Neurohypophysial Hormones Associated with Osmotic Challenges in the Brain and Pituitary of the Euryhaline Black Porgy, Acanthopagrus schlegelii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Experimental Design
2.3. Analysis of Serum Cortisol by Enzyme Immunoassay (EIA)
2.4. Analysis of Serum Osmolality with a Vapor Pressure Osmometer
2.5. RNA Extraction, First Strand cDNA Synthesis, and Cloning
2.6. Phylogenetic Analysis of itr and avtr
2.7. Quantification of avt, avtr, it, and itr by Quantitative Real-Time PCR Analysis
2.8. In Situ Hybridization
2.9. Data Analysis
3. Results
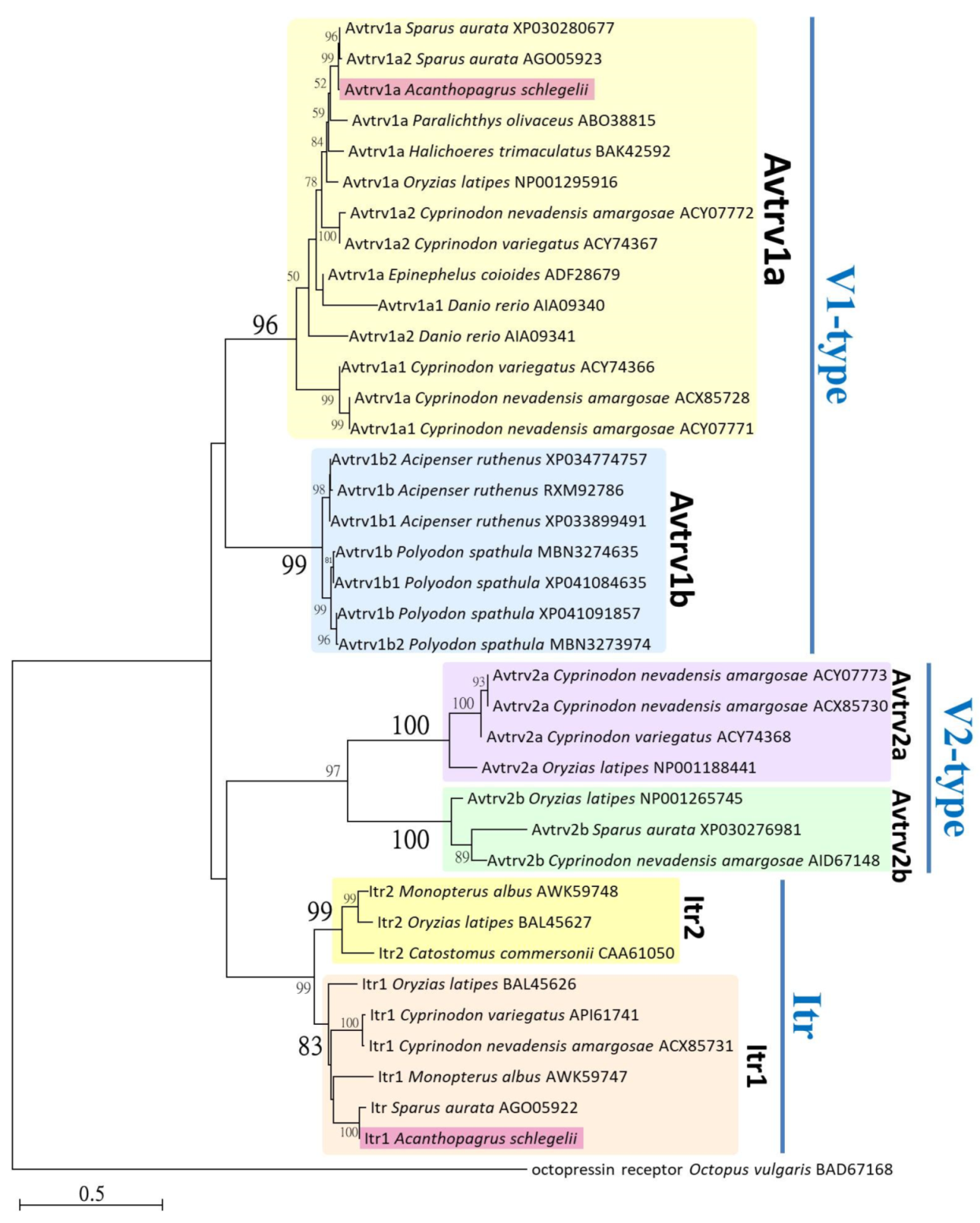
3.1. Sequence Analysis of it, itr, avt, and avtr, and Phylogenetic Tree of Neurohypophysial Hormone Receptors
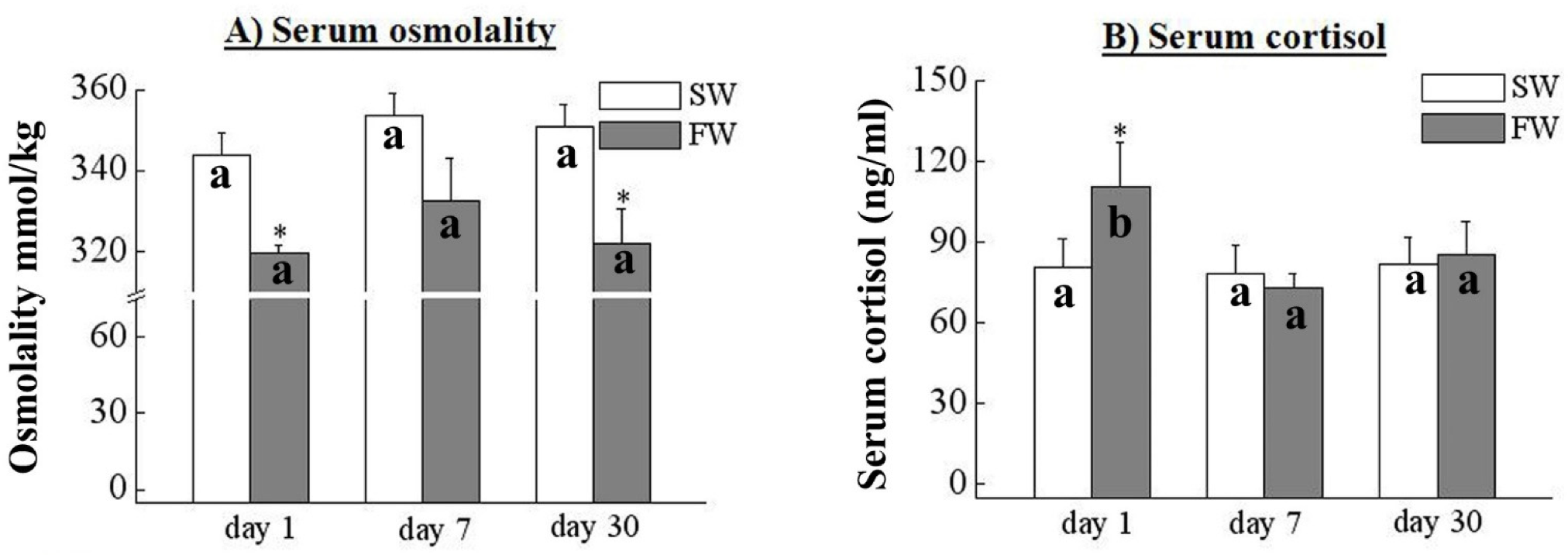
3.2. Serum Osmolality and Cortisol
3.3. The Expression of avt Transcripts in the Brain
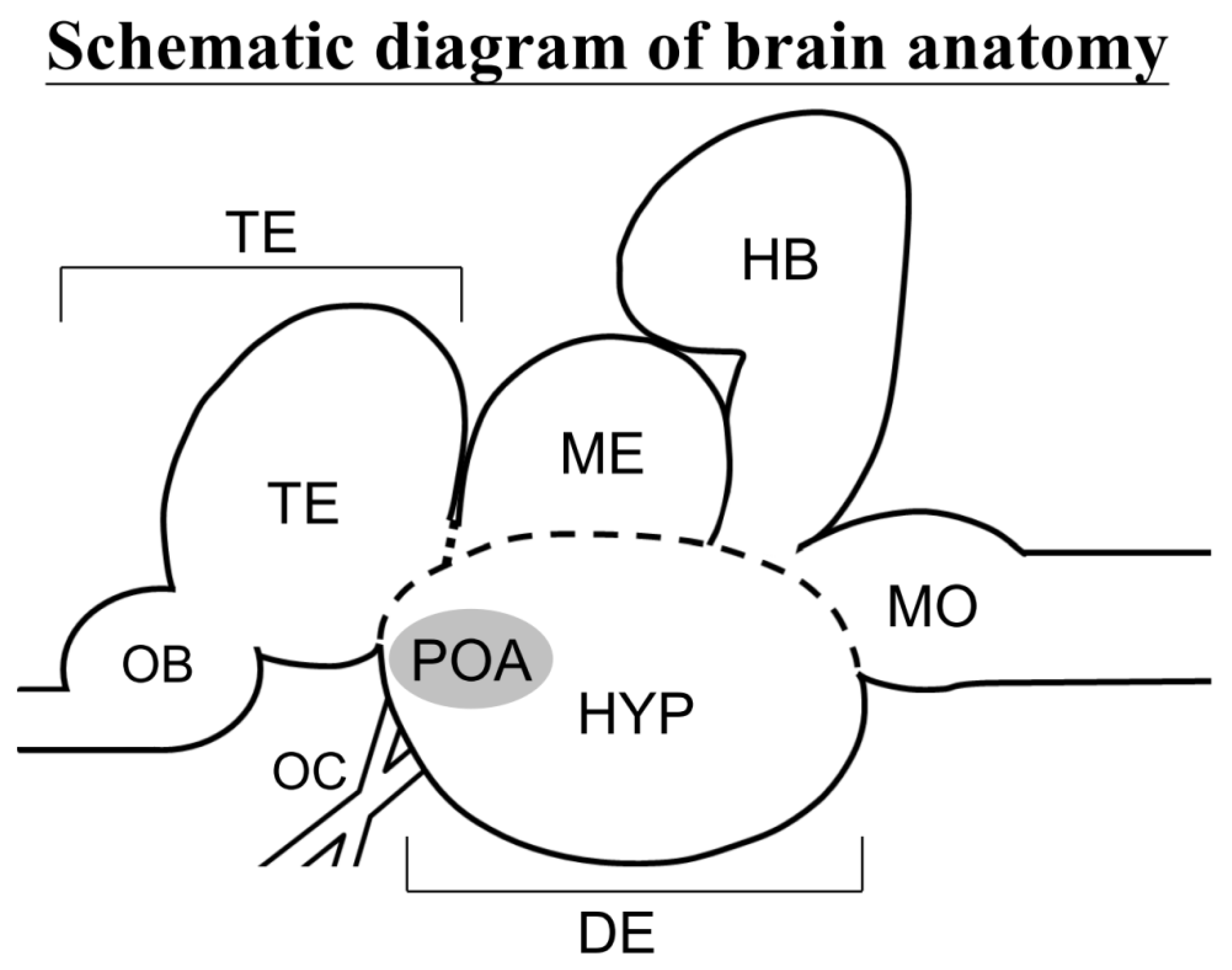
3.4. Localization of avt Transcripts in the SW and FW Black Porgies’ Preoptic Areas
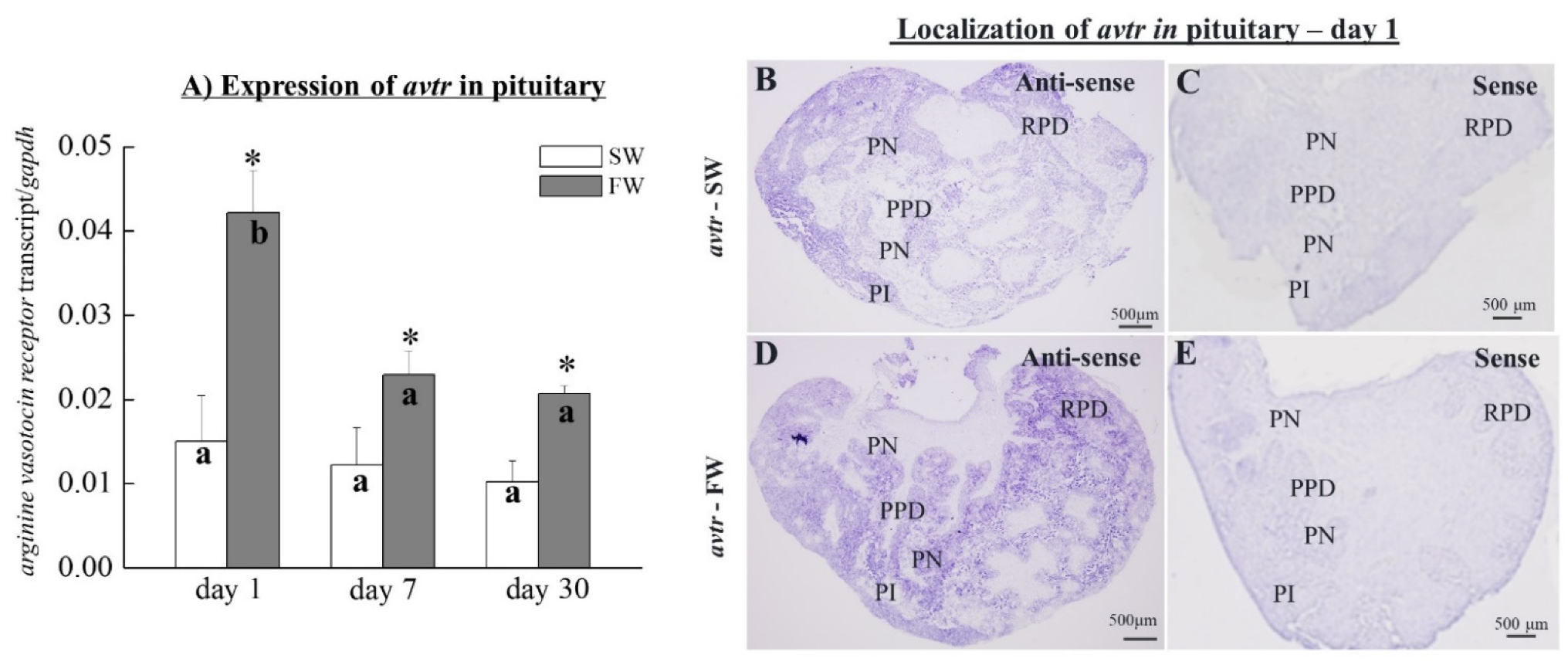
3.5. The Expression of avtr Transcripts in the Pituitary
3.6. Localization of avtr Transcripts in Black Porgy Pituitary Glands
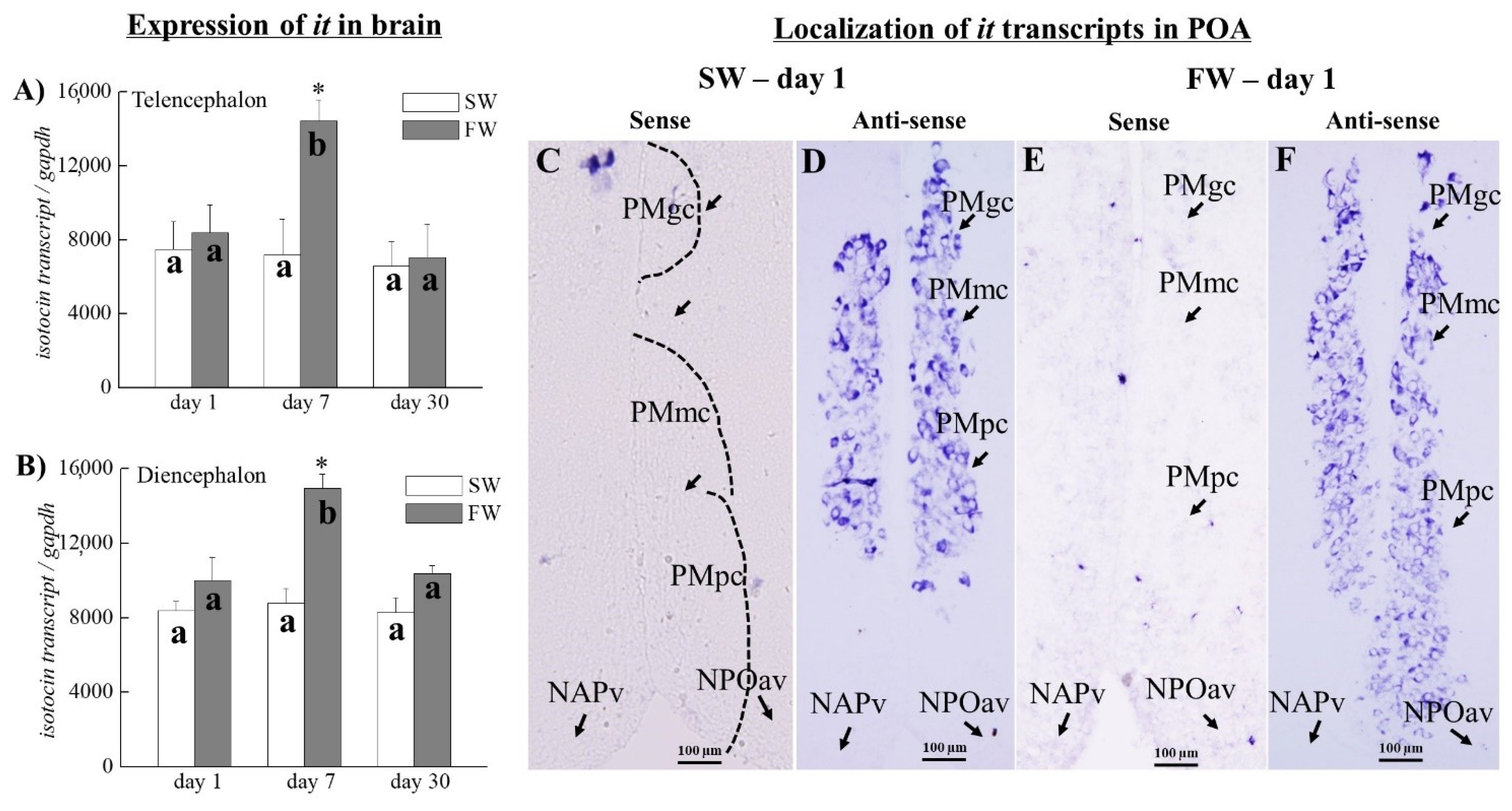
3.7. The Expression of it Transcripts in the Brain
3.8. Localization of it Transcripts in the SW and FW Black Porgies’ Preoptic Areas
3.9. The Expression of itr Transcripts in the Pituitary
3.10. Localization of itr Transcripts in the SW and FW Black Porgies’ Pituitary Glands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulczykowska, E.; Gozdowska, M.; Martos-Sitcha, J.; Kalamarz, H.; Nietrzeba, M.; Mancera, J.; Martínez-Rodríguez, G. Melatonin, vasotocin and isotocin as biomarkers of the condition of fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, S18. [Google Scholar] [CrossRef]
- Du Vigneaud, V. Hormones of the posterior pituitary gland: Oxytocin and vasopressin. Harvey Lect. 1954, 50, 1–26. [Google Scholar]
- Du Vigneaud, V.; Ressler, C.; Swan, C.J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Balment, R.; Lu, W.; Weybourne, E.; Warne, J. Arginine vasotocin a key hormone in fish physiology and behaviour: A review with insights from mammalian models. Gen. Comp. Endocrinol. 2006, 147, 9–16. [Google Scholar] [CrossRef]
- Goodson, J.L. Nonapeptides and the evolutionary patterning of sociality. Prog. Brain Res. 2008, 170, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Kline, R.J.; O’Connell, L.; Hofmann, H.; Holt, G.J.; Khan, I.A. The distribution of an AVT V1a receptor in the brain of a sex changing fish, Epinephelus adscensionis. J. Chem. Neuroanat. 2011, 42, 72–88. [Google Scholar] [CrossRef]
