Increased Level of IFN-γ and IL-4 Spot-Forming Cells on ELISPOT Assay as Biomarkers for Acute Graft-Versus-Host Disease and Concurrent Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
| Characteristic | aGVHD grade 0 (n = 49) | aGVHD grade I-IV (n = 31) | P | |
|---|---|---|---|---|
| Median age (range) | 15 (1–61) | 14 (1–49) | NS* | |
| Male: Female | 33:16 | 19:12 | NS# | |
| Donor type | ||||
| Related | 23 | 13 | NS# | |
| Unrelated | 26 | 18 | ||
| Stem cell source | ||||
| Bone marrow | 37 | 22 | ||
| Peripheral blood | 5 | 5 | NS† | |
| Cord blood | 7 | 4 | ||
| GVHD prophylaxis | ||||
| Cyclosporin A ± MTX | 40 | 24 | NS# | |
| Tacrolimus ± MTX | 9 | 7 | ||
| Conditioning regimen | ||||
| TBI based | 26 | 19 | ||
| BU based | 15 | 9 | NS† | |
| Others | 8 | 3 | ||
| HLA histocompatibility | ||||
| 6/6 | 38 | 26 | ||
| 5/6 | 6 | 4 | NS† | |
| 4/6, 3/6 | 5 | 1 | ||
| Pretransplant CMV serostatus | ||||
| (Donor/recipient) | ||||
| +/+ | 24 | 14 | NS† | |
| −/+ | 9 | 8 | ||
| +/− | 11 | 4 | ||
| −/− | 5 | 5 | ||
| Posttransplant CMV antigenemia | ||||
| Positive | 16 | 10 | NS# | |
| Negative | 33 | 21 | ||
2.2. Evaluation of Events and Sample Collection
2.3. ELISA
2.4. ELISPOT Assay
2.5. Statistical Analysis
3. Results
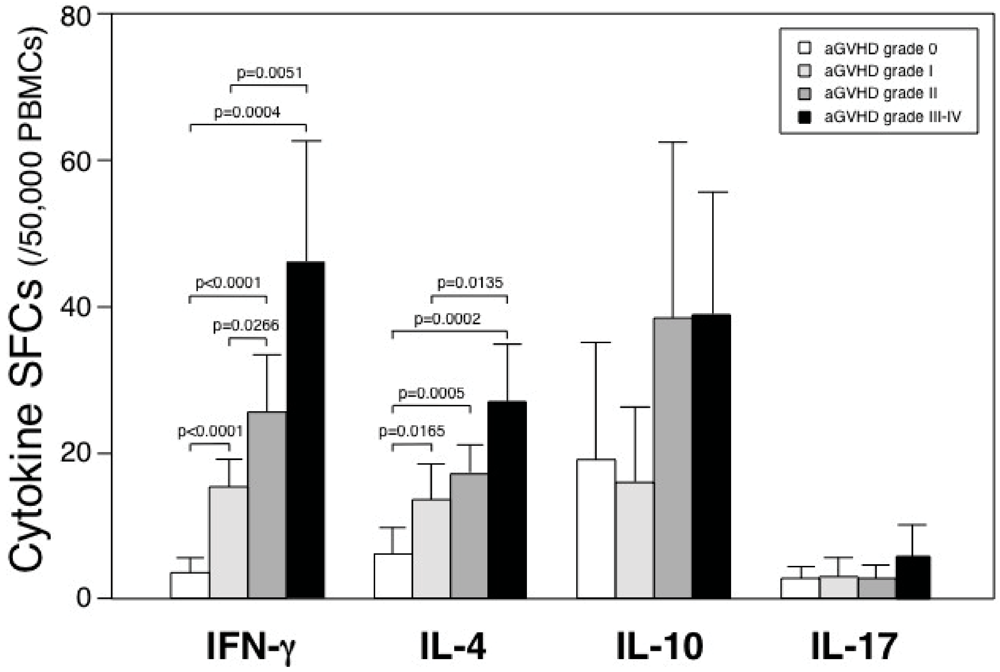
3.1. Spot-Forming Cells in Patients Who Underwent Allogeneic HSCT

3.2. Plasma Cytokine Levels in Patients Who Underwent Allogeneic HSCT
3.3. ELISPOT Assay Discriminates aGVHD from Infection


4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Mitchell, A.E.; Derrington, P.; Turner, P.; Hunt, L.P.; Oakhill, A.; Marks, D.I. Gram-negative bacteraemia (GNB) after 428 unrelated donor bone marrow transplants (UD-BMT): Risk factors, prophylaxis, therapy and outcome. Bone Marrow Transplant. 2004, 33, 303–310. [Google Scholar] [CrossRef]
- Cooke, K.R.; Gerbitz, A.; Crawford, J.M.; Teshima, T.; Hill, G.R.; Tesolin, A.; Rossignol, D.P.; Ferrara, J.L. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J. Clin. Invest. 2001, 107, 1581–1589. [Google Scholar] [CrossRef]
- Krenger, W.; Ferrara, J.L. Graft-versus-host disease and the Th1/Th2 paradigm. Immunol. Res. 1996, 15, 50–73. [Google Scholar] [CrossRef]
- Liem, L.M.; van Lopik, T.; van Nieuwenhuijze, A.E.; van Houwelingen, H.C.; Aarden, L.; Goulmy, E. Soluble Fas levels in sera of bone marrow transplantation recipients are increased during acute graft-versus-host disease but not during infections. Blood 1998, 91, 1464–1468. [Google Scholar]
- Fujimori, Y.; Takatsuka, H.; Takemoto, Y.; Hara, H.; Okamura, H.; Nakanishi, K.; Kakishita, E. Elevated interleukin (IL)-18 levels during acute graft-versus-host disease after allogeneic bone marrow transplantation. Br. J. Haematol. 2000, 109, 652–657. [Google Scholar] [CrossRef]
- Visentainer, J.E.; Lieber, S.R.; Persoli, L.B.; Vigorito, A.C.; Aranha, F.J.; de Brito Eid, K.A.; Oliveira, G.B.; Miranda, E.C.; de Souza, C.A. Serum cytokine levels and acute graft-versus-host disease after HLA-identical hematopoietic stem cell transplantation. Exp. Hematol. 2003, 31, 1044–1050. [Google Scholar]
