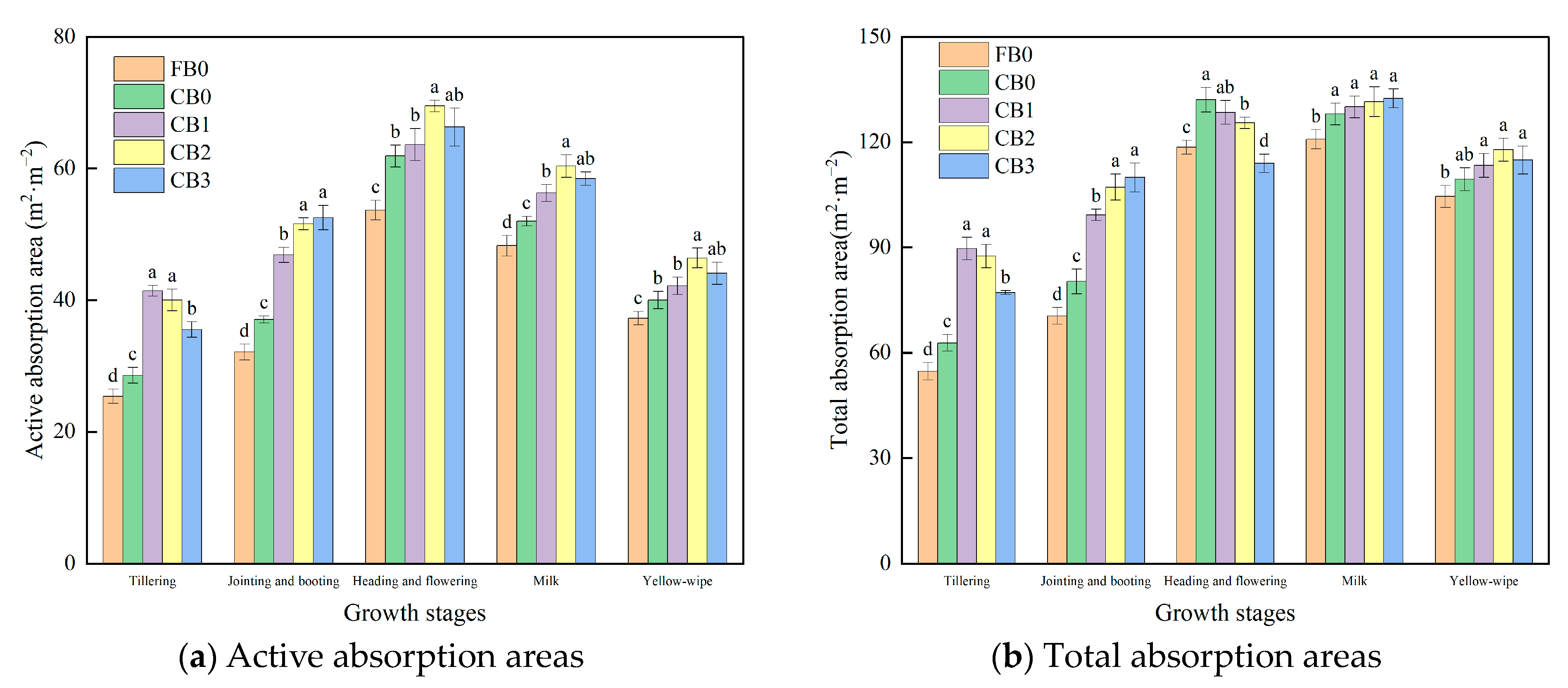
3.3.1. Root Active Absorption Areas and Total Absorption Areas
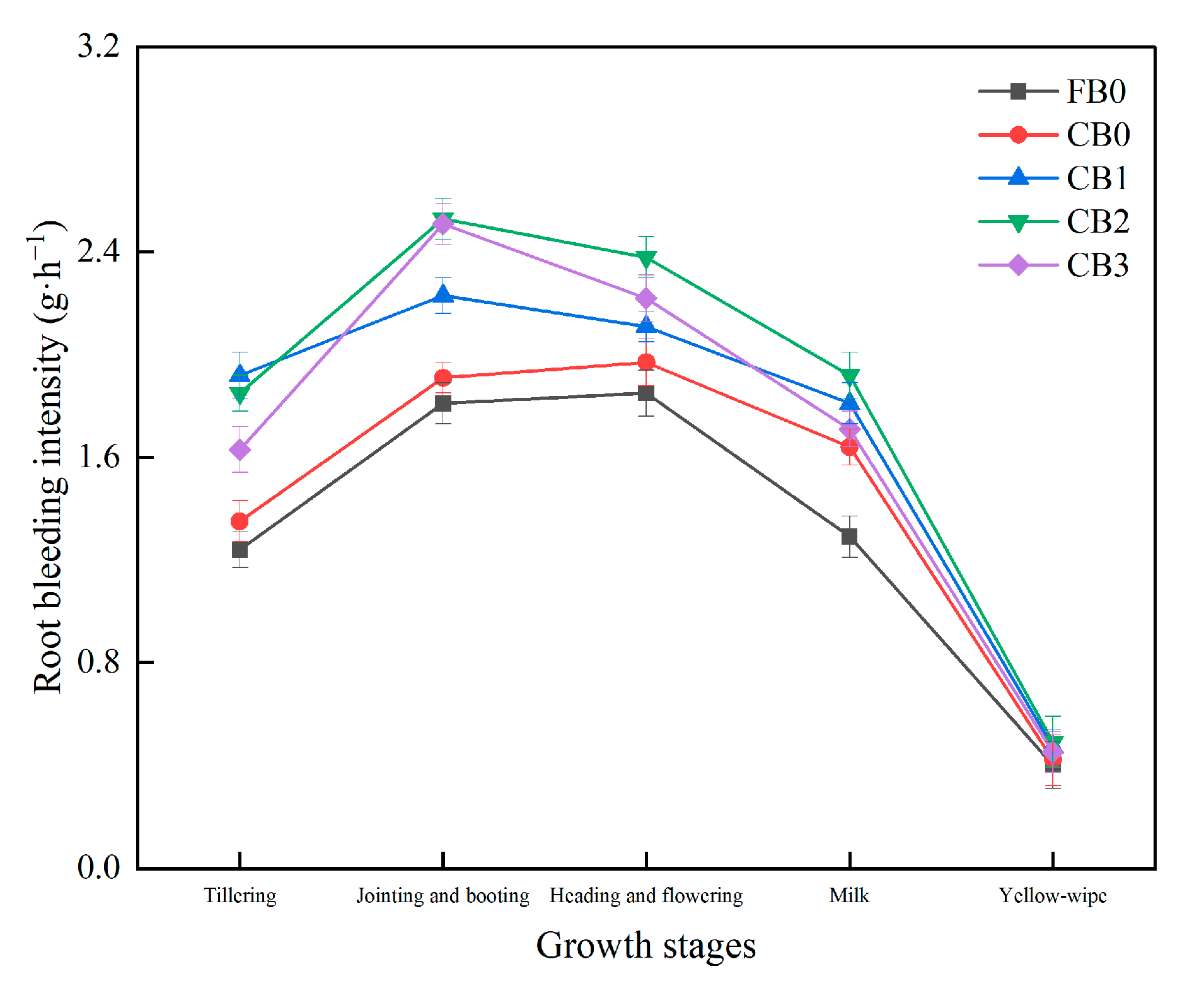
3.3.2. Root Bleeding Intensity
3.4. Absorption of 15N-Fertilizer and Soil Nitrogen by Rice Roots
3.5. Nitrogen Use Efficiency
4. Discussion
4.1. Relationships between Rice Root Morphological and Physiological Characteristics with Nitrogen Uptake
4.2. Effects of Biochar Application on Root Growth under WSI Conditions
4.3. Effects of Biochar Application on NUE and Yields under WSI Conditions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, S.; Zhao, Q.; Wu, K.; Ali, I.; Liang, H.; Iqbal, A.; Wei, S.; Cheng, F.; Ahmad, S.; Jiang, L.; et al. Biochar application to rice with 15N-labelled fertilizers, enhanced leaf nitrogen concentration and assimilation by improving morpho-physiological traits and soil quality. Saudi J. Biol. Sci. 2021, 28, 3399–3413. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2021. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 19 December 2023.).