- Acher, R. Neurohypophysial peptide systems: Processing machinery, hydroosmotic regulation, adaptation and evolution. Regul. Pept. 1993, 45, 1–13. [Google Scholar] [CrossRef]
- Babiker, M.M.; Rankin, J.C. Neurohypophysial hormonal control of kidney function in the European eel (Anguilla anguilla L.) adapted to sea-water or fresh water. J. Endocrinol. 1978, 76, 347–358. [Google Scholar] [CrossRef]
- Bentley, P.J. Endocrines and osmoregulation: A comparative account of the regulation of water and salt in vertebrates. In Zoophysiology and Ecology; Hoar, W.S., Jacobs, J., Langer, H., Lindauer, M., Eds.; Springer: New York, NY, USA, 1971; Volume 1, pp. 199–254. [Google Scholar]
- Goodson, J.L.; Bass, A.H. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Rev. 2001, 35, 246–265. [Google Scholar] [CrossRef]
- Warne, J.; Harding, K.; Balment, R. Neurohypophysial hormones and renal function in fish and mammals. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 132, 231–237. [Google Scholar] [CrossRef]
- Bentley, P.J. Endocrines and Osmoregulation: A Comparative Account in Vertebrates, 2nd ed.; Bradshaw, S.D., Burggren, W., Heller, H.C., Ishii, S., Langer, H., Neuweiler, G., Randall, D.J., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2002; Volume 1. [Google Scholar]
- Holmgvist, B.I.; Ekström, P. Hypophysiotrophic systems in the brain of the Atlantic salmon. Neuronal innervation of the pituitary and the origin of pituitary dopamine and nonapeptides identified by means of combined carbocyanine tract tracing and immunocytochemistry. J. Chem. Neuroanat. 1995, 8, 125–145. [Google Scholar] [CrossRef]
- Hausmann, H.; Meyerhof, W.; Zwiers, H.; Lederis, K.; Richter, D. Teleost isotocin receptor: Structure, functional expression, mRNA distribution and phylogeny. FEBS Lett. 1995, 370, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Lema, S.C. Identification of multiple vasotocin receptor cDNAs in teleost fish: Sequences, phylogenetic analysis, sites of expression, and regulation in the hypothalamus and gill in response to hyperosmotic challenge. Mol. Cell. Endocrinol. 2010, 321, 215–230. [Google Scholar] [CrossRef] [Green Version]
- Almeida, O.; Gozdowska, M.; Kulczykowska, E.; Oliveira, R. Brain levels of arginine-vasotocin and isotocin in dominant and subordinate males of a cichlid fish. Horm. Behav. 2012, 61, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Lema, S.C.; Sanders, K.E.; Walti, K.A. Arginine Vasotocin, Isotocin and Nonapeptide Receptor Gene Expression Link to Social Status and Aggression in Sex-Dependent Patterns. J. Neuroendocr. 2015, 27, 142–157. [Google Scholar] [CrossRef]