- Hori, T.; Naishiro, Y.; Sohma, H.; Suzuki, N.; Hatakeyama, N.; Yamamoto, M.; Sonoda, T.; Mizue, Y.; Imai, K.; Tsutsumi, H.; Kokai, Y. CCL8 is a potential molecular candidate for the diagnosis of graft-versus-host disease. Blood 2008, 111, 4403–4412. [Google Scholar]
- Paczesny, S.; Krijanovski, O.I.; Braun, T.M.; Choi, S.W.; Clouthier, S.G.; Kuick, R.; Misek, D.E.; Cooke, K.R.; Kitko, C.L.; Weyand, A.; Bickley, D.; Jones, D.; Whitfield, J.; Reddy, P.; Levine, J.E.; Hanash, S.M.; Ferrara, J.L. A biomarker panel for acute graft-versus-host disease. Blood 2009, 113, 273–278. [Google Scholar]
- Lin, M.T.; Storer, B.; Martin, P.J.; Tseng, L.H.; Gooley, T.; Chen, P.J.; Hansen, J.A. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. New Engl. J. Med. 2003, 349, 2201–2210. [Google Scholar] [CrossRef]
- Hattori, H.; Matsuzaki, A.; Suminoe, A.; Ihara, K.; Nagatoshi, Y.; Sakata, N.; Kawa, K.; Okamura, J.; Hara, T. Polymorphisms of transforming growth factor-beta1 and transforming growth factor-beta1 type II receptor genes are associated with acute graft-versus-host disease in children with HLA-matched sibling bone marrow transplantation. Bone Marrow Transplant. 2002, 30, 665–671. [Google Scholar] [CrossRef]
- Theobald, M.; Nierle, T.; Bunjes, D.; Arnold, R.; Heimpel, H. Host-specific interleukin-2-secreting donor T-cell precursors as predictors of acute graft-versus-host disease in bone marrow transplantation between HLA-identical siblings. New Engl. J. Med. 1992, 327, 1613–1617. [Google Scholar] [CrossRef]
- Hempel, L.; Korholz, D.; Nussbaum, P.; Bonig, H.; Burdach, S.; Zintl, F. High interleukin-10 serum levels are associated with fatal outcome in patients after bone marrow transplantation. Bone Marrow Transplant. 1997, 20, 365–368. [Google Scholar]
- El Kassar, N.; Legouvello, S.; Joseph, C.M.; Salesses, P.; Rieux, C.; Cordonnier, C.; Vernant, J.P.; Farcet, J.P.; Bierling, P.; Kuentz, M. High resolution HLA class I and II typing and CTLp frequency in unrelated donor transplantation: A single-institution retrospective study of 69 BMTs. Bone Marrow Transplant. 2001, 27, 35–43. [Google Scholar] [CrossRef]
- Tanguay, S.; Killion, J.J. Direct comparison of ELISPOT and ELISA-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res. 1994, 13, 259–263. [Google Scholar]
- Han, P.; Hodge, G. Intracellular cytokine production and cytokine receptor interaction of cord mononuclear cells: Relevance to cord blood transplantation. Br. J. Haematol. 1999, 107, 450–457. [Google Scholar] [CrossRef]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Gondo, H.; Minematsu, T.; Harada, M.; Akashi, K.; Hayashi, S.; Taniguchi, S.; Yamasaki, K.; Shibuya, T.; Takamatsu, Y.; Teshima, T.; et al. Cytomegalovirus (CMV) antigenaemia for rapid diagnosis and monitoring of CMV-associated disease after bone marrow transplantation. Br. J. Haematol. 1994, 86, 130–137. [Google Scholar]
- Tanaka, Y.; Kanda, Y.; Kami, M.; Mori, S.; Hamaki, T.; Kusumi, E.; Miyakoshi, S.; Nannya, Y.; Chiba, S.; Arai, Y.; Mitani, K.; Hirai, H.; Mutou, Y. Monitoring cytomegalovirus infection by antigenemia assay and two distinct plasma real-time PCR methods after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002, 30, 315–319. [Google Scholar] [CrossRef]