- Niu, Y.; Xie, G.; Xiao, Y.; Liu, J.; Zou, H.; Qin, K.; Wang, Y.; Huang, M. The story of grain self-sufficiency: China’s food security and food for thought. Food Energy Secur. 2022, 11, e344. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Z.; Meng, J.; Liu, Z.; Wei, J.; Liu, X.; Ge, Z.; Dai, W.; Lin, L.; Chen, W. The positive effects of biochar application on Rhizophagus irregularis, rice seedlings, and phosphorus cycling in paddy soil. Pedosphere 2023, 33, 1–16. [Google Scholar] [CrossRef]
- Xin, F.; Xiao, X.; Dong, J.; Zhang, G.; Zhang, Y.; Wu, X.; Li, X.; Zou, Z.; Ma, J.; Du, G.; et al. Large increases of paddy rice area, gross primary production, and grain production in Northeast china during 2000–2017. Sci. Total Environ. 2020, 711, 135183. [Google Scholar] [CrossRef]
- Li, T.; Nie, T.; Chen, P.; Zhang, Z.; Lan, J.; Zhang, Z.; Qi, Z.; Han, Y.; Jiang, L. Carbon budget of paddy fields after implementing water-saving irrigation in Northeast China. Agronomy 2022, 12, 1481. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.; Liu, Q. Agricultural water consumption and suitable paddy rice plantareas of the Three-River-Plain. Trans. CSAE 2004, 20, 50–53. [Google Scholar]
- Tian, Q.; Wang, F.; Tian, Y.; Jiang, Y.; Weng, P.; Li, J. Copula-based comprehensive drought identification and evaluation over the Xijiang River Basin in South China. Ecol. Indic. 2023, 154, 110503. [Google Scholar] [CrossRef]
- Carracelas, G.; Hornbuckleb, J.; Rosas, J.; Roel, A. Irrigation management strategies to increase water productivity in Oryza sativa (rice) in Uruguay. Agric. Water Manag. 2019, 222, 161–172. [Google Scholar] [CrossRef]
- Nie, T.; Huang, J.; Zhang, Z.; Chen, P.; Li, T.; Dai, C. The inhibitory effect of a water-saving irrigation regime on CH4 emission in Mollisols under straw incorporation for 5 consecutive years. Agric. Water Manag. 2023, 278, 108163. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, L.; Li, S.; Liu, H.; Zhai, L.; Zhou, F.; Ye, Y.; Ruan, S.; Wen, W. Effects and potential of water-saving irrigation for rice production in China. Agric. Water Manag. 2019, 217, 374–382. [Google Scholar] [CrossRef]
- China Water. 2019. Available online: http://slt.hlj.gov.cn/contents/9/4645.html (accessed on 23 July 2019).
- Xu, G.; Lu, D.; Wang, H.; Li, Y. Morphological and physiological traits of rice roots and their relationships to yield and nitrogen utilization as influenced by irrigation regime and nitrogen rate. Agric. Water Manag. 2018, 203, 385–394. [Google Scholar] [CrossRef]
- Akter, M.; Deroo, H.; Kamal, A.M.; Kader, M.A.; Verhoeven, E.; Decock, C.; Boeckx, P.; Sleutel, S. Impact of irrigation management on paddy soil N supply and depth distribution of abiotic drivers. Agric. Ecosyst. Environ. 2018, 261, 12–24. [Google Scholar] [CrossRef]
- Li, T.; Feng, Y.; Zhu, A.; Huang, J.; Wang, H.; Li, S.; Liu, K.; Peng, R.; Zhang, H.; Liu, L. Effects of main water-saving irrigation methods on morphological and physiological traits of rice roots. Chin. J. Rice Sci. 2019, 33, 293–302. [Google Scholar]
- Wang, M.; Yu, S.; Shao, G.; Gao, S.; Wang, J.; Zhang, Y. Impact of alternate drought and flooding stress on water use, and nitrogen and phosphorus losses in a paddy field. Pol. J. Environ. Stud. 2018, 27, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Nie, T.; Chen, S.; Zhang, Z.; Qi, Z.; Liu, W. Recovery efficiency and loss of 15N-labelled urea in a rice-soil system under water saving irrigation in the Songnen Plain of Northeast China. Agric. Water Manag. 2019, 222, 139–153. [Google Scholar] [CrossRef]