- Lema, S.C.; Wagstaff, L.J.; Gardner, N.M. Diurnal rhythms of behavior and brain mRNA expression for arginine vasotocin, isotocin, and their receptors in wild Amargosa pupfish (Cyprinodon nevadensis amargosae). Mar. Freshw. Behav. Physiol. 2010, 43, 257–281. [Google Scholar] [CrossRef] [Green Version]
- Acher, R. Molecular Evolution of Fish Neurohypophysial hormones: Neutral and selective evolutionary mechanisms. Gen. Comp. Endocrinol. 1996, 102, 157–172. [Google Scholar] [CrossRef]
- Balment, R.J.; Warne, J.M.; Tierney, M.; Hazon, N. Arginine vasotocin and fish osmoregulation. Fish Physiol. Biochem. 1993, 11, 189–194. [Google Scholar] [CrossRef]
- Gilchriest, B.J.; Tipping, D.R.; Hake, L.; Levy, A.; Baker, B.I. The effects of acute and chronic stresses on vasotocin gene transcripts in the brain of the rainbow trout (Oncorhynchus mykiss). J. Neuroendocr. 2001, 12, 795–801. [Google Scholar] [CrossRef]
- Godwin, J.; Sawby, R.; Warner, R.; Crews, D.; Grober, M.S. Hypothalamic arginine vasotocin mRNA abundance variation across sexes and with sex change in a coral reef fish. Brain Behav. Evol. 2000, 55, 77–84. [Google Scholar] [CrossRef]
- Haruta, K.; Yamashita, T.; Kawashima, S. Changes in arginine vasotocin content in the pituitary of the medaka (Oryzia latipes) during osmotic stress. Gen. Comp. Endocrinol. 1991, 83, 327–336. [Google Scholar] [CrossRef]
- Hyodo, S.; Urano, A. Changes in expression of provasotocin and proisotocin genes during adaptation to hyper- and hypo-osmotic environments in rainbow trout. J. Comp. Physiol. B 1991, 161, 549–556. [Google Scholar] [CrossRef]
- Maetz, J.; Lahlou, B. Actions of neurohypophysial hormones in fishes. In Handbook of Physiology-Section 7 Endocrinology; American Physiology Society: Washington, DC, USA, 1974; Volume 4, pp. 521–544. [Google Scholar]
- Perrott, M.; Carrick, S.; Balment, R. Pituitary and plasma arginine vasotocin levels in teleost fish. Gen. Comp. Endocrinol. 1991, 83, 68–74. [Google Scholar] [CrossRef]
- Warne, J.; Balment, R. Changes in Plasma Arginine Vasotocin (AVT) Concentration and dorsal aortic blood pressure following AVT injection in the teleost Platichthys flesus. Gen. Comp. Endocrinol. 1997, 105, 358–364. [Google Scholar] [CrossRef]
- Warne, J.; Hyodo, S.; Harding, K.; Balment, R. Cloning of pro-vasotocin and pro-isotocin cDNAs from the flounder Platichthys flesus; Levels of hypothalamic mRNA following acute osmotic challenge. Gen. Comp. Endocrinol. 2000, 119, 77–84. [Google Scholar] [CrossRef]