- Kobayashi, M.; Azuma, E.; Ido, M.; Hirayama, M.; Jiang, Q.; Iwamoto, S.; Kumamoto, T.; Yamamoto, H.; Sakurai, M.; Komada, Y. A pivotal role of Rho GTPase in the regulation of morphology and function of dendritic cells. J. Immunol. 2001, 167, 3585–3591. [Google Scholar]
- Hirayama, M.; Azuma, E.; Kumamoto, T.; Iwamoto, S.; Yamada, H.; Nashida, Y.; Araki, M.; Kageyama, S.; Tamaki, S.; Kawakami, K.; Yamamoto, H.; Komada, Y. Prediction of acute graft-versus-host disease and detection of distinct end-organ targets by enumeration of peripheral blood cytokine spot-forming cells. Transplantation 2005, 80, 58–65. [Google Scholar]
- Emery, V.C.; Sabin, C.A.; Cope, A.V.; Gor, D.; Hassan-Walker, A.F.; Griffiths, P.D. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000, 355, 2032–2036. [Google Scholar]
- Li, C.R.; Greenberg, P.D.; Gilbert, M.J.; Goodrich, J.M.; Riddell, S.R. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: Correlation with CMV disease and effect of ganciclovir prophylaxis. Blood 1994, 83, 1971–1979. [Google Scholar]
- Ljungman, P.; Aschan, J.; Lewensohn-Fuchs, I.; Carlens, S.; Larsson, K.; Lonnqvist, B.; Mattsson, J.; Sparrelid, E.; Winiarski, J.; Ringden, O. Results of different strategies for reducing cytomegalovirus-associated mortality in allogeneic stem cell transplant recipients. Transplantation 1998, 66, 1330–1334. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Cooke, K.R.; Pan, L.; Krenger, W. The immunopathophysiology of acute graft-versus-host-disease. Stem Cells 1996, 14, 473–489. [Google Scholar] [CrossRef]
- Williamson, E.; Garside, P.; Bradley, J.A.; More, I.A.; Mowat, A.M. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes the cytokine profile toward a Th2-type alloimmune response and confers long term protection from disease. J. Immunol. 1997, 159, 1208–1215. [Google Scholar]
- Blazar, B.R.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Vallera, D.A. Rapamycin inhibits the generation of graft-versus-host disease- and graft-versus-leukemia-causing T cells by interfering with the production of Th1 or Th1 cytotoxic cytokines. J. Immunol. 1998, 160, 5355–5365. [Google Scholar]
- Murphy, W.J.; Welniak, L.A.; Taub, D.D.; Wiltrout, R.H.; Taylor, P.A.; Vallera, D.A.; Kopf, M.; Young, H.; Longo, D.L.; Blazar, B.R. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J. Clin. Invest. 1998, 102, 1742–1748. [Google Scholar] [CrossRef]
- Weston, L.E.; Geczy, A.F.; Briscoe, H. Production of IL-10 by alloreactive sibling donor cells and its influence on the development of acute GVHD. Bone Marrow Transplant. 2006, 37, 207–212. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Fili, L.; Ferri, S.; Frosali, F.; Giudici, F.; Romagnani, P.; Parronchi, P.; Tonelli, F.; Maggi, E.; Romagnani, S. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar]
- Dander, E.; Balduzzi, A.; Zappa, G.; Lucchini, G.; Perseghin, P.; Andre, V.; Todisco, E.; Rahal, D.; Migliavacca, M.; Longoni, D.; Solinas, G.; Villa, A.; Berti, E.; Mina, P.D.; Parma, M.; Allavena, P.; Biagi, E.; Rovelli, A.; Biondi, A.; D’Amico, G. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation 2009, 88, 1261–1272. [Google Scholar]