- Xu, J.; Yang, S.; Peng, S.; Wei, Q.; Gao, X. Solubility and leaching risks of organic carbon in paddy soils as affected by irrigation managements. Sci. World J. 2013, 2013, 546750. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Agronomic and remedial benefits and risks of applying biochar to soil: Current knowledge and future research directions. Environ. Int. 2016, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Sun, J.; Chu, L.; Cui, L.; Quan, G.; Yan, J.; Hussain, Q.; Iqbal, M. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Dong, C.; Su, J.; Wang, P.; Chen, C.; Chang, J.; Kim, H.; Huang, C.; Hung, C. The role of biochar in regulating the carbon, phosphorus, and nitrogen cycles exemplified by soil systems. Sustainability 2021, 13, 5612. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Hu, T.; Mahmoud, A.; Li, J.; Zhu, R.; Jiao, X.; Jing, P. A quantitative review of the effects of biochar application on rice yield and nitrogen use efficiency in paddy fields: A meta-analysis. Sci. Total Environ. 2022, 830, 154792. [Google Scholar] [CrossRef]
- Wu, C.; Hou, Y.; Bie, Y.; Chen, X.; Dong, Y.; Lin, L. Effects of biochar on soil water soluble sodium, calcium, magnesium and soil enzyme activity of peach seedlings. IOP Conf. Ser. Earth Environ. Sci. 2020, 446, 032007. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Sun, H.; Lü, F.; He, P. Three-year rice grain yield responses to coastal mudflat soil properties amended with straw biochar. J. Environ. Manag. 2019, 239, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; Pasquale, C. Effect of biochar on the physical and structural properties of a sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrol. 2021, 155, 105081. [Google Scholar] [CrossRef]
- Cui, X.; Yuan, J.; Yang, X.; Wei, C.; Bi, Y.; Sun, Q.; Meng, J.; Han, X. Biochar application alters soil metabolites and nitrogen cycle-related microorganisms in a soybean continuous cropping system. Sci. Total Environ. 2024, 917, 170522. [Google Scholar] [CrossRef] [PubMed]
- Asadyar, L.; Xu, C.; Wallace, H.M.; Xu, Z.; Reverchon, F.; Bai, S.H. Soil-plant nitrogen isotope composition and nitrogen cycling after biochar applications. Environ. Sci. Pollut. Res. 2021, 28, 6684–6690. [Google Scholar] [CrossRef]
- Be, S.; Vinitnantharat, S.; Pinisakul, A. Effect of mangrove biochar residue amended shrimp pond sediment on nitrogen adsorption and leaching. Sustainability 2021, 13, 7230. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Li, H.; Zhang, A.; Rengel, Z. Long-term biochar application promotes rice productivity by regulating root dynamic development and reducing nitrogen leaching. GCB Bioenergy 2021, 13, 257–268. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mosa, A.; Zhan, L.; Gao, B. Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: A critical review. Chemosphere 2021, 278, 130378. [Google Scholar] [CrossRef]
- Ul Islam, M.; Jiang, F.; Guo, Z.; Peng, X. Does biochar application improve soil aggregation? A meta-analysis. Soil Till. Res. 2021, 209, 104926. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Mo, C.; Jiang, Z.; Yang, J.; Lin, J. Microbial mechanism of biochar addition on nitrogen leaching and retention in tea soils from different plantation ages. Sci. Total Environ. 2021, 757, 143817. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between plant roots, rhizosphere microorganisms, and nitrogen and its special focus on rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Jiao, X.; Li, J.; Liu, K.; Wu, T.; Zhang, Z.; Luo, D. Response of soil nutrients retention and rice growth to biochar in straw returning paddy fields. Chemosphere 2023, 312, 137244. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, B.; Shen, J.; Zhu, X.; Yi, W.; Li, Y.; Wu, J. Contrasting effects of straw and straw-derived biochar applications on soil carbon accumulation and nitrogen use efficiency in double-rice cropping systems. Agric. Ecosyst. Environ. 2021, 311, 107286. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Cheng, Y.; Wang, C.; Liu, G.; Yang, J. The effects of water and nitrogen on the roots and yield of upland and paddy rice. J. Integr. Agric. 2020, 19, 1363–1374. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Response of soil alkaline phosphatase to biochar amendments: Changes in kinetic and thermodynamic characteristics. Geoderma 2019, 377, 44–54. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soil. Sediment. 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Mehmood, I.; Qiao, L.; Chen, H.; Tang, Q.; Woolf, D.; Fan, M. Biochar addition leads to more soil organic carbon sequestration under a maize-rice cropping system than continuous flooded rice. Agric. Ecosyst. Environ. 2020, 298, 106965. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, X.; Zhang, X.; Wan, L.; Wang, Z. Effects of biochar application on soil nitrogen and phosphorous leaching loss and oil peony growth. Agric. Water Manag. 2021, 255, 107022. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, L.; Zhao, B.; Li, Y. Effects of single-sided application of humic acid on maize root growth. Sci. Agric. Sin. 2022, 55, 339–349. [Google Scholar]
- Sun, H.; Li, X.; Ren, T.; Cong, R.; Lu, J. Effects of fertilizer in shallow soils on growth and distribution of rice roots at seedling stage. Sci. Agric. Sin. 2014, 47, 2476–2484. [Google Scholar]
- Zou, Z.; Fan, L.; Li, X.; Dong, C.; Zhang, L.; Zhang, L.; Fu, J.; Han, W.; Yan, P. Response of plant root growth to biochar amendment: A meta-analysis. Agronomy 2021, 11, 2442. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Gao, Y.; Liu, H.; Huang, Z.; Wang, G.; Liang, Y.; Duan, A.-W. Root development and water uptake in winter wheat under different irrigation methods and scheduling for North China. Agric. Water Manag. 2017, 182, 139–150. [Google Scholar] [CrossRef]
- Dong, D.; Feng, Q.; McGrouther, K.; Yang, M.; Wang, H.; Wu, W. Effects of biochar amendment on rice growth and nitrogen retention in a waterlogged paddy field. J. Soil Sediment. 2015, 15, 153–162. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Song, N.; Chen, Q.; Sun, H.; Peng, T.; Huang, S.; Zhao, Q. Response of grain-filling rate and grain quality of mid-season indica rice to nitrogen application. J. Integr. Agric. 2021, 20, 1465–1473. [Google Scholar] [CrossRef]
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of biochar application on root traits: A meta-analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Yan, S.; Niu, Z.; Yan, H.; Yun, F.; Peng, G.; Yang, Y.; Liu, G. Biochar application significantly affects the N pool and microbial community structure in purple and paddy soils. PeerJ 2019, 7, e7576. [Google Scholar] [CrossRef]
- Kamali, M.; Jahaninafard, D.; Mostafaie, A.; Davarazar, M.; Gomes, A.P.D.; Tarelho, L.A.C.; Dewil, R.; Aminabhavi, T.M. Scientometric analysis and scientific trends on biochar application as soil amendment. Chem. Eng. J. 2020, 395, 125128. [Google Scholar] [CrossRef]
- Ahmed, R.; Li, Y.; Mao, L.; Xu, C.; Lin, W.; Ahmed, S.; Ahmed, W. Biochar effects on mineral nitrogen leaching, moisture content, and evapotranspiration after 15N urea fertilization for vegetable crop. Agronomy 2019, 9, 331. [Google Scholar] [CrossRef]