- Kulczykowska, E. Responses of circulating arginine vasotocin, isotocin, and melatonin to osmotic and disturbance stress in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2001, 24, 201–206. [Google Scholar] [CrossRef]
- Kulczykowska, E. Response of circulating arginine vasotocin and isotocin to rapid osmotic challenge in rainbow trout. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 773–778. [Google Scholar] [CrossRef]
- Warne, J.; Bond, H.; Weybourne, E.; Sahajpal, V.; Lu, W.; Balment, R. Altered plasma and pituitary arginine vasotocin and hypothalamic provasotocin expression in flounder (Platichthys flesus) following hypertonic challenge and distribution of vasotocin receptors within the kidney. Gen. Comp. Endocrinol. 2005, 144, 240–247. [Google Scholar] [CrossRef]
- Guibbolini, M.E.; Henderson, I.W.; Mosley, W.; Lahlou, B. Arginine vasotocin binding to isolated branchial cells of the eel: Effect of salinity. J. Mol. Endocrinol. 1988, 1, 125–130. [Google Scholar] [CrossRef]
- Guibbolini, M.E.; Avella, M. Neurohypophysial hormone regulation of Cl-secretion: Physiological evidence for V1-type receptors in sea bass gill respiratory cells in culture. J. Endocrinol. 2003, 176, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Chou, M.-Y.; Hung, J.-C.; Wu, L.-C.; Hwang, S.-P.L.; Hwang, P.-P. Isotocin controls ion regulation through regulating ionocyte progenitor differentiation and proliferation. Cell. Mol. Life Sci. 2010, 68, 2797–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martos-Sitcha, J.A.; Cádiz, L.; Gozdowska, M.; Kulczykowska, E.; Martinez-Rodriguez, G.; Mancera, J.M. Arginine vasotocin and cortisol co-regulate vasotocinergic, isotocinergic, stress, and thyroid pathways in the gilthead sea bream (Sparus aurata). Front. Physiol. 2019, 10, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrzyńska, A.K.; Maiorano, E.; Bastaroli, M.; Naderi, F.; Míguez, J.M.; Martinez-Rodriguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Impact of air exposure on vasotocinergic and isotocinergic systems in gilthead sea bream (Sparus aurata): New insights on fish stress response. Front. Physiol. 2018, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadiz, L.; Roman-Padilla, J.; Gozdowska, M.; Kulczykowska, E.; Martinez-Rodriguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Cortisol modulates vasotocinergic and isotocinergic pathways in the gilthead sea bream (Sparus aurata, Linnaeus 1758). J. Exp. Biol. 2015, 218, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.-C.; Chang, C.-F. Primary males guide the femaleness through the regulation of testicular Dmrt1 and ovarian Cyp19a1a in protandrous black porgy. Gen. Comp. Endocrinol. 2018, 261, 198–202. [Google Scholar] [CrossRef]