- Sakuma, S.; Higashi, Y.; Sato, N.; Sasakawa, T.; Sengoku, T.; Ohkubo, Y.; Amaya, T.; Goto, T. Tacrolimus suppressed the production of cytokines involved in atopic dermatitis by direct stimulation of human PBMC system. (Comparison with steroids). Int. Immunopharmacol. 2001, 1, 1219–1226. [Google Scholar] [CrossRef]
- Rentenaar, R.J.; Heydendael, V.M.; Diepen, F.N.; Rie, M.A.; Berge, I.J. Systemic treatment with either cyclosporin A or methotrexate does not influence the T Helper 1/t Helper 2 balance in psoriatic patients. J. Clin. Immunol. 2004, 24, 361–369. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Y.Y.; Zhou, Y.L.; Zhu, Z.J.; Tang, Z.Q.; Jiang, Y.; Peng, L.; Li, G.; Zhang, X.H. T-helper and T-cytotoxic cell subsets monitoring during active cytomegalovirus infection in liver transplantation. Transplant. Proc. 2004, 36, 1498–1499. [Google Scholar]
- Bitmansour, A.D.; Waldrop, S.L.; Pitcher, C.J.; Khatamzas, E.; Kern, F.; Maino, V.C.; Picker, L.J. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J. Immunol. 2001, 167, 1151–1163. [Google Scholar]
- Cwynarski, K.; Ainsworth, J.; Cobbold, M.; Wagner, S.; Mahendra, P.; Apperley, J.; Goldman, J.; Craddock, C.; Moss, P.A. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood 2001, 97, 1232–1240. [Google Scholar] [CrossRef]
- Luo, X.H.; Huang, X.J.; Liu, K.Y.; Xu, L.P.; Liu, D.H. Protective immunity transferred by infusion of cytomegalovirus-specific CD8(+) T cells within donor grafts: Its associations with cytomegalovirus reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2010, 16, 994–1004. [Google Scholar] [CrossRef]
- Atkinson, K. Reconstruction of the haemopoietic and immune systems after marrow transplantation. Bone Marrow Transplant 1990, 5, 209–226. [Google Scholar]
- Paganelli, R.; Scala, E.; Ansotegui, I.J.; Ausiello, C.M.; Halapi, E.; Fanales-Belasio, E.; D’Offizi, G.; Mezzaroma, I.; Pandolfi, F.; Fiorilli, M.; Cassone, A.; Aiuti, F. CD8+ T lymphocytes provide helper activity for IgE synthesis in human immunodeficiency virus-infected patients with hyper-IgE. J. Exp. Med. 1995, 181, 423–428. [Google Scholar]
- Li, L.; Sad, S.; Kagi, D.; Mosmann, T.R. CD8Tc1 and Tc2 cells secrete distinct cytokine patterns in vitro and in vivo but induce similar inflammatory reactions. J. Immunol. 1997, 158, 4152–4161. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hirayama, M.; Azuma, E.; Komada, Y. Increased Level of IFN-γ and IL-4 Spot-Forming Cells on ELISPOT Assay as Biomarkers for Acute Graft-Versus-Host Disease and Concurrent Infections. Cells 2012, 1, 61-73. https://doi.org/10.3390/cells1020061
Hirayama M, Azuma E, Komada Y. Increased Level of IFN-γ and IL-4 Spot-Forming Cells on ELISPOT Assay as Biomarkers for Acute Graft-Versus-Host Disease and Concurrent Infections. Cells. 2012; 1(2):61-73. https://doi.org/10.3390/cells1020061
Chicago/Turabian StyleHirayama, Masahiro, Eiichi Azuma, and Yoshihiro Komada. 2012. "Increased Level of IFN-γ and IL-4 Spot-Forming Cells on ELISPOT Assay as Biomarkers for Acute Graft-Versus-Host Disease and Concurrent Infections" Cells 1, no. 2: 61-73. https://doi.org/10.3390/cells1020061