- Muhammad, N.; Aziz, R.; Brookes, P.C.; Xu, J. Impact of wheat straw biochar on yield of rice and some properties of Psammaquent and Plinthudult. J. Soil Sci. Plant Nut. 2017, 17, 808–823. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gou, J. Biochar enhances the retention capacity of nitrogen fertilizer and affects the diversity of nitrifying functional microbial communities in karst soil of southwest China. Ecotox. Environ. Safe 2021, 226, 112819. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, Y.; Wang, Y.; Li, Y.; Shen, J.; Liu, X.; Wu, J. Pathways and mechanisms by which biochar application reduces nitrogen and phosphorus runoff losses from a rice agroecosystem. Sci. Total Environ. 2021, 797, 149193. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Yao, R.; Li, H.; Yang, J.; Zhu, W.; Yin, C.; Wang, X.; Xie, W.; Zhang, X. Combined application of biochar and N fertilizer shifted nitrification rate and amoa gene abundance of ammonia-oxidizing microorganisms in salt-affected anthropogenic-alluvial soil. Appl. Soil Ecol. 2022, 171, 104348. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Zhang, Y.; Hu, T.; Lin, Z.; Liu, G.; Wang, X.; Ma, J.; Wang, H.; Jin, H.; et al. Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands:options and mitigation strength in a global perspective. Glob. Chang. Biol. 2019, 25, 2077–2093. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Zhang, W.; Sui, Y.; Jin, D.; Xin, W.; Yi, J.; He, D. Biochar prepared at different pyrolysis temperatures affects urea-nitrogen immobilization and N2O emissions in paddy fields. PeerJ 2019, 7, e7027. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Torabian, S.; Qin, R.; Noulas, C.; Lu, Y.; Gao, S. Biochar effects on yield of cereal and legume crops using meta-analysis. Sci. Total Environ. 2021, 775, 145869. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, W.; Gulaqa, A.; Cui, Y.; Zhou, Y.; Weng, W.; Wang, X.; Liu, Q.; Jin, F. Effects of peanut shell biochar on soil nutrients, soil enzyme activity, and rice yield in heavily saline-sodic paddy field. J. Soil Sci. Plant Nut. 2021, 21, 655–664. [Google Scholar] [CrossRef]

| Irrigation Regime | Control Index | Turning Green | Early Tillering | Middle Tillering | Later Tillering | Jointing and Booting | Heading and Flowering | Grouting | Yellow-Ripe |
|---|---|---|---|---|---|---|---|---|---|
| Controlled irrigation (CI) | Upper irrigation threshold | 30 mm | 30 mm | 30 mm | Drainage | 30 mm | 30 mm | 0 mm | Naturally drying |
| Lower irrigation threshold | 0 | 0.7θs | 0.7θs | 0.8θs | 0.8θs | 0.7θs | |||
| Flooded irrigation (FI) | Upper irrigation threshold | 30 mm | 50 mm | 50 mm | Drainage | 50 mm | 50 mm | 50 mm | Naturally drying |
| Lower irrigation threshold | 10 mm | 10 mm | 10 mm | 10 mm | 10 mm | 10 mm |
| Treatments | Growth Stages | |||||
|---|---|---|---|---|---|---|
| Tillering | Jointing and Booting | Heading and Flowering | Grouting | Yellow-Ripe | ||
| pH | FB0 | 6.41 c | 6.55 d | 6.08 c | 6.74 c | 6.21 c |
| CB0 | 6.43 c | 6.64 d | 6.14 c | 6.86 c | 6.37 c | |
| CB1 | 6.51 c | 6.82 c | 6.27 c | 7.12 b | 6.73 b | |
| CB2 | 6.92 b | 7.23 b | 6.62 b | 7.51 a | 7.11 a | |
| CB3 | 7.34 a | 7.84 a | 6.95 a | 7.64 a | 7.28 a | |
| NH4+–N (mg g–1) | FB0 | 15.58 d | 9.79 d | 7.36 d | 8.23 d | 11.38 d |
| CB0 | 17.35 c | 10.26 d | 7.15 d | 9.78 c | 13.02 c | |
| CB1 | 19.21 b | 12.71 c | 8.05 c | 10.37 c | 14.86 b | |
| CB2 | 21.86 a | 15.13 b | 9.57 b | 12.84 b | 16.78 a | |
| CB3 | 20.03 b | 16.48 a | 11.79 a | 14.67 a | 16.35 a | |
| NO3––N (mg g–1) | FB0 | 7.23 e | 11.69 c | 8.13 d | 5.47 d | 7.64 d |
| CB0 | 8.48 d | 12.25 c | 9.58 c | 7.30 c | 10.31 c | |
| CB1 | 10.47 c | 13.92 b | 11.81 b | 8.76 b | 10.85 c | |