- Wu, G.-C.; Dufour, S.; Chang, C.-F. Molecular and cellular regulation on sex change in hermaphroditic fish, with a special focus on protandrous black porgy, Acanthopagrus schlegelii. Mol. Cell. Endocrinol. 2021, 520, 111069. [Google Scholar] [CrossRef] [PubMed]
- Tomy, S.; Chang, Y.-M.; Chen, Y.-H.; Cao, J.-C.; Wang, T.-P.; Chang, C.-F. Salinity effects on the expression of osmoregulatory genes in the euryhaline black porgy Acanthopagrus schlegeli. Gen. Comp. Endocrinol. 2009, 161, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Aruna, A.; Wang, T.S.; Cao, J.C.; Lan, D.S.; Nagarajan, G.; Chang, C.F. Differntiation expresssion of hypothalamic- and gill-crh system with osmotic stress in the erythaline black porgy, Acanthopagrus schlegeli. Front. Physiol. 2021, in press. [Google Scholar] [CrossRef]
- Nagarajan, G.; Aruna, A.; Chang, C.-F. Neuropeptide arginine vasotocin positively affects neurosteroidogenesis in the early brain of grouper, Epinephelus coioides. J. Neuroendocr. 2015, 27, 718–736. [Google Scholar] [CrossRef]
- Aruna, A.; Nagarajan, G.; Chang, C.-F. Involvement of corticotrophin-releasing hormone and corticosteroid receptors in the brain-pituitary-gill of tilapia during the course of seawater acclimation. J. Neuroendocr. 2012, 24, 818–830. [Google Scholar] [CrossRef]
- Aruna, A.; Nagarajan, G.; Chang, C.-F. Differential expression patterns and localization of glucocorticoid and mineralocorticoid receptor transcripts in the osmoregulatory organs of tilapia during salinity stress. Gen. Comp. Endocrinol. 2012, 179, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cueto, J.A.; Sarasquete, C.; Zohar, Y.; Kah, O. An Atlas of the Brain of the Gilthead Seabream (Sparus aurata); Maryland Sea Grant: College Park, MD, USA, 2001. [Google Scholar]
- Lin, C.-J.; Fan-Chiang, Y.-C.; Dufour, S.; Chang, C.-F. Activation of brain steroidogenesis and neurogenesis during the gonadal differentiation in protandrous black porgy, Acanthopagrus schlegelii. Dev. Neurobiol. 2016, 76, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Martos-Sitcha, J.A.; Martinez-Rodriguez, G.; Mancera, J.M.; Fuentes, J. AVT and IT regulate ion transport across the opercular epithelium of killifish (Fundulus heteroclitus) and gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 182, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Martos-Sitcha, J.; Fuentes, J.; Mancera, J.; Martinez-Rodriguez, G. Variations in the expression of vasotocin and isotocin receptor genes in the gilthead sea bream Sparus aurata during different osmotic challenges. Gen. Comp. Endocrinol. 