Single Cell Analysis of Stored Red Blood Cells Using Ultra-High Throughput Holographic Cytometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Collection and Preparation
2.2. Development of Microfluidic Channels
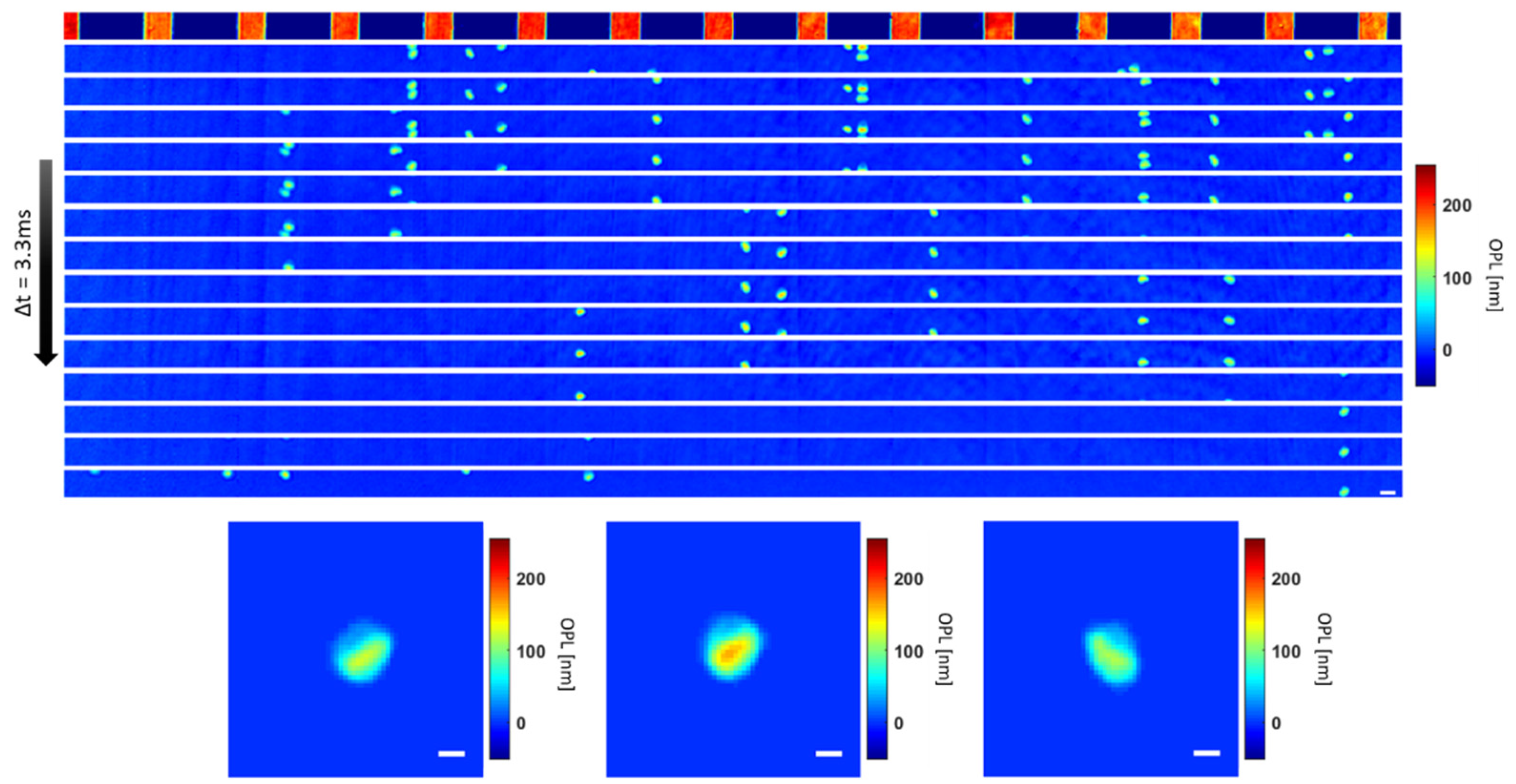
2.3. Holographic Cytometry
3. Results
3.1. Number of Individual Cells Imaged
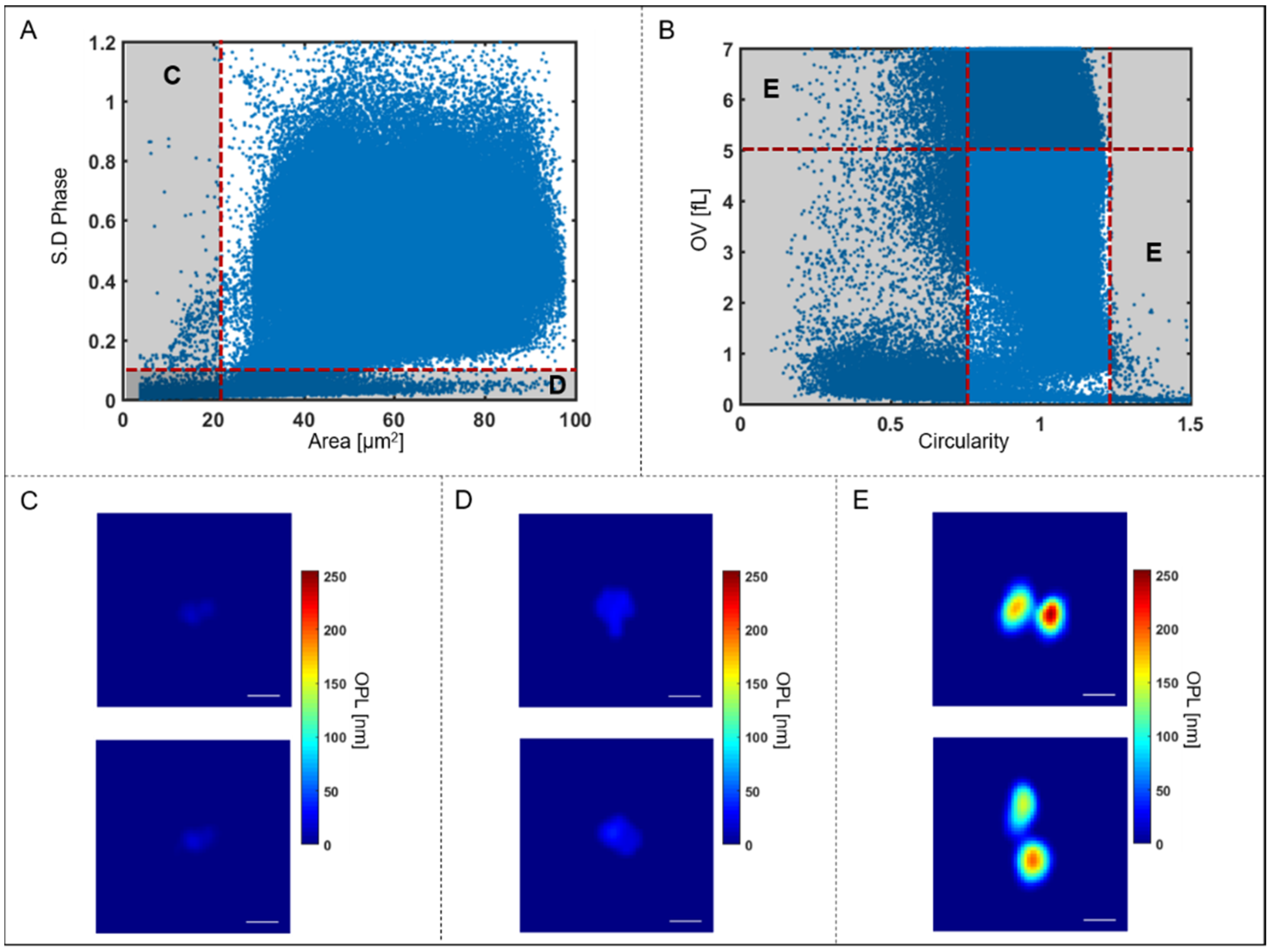
3.2. Characterization of Morphological Changes over Time
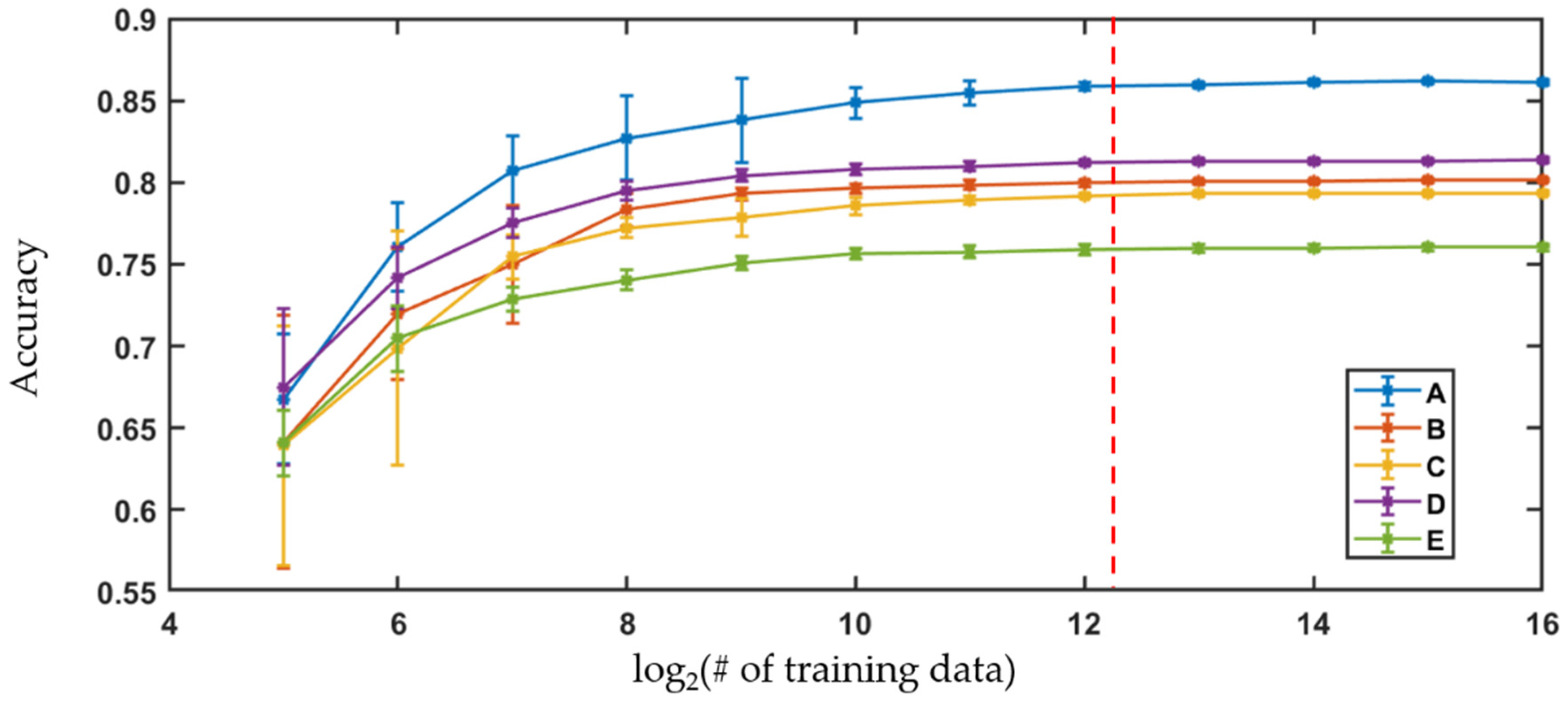
3.3. Classification of RBCs Using Logistic Regression
3.4. Self-Trained Binary Classification: Week 1 vs. Week 5
3.5. All Sample Binary Classification: Week 1 vs. Week 5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.J. Principles and applications of flow cytometry and cell sorting in companion animal medicine. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 53–71. [Google Scholar] [CrossRef]
- Macey, M.G. Principles of flow cytometry. In Flow Cytometry: Principles and Applications; Macey, M.G., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 1–15. [Google Scholar]
- Barteneva, N.S.; Fasler-Kan, E.; Vorobjev, I.A. Imaging flow cytometry: Coping with heterogeneity in biological systems. J. Histochem. Cytochem. 2012, 60, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Gu, Y.; Zhang, A.C.; Lo, Y.-H. Review: Imaging Technologies for Flow Cytometry. Lab Chip 2016, 16, 4639–4647. [Google Scholar] [CrossRef]