| CB2 | 13.84 a | 16.52 a | 13.76 a | 10.01 a | 12.37 b | |
| CB3 | 12.04 b | 16.06 a | 12.17 b | 9.58 a | 14.14 a | |
| Treatments | Growth Stages | |||||
|---|---|---|---|---|---|---|
| Tillering | Jointing and Booting | Heading and Flowering | Grouting | Yellow-Ripe | ||
| Longest root length (cm) | FB0 | 29.26 d | 48.84 d | 52.27 b | 46.27 c | 43.93 b |
| CB0 | 32.76 c | 54.15 c | 53.40 b | 50.46 b | 45.50 b | |
| CB1 | 40.85 a | 61.56 a | 59.82 a | 53.81 a | 49.56 a | |
| CB2 | 37.88 b | 63.15 a | 61.06 a | 54.78 a | 51.80 a | |
| CB3 | 36.07 b | 58.82 b | 58.45 a | 54.80 a | 52.01 a | |
| Root volume (cm3 hill–1) | FB0 | 34.23 d | 53.79 d | 97.82 c | 80.95 c | 76.72 b |
| CB0 | 42.27 c | 63.41 c | 110.25 b | 89.16 b | 81.54 b | |
| CB1 | 62.45 a | 79.01 a | 115.99 b | 91.02 b | 83.81 b | |
| CB2 | 58.84 a | 83.91 a | 127.05 a | 99.71 a | 93.22 a | |
| CB3 | 48.22 b | 70.81 b | 105.58 bc | 102.24 a | 94.18 a | |
| Root fresh weight (g hill–1) | FB0 | 28.12 d | 40.51 d | 62.36 c | 56.05 c | 52.35 c |
| CB0 | 33.43 c | 45.48 c | 67.98 b | 63.09 b | 57.85 b | |
| CB1 | 51.37 a | 50.47 b | 70.82 b | 65.31 b | 63.08 a | |
| CB2 | 47.19 ab | 58.12 a | 75.49 a | 70.91 a | 65.53 a | |
| CB3 | 37.47 b | 51.84 b | 68.23 b | 70.18 a | 66.36 a | |
| Treatments | Tillering | Jointing and Booting | Heading and Flowering | Grouting | Yellow-Ripe | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15N-Fertilizer | Soil Nitrogen | 15N-Fertilizer | Soil Nitrogen | 15N-Fertilizer | Soil Nitrogen | 15N-Fertilizer | Soil Nitrogen | 15N-Fertilizer | Soil Nitrogen | |
| FB0 | 4.28 e | 30.18 b | 11.47 d | 52.75 c | 9.42 d | 35.06 d | 3.63 d | 23.79 c | 1.92 a | 14.22 a |
| DB0 | 4.74 d | 30.01 b | 12.14 c | 57.44 bc | 10.11 c | 39.62 c | 4.14 c | 26.12 b | 1.68 b | 11.78 b |
| DB1 | 5.12 c | 31.44 b | 12.95 b | 59.29 b | 10.63 c | 40.69 c | 4.57 c | 28.75 ab | 1.52 bc | 8.99 d |
| DB2 | 6.21 a | 33.43 a | 16.53 a | 65.29 a | 13.44 a | 47.04 a | 5.32 b | 30.21 a | 1.33 c | 8.42 d |
| DB3 | 5.65 b | 31.22 b | 14.12 b | 60.13 b | 12.13 b | 44.43 b | 6.05 a | 30.72 a | 2.21 a | 10.41 c |
| Nitrogen Utilization | Longest RL | RV | RFW | Root–Shoot Ratio | Root Bleeding Intensity | Root Active Absorption Area | RA |
|---|---|---|---|---|---|---|---|
| 15N-fertilizer | 0.604 ** | 0.290 | 0.155 | 0.187 | 0.864 ** | 0.411 * | 0.866 ** |
| Soil nitrogen | 0.458 * | 0.133 | 0.251 | 0.309 | 0.885 ** | 0.215 | 0.806 ** |
| Treatment | Yield (kg ha–1) | NUE (%) | NAE (kg kg–1) | NPFP (kg kg–1) |
|---|---|---|---|---|
| FB0 | 8189.67 c | 27.93 d | 23.68 c | 74.45 c |
| CB0 | 8324.15 c | 29.83 c | 24.36 c | 75.67 c |
| CB1 | 8608.74 b | 31.63 c | 26.56 b | 78.26 b |
| CB2 | 9536.50 a | 38.94 a | 29.47 a | 86.70 a |
| CB3 | 8807.11 b | 36.51 b | 28.18 a | 80.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, Z.; Gong, Z.; Li, T.; Nie, T.; Chen, P.; Han, Y.; Xue, L. Biochar Application Combined with Water-Saving Irrigation Enhances Rice Root Growth and Nitrogen Utilization in Paddy Fields. Agronomy 2024, 14, 889. https://doi.org/10.3390/agronomy14050889
Zhang Z, Zhang Z, Gong Z, Li T, Nie T, Chen P, Han Y, Xue L. Biochar Application Combined with Water-Saving Irrigation Enhances Rice Root Growth and Nitrogen Utilization in Paddy Fields. Agronomy. 2024; 14(5):889. https://doi.org/10.3390/agronomy14050889
Chicago/Turabian StyleZhang, Zuohe, Zhongxue Zhang, Zhenping Gong, Tiecheng Li, Tangzhe Nie, Peng Chen, Yu Han, and Li Xue. 2024. "Biochar Application Combined with Water-Saving Irrigation Enhances Rice Root Growth and Nitrogen Utilization in Paddy Fields" Agronomy 14, no. 5: 889. https://doi.org/10.3390/agronomy14050889