2014, 197, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, K.W.; Na Kim, N.; Choi, C.Y. Cloning and expression of aquaporin 1 and arginine vasotocin receptor mRNA from the black porgy, Acanthopagrus schlegeli: Effect of freshwater acclimation. Fish Physiol. Biochem. 2007, 34, 185–194. [Google Scholar] [CrossRef]
- Saoud, I.P.; Kreydiyyeh, S.; Chalfoun, A.; Fakih, M. Influence of salinity on survival, growth, plasma osmolality and gill Na+–K+–ATPase activity in the rabbitfish Siganus rivulatus. J. Exp. Mar. Biol. Ecol. 2007, 348, 183–190. [Google Scholar] [CrossRef]
- Shui, C.; Shi, Y.; Hua, X.; Zhang, Z.; Zhang, H.; Lu, G.; Xie, Y. Serum osmolality and ions, and gill Na+/K+-ATPase of spottedtail goby Synechogobius ommaturus (R.) in response to acute salinity changes. Aquac. Fish. 2018, 3, 79–83. [Google Scholar] [CrossRef]
- Kammerer, B.D.; Cech, J.J.; Kültz, D. Rapid changes in plasma cortisol, osmolality, and respiration in response to salinity stress in tilapia (Oreochromis mossambicus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 260–265. [Google Scholar] [CrossRef]
- Aruna, A.; Nagarajan, G.; Chang, C.-F. The acute salinity changes activate the dual pathways of endocrine responses in the brain and pituitary of tilapia. Gen. Comp. Endocrinol. 2015, 211, 154–164. [Google Scholar] [CrossRef]
- Greenwood, A.K.; Wark, A.R.; Fernald, R.D.; Hofmann, H.A. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc. R. Soc. B Boil. Sci. 2008, 275, 2393–2402. [Google Scholar] [CrossRef] [Green Version]
- Hur, S.-P.; Takeuchi, Y.; Esaka, Y.; Nina, W.; Park, Y.-J.; Kang, H.-C.; Jeong, H.-B.; Lee, Y.-D.; Kim, S.-J.; Takemura, A. Diurnal expression patterns of neurohypophysial hormone genes in the brain of the threespot wrasse Halichoeres trimaculatus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 490–497. [Google Scholar] [CrossRef]
- Rodriguez-Santiago, M.; Nguyen, J.; Winton, L.S.; Weitekamp, C.; Hofmann, H.A. Arginine vasotocin preprohormone is expressed in surprising regions of the teleost forebrain. Front. Endocrinol. 2017, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Kalamarz-Kubiak, H.; Meiri-Ashkenazi, I.; Kleszczyńska, A.; Rosenfeld, H. In vitro effect of cortisol and urotensin I on arginine vasotocin and isotocin secretion from pituitary cells of gilthead sea bream Sparus aurata. J. Fish Biol. 2014, 84, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.; Warne, J.; Hyodo, S.; Balment, R. Pituitary and plasma AVT content in the flounder (Platichthys flesus). Fish Physiol. Biochem. 1997, 17, 357–362. [Google Scholar] [CrossRef]