- Herzenberg, L.A.; Parks, D.; Sahaf, B.; Perez, O.; Roederer, M.; Herzenberg, L.A. The history and future of the fluorescence activated cell sorter and flow cytometry: A view from Stanford. Clin. Chem. 2002, 48, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Forment, J.V.; Jackson, S.P. A flow cytometry-based method to simplify the analysis and quantification of protein association to chromatin in mammalian cells. Nat. Protoc. 2015, 10, 1297–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Headland, S.E.; Jones, H.R.; D’Sa, A.S.V.; Perretti, M.; Norling, L.V. Cutting-edge analysis of extracellular microparticles using imagestream(x) imaging flow cytometry. Sci. Rep. 2014, 4, 5237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsel, L.; Dagur, P.K.; Raghavachari, N.; Seamon, C.; Kato, G.J.; McCoy, J.P. Imaging flow cytometry for morphologic and phenotypic characterization of rare circulating endothelial cells. Cytom. B Clin. Cytom. 2013, 84, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Aghaeepour, N.; Finak, G.; Flow, C.A.P.C.; Consortium, D.; Hoos, H.; Mosmann, T.R.; Brinkman, R.; Gottardo, R.; Scheuermann, R.H. Critical assessment of automated flow cytometry data analysis techniques. Nat. Methods 2013, 10, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Pedreira, C.E.; Costa, E.S.; Lecrevisse, Q.; van Dongen, J.J.M.; Orfao, A.; EuroFlow, C. Overview of clinical flow cytometry data analysis: Recent advances and future challenges. Trends Biotechnol. 2013, 31, 415–425. [Google Scholar] [CrossRef]
- Lugli, E.; Roederer, M.; Cossarizza, A. Data analysis in flow cytometry: The future just started. Cytom. Part A 2010, 77, 705–713. [Google Scholar] [CrossRef]
- Eldridge, W.J.; Sheinfeld, A.; Rinehart, M.T.; Wax, A. Imaging deformation of adherent cells due to shear stress using quantitative phase imaging. Opt. Lett. 2016, 41, 352. [Google Scholar] [CrossRef]
- Eldridge, W.J.; Steelman, Z.A.; Loomis, B.; Wax, A. Optical phase measurements of disorder strength link microstructure to cell stiffness. Biophys. J. 2017, 112, 692–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.S.; Rinehart, M.T.; Walzer, K.A.; Chi, J.-T.A.; Wax, A. Automated detection of P. falciparum using machine learning algorithms with quantitative phase images of unstained cells. PLoS ONE 2016, 11, e0163045. [Google Scholar] [CrossRef] [Green Version]
- Rinehart, M.T.; Park, H.S.; Walzer, K.A.; Chi, J.-T.A.; Wax, A. Hemoglobin consumption by P. falciparum in individual erythrocytes imaged via quantitative phase spectroscopy. Sci. Rep. 2016, 6, 24461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimring, J.C. Established and theoretical factors to consider in assessing the red cell storage lesion. Blood 2015, 125, 2185–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hod, E.A.; Brittenham, G.M.; Billote, G.B.; Francis, R.O.; Ginzburg, Y.Z.; Hendrickson, J.E.; Jhang, J.; Schwartz, J.; Sharma, S.; Sheth, S.; et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non–transferrin-bound iron. Blood 2011, 118, 6675–6682. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly (dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Ceballos, S.; Eldridge, W.J.; Wax, A. Invited article: Digital refocusing in quantitative phase imaging for flowing red blood cells. APL Photonics 2018, 3, 110802. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Eldridge, W.J.; Yang, W.-H.; Crose, M.; Ceballos, S.; Roback, J.D.; Chi, J.-T.A.; Wax, A. Quantitative phase imaging of erythrocytes under microfluidic constriction in a high refractive index medium reveals water content changes. Microsyst. Nanoeng. 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- MathWorks. MATLAB Computer Vision Toolbox, Windows: Natick, MA, USA, 2019.
- Convex Hull of Delaunay Triangulation—Matlab Convexhull. Available online: https://www.mathworks.com/help/matlab/ref/delaunaytriangulation.convexhull.html (accessed on 5 September 2021).