- Hyodo, S.; Tsukada, T.; Takei, Y. Neurohypophysial hormones of dogfish, Triakis scyllium: Structures and salinity-dependent secretion. Gen. Comp. Endocrinol. 2004, 138, 97–104. [Google Scholar] [CrossRef]
- Baker, B.I.; Bird, D.J.; Buckingham, J.C. In the trout, CRH and AVT synergize to stimulate ACTH release. Regul. Pept. 1996, 67, 207–210. [Google Scholar] [CrossRef]
- Bond, H.; Warne, J.; Balment, R. Effect of acute restraint on hypothalamic pro-vasotocin mRNA expression in flounder, Platichthys flesus. Gen. Comp. Endocrinol. 2007, 153, 221–227. [Google Scholar] [CrossRef]
- Olivereau, M.; Olivereau, J. Effect of pharmacological adrenalectomy on corticotropin-releasing factor-like and arginine vasotocin immunoreactivities in the brain and pituitary of the eel: Immunocytochemical study. Gen. Comp. Endocrinol. 1990, 80, 199–215. [Google Scholar] [CrossRef]
- Seth, R.; Köhler, A.; Grossmann, R.; Chaturvedi, C.M. Expression of hypothalamic arginine vasotocin gene in response to water deprivation and sex steroid administration in female Japanese quail. J. Exp. Biol. 2004, 207, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Batten, T.F.; Moons, L.; Vandesande, F. Innervation and control of the adenohypophysis by hypothalamic peptidergic neurons in teleost fishes: EM immunohistochemical evidence. Microsc. Res. Tech. 1999, 44, 19–35. [Google Scholar] [CrossRef]
- Yulis, C.; Lederis, K. Co-localization of the immunoreactivities of corticotropin-releasing factor and arginine vasotocin in the brain and pituitary system of the teleost Catostomus commersoni. Cell Tissue Res. 1987, 247, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Rawat, A.; Chaube, R.; Joy, K.P. In situ localization of vasotocin receptor gene transcripts in the brain-pituitary-gonadal axis of the catfish Heteropneustes fossilis: A morpho-functional study. Fish Physiol. Biochem. 2018, 45, 885–905. [Google Scholar] [CrossRef] [PubMed]
- Antoni, F.A. Hypothalamic Control of adrenocorticotropin secretion: Advances since the discovery of 41-residue corticotropin-releasing factor. Endocr. Rev. 1986, 7, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Jurkevich, A.; Berghman, L.R.; Cornett, L.E.; Kuenzel, W.J. Immunohistochemical characterization of chicken pituitary cells containing the vasotocin VT2 receptor. Cell Tissue Res. 2008, 333, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, S.; Do-Rego, J.; Oh, D.; Moon, J.; Ahn, R.; Lee, K.; Bai, D.; Vaudry, H.; Kwon, H.; Seong, J. Molecular cloning, pharmacological characterization, and histochemical distribution of frog vasotocin and mesotocin receptors. J. Mol. Endocrinol. 