- Franco, R.S.; Puchulu-Campanella, M.E.; Barber, L.A.; Palascak, M.B.; Joiner, C.H.; Low, P.S.; Cohen, R.M. Changes in the properties of normal human red blood cells during in vivo aging. Am. J. Hematol. 2013, 88, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardyn, M.; Rappaz, B.; Jaferzadeh, K.; Crettaz, D.; Tissot, J.-D.; Moon, I.; Turcatti, G.; Lion, N.; Prudent, M. Red blood cells ageing markers: A multi-parametric analysis. Blood Transfus. 2017, 15, 239–248. [Google Scholar] [CrossRef]
- Nah, E.H.; Kim, S.; Cho, S.; Cho, H.I. Complete blood count reference intervals and patterns of changes across pediatric, adult, and geriatric ages in korea. Ann. Lab. Med. 2018, 38, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.K.; Gasser, R.A.; Magill, A.J.; Miller, R.S. Update on rapid diagnostic testing for malaria. Clin. Microbiol. Rev. 2008, 21, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Cluitmans, J.C.A.; Hardeman, M.R.; Dinkla, S.; Brock, R.; Bosman, G.J.C.G.M. Red blood cell deformability during storage: Towards functional proteomics and metabolomics in the blood bank. Blood Transfus. 2012, 10, s12–s18. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Dussiot, M.; Marin, M.; Morel, A.; Ndour, P.A.; Duez, J.; Le Van Kim, C.; Hermine, O.; Colin, Y.; Buffet, P.A.; et al. Spherocytic shift of red blood cells during storage provides a quantitative whole cell-based marker of the storage lesion. Transfusion 2017, 57, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H.; Piety, N.Z.; Deuel, J.W.; Makhro, A.; Schulzki, T.; Bogdanov, N.; Goede, J.S.; Bogdanova, A.; Abidi, R.; Shevkoplyas, S.S. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion 2015, 55, 1872–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doss, J.F.; Corcoran, D.L.; Jima, D.D.; Telen, M.J.; Dave, S.S.; Chi, J.-T. A comprehensive joint analysis of the long and short rna transcriptomes of human erythrocytes. BMC Genom. 2015, 16, 952. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-H.; Doss, J.F.; Walzer, K.A.; McNulty, S.M.; Wu, J.; Roback, J.D.; Chi, J.-T. Angiogenin-mediated trna cleavage as a novel feature of stored red blood cells. Br. J. Haematol. 2019, 185, 760–764. [Google Scholar] [CrossRef]




| Sex | Year of Birth | |
|---|---|---|
| Sample A | Male | 1985 |
| Sample B | Female | 2000 |
| Sample C | Male | 1988 |
| Sample D | Female | 1964 |
| Sample E | Male | 1955 |
| Sample A | Sample B | Sample C | Sample D | Sample E | |
|---|---|---|---|---|---|
| Day 01 | 653,835 | 585,142 | 742,151 | 342,148 | 144,187 |
| Day 15 | 788,569 | 1,387,083 | 704,062 | 117,985 | 135,920 |
| Day 29 | 813,324 | 1,046,341 | 845,081 | 709,039 | 422,482 |
| Classification Performance Avg ± std [%] | Sample A | Sample B | Sample C | Sample D | Sample E |
|---|---|---|---|---|---|
| Week 01 | 90.8 ± 0.3 | 82.0 ± 0.4 | 83.2 ± 0.4 | 77.3 ± 0.3 | 71.6 ± 0.2 |
| Week 05 | 81.5 ± 0.3 | 78.1 ± 0.3 | 75.3 ± 0.4 | 85.1 ± 0.3 | 80.4 ± 0.2 |
| Accuracy | 85.6 ± 0.1 | 79.5 ± 0.1 | 79.0 ± 0.1 | 82.6 ± 0.1 | 78.2 ± 0.1 |
| Classification Performance Avg ± std [%] | Week 01 | Week 05 |
|---|---|---|
| Week 01 | 76.1 ± 0.4 | 23.9 ± 0.4 |
| Week 05 | 34.9 ± 0.6 | 65.1 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Price, H.; Ceballos, S.; Chi, J.-T.; Wax, A. Single Cell Analysis of Stored Red Blood Cells Using Ultra-High Throughput Holographic Cytometry. Cells 2021, 10, 2455. https://doi.org/10.3390/cells10092455
Park H-S, Price H, Ceballos S, Chi J-T, Wax A. Single Cell Analysis of Stored Red Blood Cells Using Ultra-High Throughput Holographic Cytometry. Cells. 2021; 10(9):2455. https://doi.org/10.3390/cells10092455
Chicago/Turabian StylePark, Han-Sang, Hillel Price, Silvia Ceballos, Jen-Tsan Chi, and Adam Wax. 2021. "Single Cell Analysis of Stored Red Blood Cells Using Ultra-High Throughput Holographic Cytometry" Cells 10, no. 9: 2455. https://doi.org/10.3390/cells10092455