2004, 33, 293–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Zhang, N.; Shi, B.; Zhang, S.; Zhang, L.; Zhang, W. Isotocin Regulates growth hormone but not prolactin release from the pituitary of ricefield eels. Front. Endocrinol. 2018, 9, 166. [Google Scholar] [CrossRef] [Green Version]


| Gene | Orientation Usage | Nucleotide Sequence (5′–3′) | Amplicon Size |
|---|---|---|---|
| avt | RT-PCR-F | ATGCCTCACTCCATGTTC | 450 bp |
| RT-PCR-R | CTGTCCTCTGGTGGCCACATGTAG | ||
| Q-PCR-F | CCCCCTGTGCGTCCTGGGACTCATC | 378 bp | |
| Q-PCR-R | CCTCCCCGAGACAGTCAGAGTC | ||
| in situ-S | CCTGTGCGTCCTGGGACTCATC | 344 bp | |
| in situ-AS | CACCCCACAGAGCTGT | ||
| avtr | RT-PCR-F | GAGGTGGCCCAAATCGAGATC | 896 bp |
| RT-PCR-R | GGCGCCCAGCAGATAATGTAC | ||
| Q-PCR-F | CCTGCTGGCAATGTACAACAC | 561 bp | |
| Q-PCR-R | GCAGCGCCGGCCATCGTTTTC | ||
| in situ-S | GTCGCGGATGCACCTCTTCATC | 463 bp | |
| in situ-AS | CCCGTAGCACATCATGAGAATG | ||
| it | RT-PCR-F | GCTGGGGGGACTTTGTGC | 342 bp |
| RT-PCR-R | CTGCAGGGTCCCATA | ||
| Q-PCR-F | CCGTGGGGAGCCAAAGCTTAC | 307 bp | |
| Q-PCR-R | CAGACCACATCTGGACAAAGAA | ||
| in situ-S | CACATGGATGAGCCTCAGC | 262 bp | |
| in situ-AS | GATGGGAGTCCAGCACATTATG | ||
| itr | RT-PCR-F | GCGTGTTACATCTCCAACTGT | 393 bp |
| RT-PCR-R | GGTGTGG CCGGCCAGAT | ||
| Q-PCR-F | CGGAGGGAAGAGATCCATCATG | 316 bp | |
| Q-PCR-R | GCCTTCGGATTGGCTGCTTTG | ||
| in situ-S | GCACCGCTGCGCAAGTGCATGTG | 325 | |
| in situ-AS | GCAGTAGCCTGAGGATGATGTC | ||
| gapdh | RT-PCR-F | GGCCCCCCTGGCCAAAGT | 523 bp |
| RT-PCR-R | TGGGTGTCACCGATGAAG | ||
| Q-PCR-F | AGGCTTCCTTAATCTCAGCATAAGAT | 490 bp | |
| Q-PCR-R | GGTGCCTGTGGCTGATGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aruna, A.; Lin, C.-J.; Nagarajan, G.; Chang, C.-F. Neurohypophysial Hormones Associated with Osmotic Challenges in the Brain and Pituitary of the Euryhaline Black Porgy, Acanthopagrus schlegelii. Cells 2021, 10, 3086. https://doi.org/10.3390/cells10113086
Aruna A, Lin C-J, Nagarajan G, Chang C-F. Neurohypophysial Hormones Associated with Osmotic Challenges in the Brain and Pituitary of the Euryhaline Black Porgy, Acanthopagrus schlegelii. Cells. 2021; 10(11):3086. https://doi.org/10.3390/cells10113086
Chicago/Turabian StyleAruna, Adimoolam, Chien-Ju Lin, Ganesan Nagarajan, and Ching-Fong Chang. 2021. "Neurohypophysial Hormones Associated with Osmotic Challenges in the Brain and Pituitary of the Euryhaline Black Porgy, Acanthopagrus schlegelii" Cells 10, no. 11: 3086. https://doi.org/10.3390/cells10113086






