Advantages and Potential Benefits of Using Organoids in Nanotoxicology
Abstract
:1. Introduction
2. Dynamics of Scientometric Indicators of Works, including Studies of Toxicity and Organoids
3. Organoids in Toxicology Studies
4. Routes of Tested Substances’ Administration into Organoids
5. Potential Benefits of Using Organoids in Nanotoxicology
5.1. 3D Cell Organization
5.2. Cell Diversity of Organoids
5.3. Ability to Observe Complex Effects
6. Current Progress in Organoid Use in Nanotoxicology
7. Importance of Nomenclature
8. Suitability of Organoids for Biomedical Testing
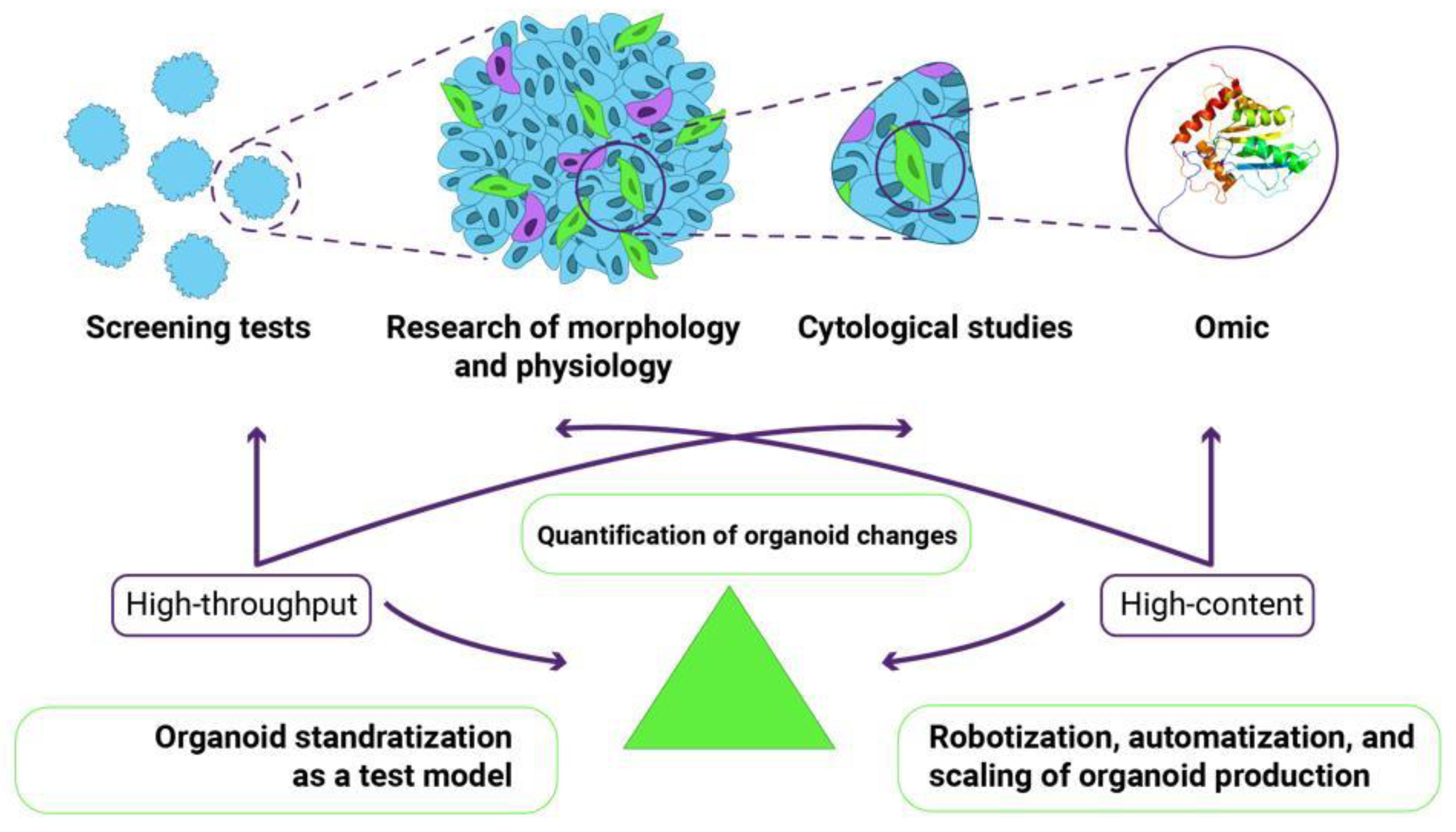
9. Methods for Assessing Organoid Characteristics
10. Methods of Advanced Organoid Production
11. Conclusions and Future Directions
- Automated 3D bioprinting has the potential for scaling up the production of organoids and tissue constructs. For post-organoid bioprinting, many hurdles need to be solved, including the improvement of bioprinting resolution, shear stress-induced cell damage due to high cell densities, the development of better bioinks for depositing cell aggregates, and effective vascularization techniques. The goal is to combine microengineering and organoid cultivation technologies to mimic a human working model to imitate any disease or for comprehensive drug testing to avoid the burden of human trials [9]. This prompted the development of other approaches. For example, the 3D bioprinting concept uses organoid-forming stem cells as building blocks, which can be deposited directly into extracellular matrices for spontaneous self-organization [157]. Another solution could be the magnetic levitational bioassembly of 3D tissue constructs [158].
- Gastruloids are 3D aggregates of embryonic stem cells cultured under defined conditions that display axial organization and gene expression patterns mimicking the earliest stages of organism development. Their use allows researchers to recreate structures analogous to those of organs in many ways. This has been demonstrated in the development of the heart and intestinal tube [8].
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| PCR | polymerase chain reaction |
| AO | acridine orange |
| ATP | adenosine triphosphate |
| CLSM | confocal laser scanning microscopy |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DHE | dihydroethidium |
| FGT | female genital tract |
| HA | hyaluronic acid |
| IHC | immunohistochemistry |
| iPSCs | induced pluripotent stem cell |
| KIM-1 | kidney injury molecule-1 |
| LTL | lotus tetragonolobus lectin |
| MWCNTs | multi-walled carbon nanotubes |
| NMs | nanomaterials |
| NO | nitric oxide |
| NPs | nanoparticles |
| QDs | quantum dots |
| PDMS | polydimethylsiloxane |
| PI | propidium iodide |
| ROS | reactive oxygen species |
| SPION | superparamagnetic iron oxide nanoparticles |
| TNFα | tumor necrosis factor-alpha |
References
- Montesano, R.; Mouron, P.; Amherdt, M.; Orci, L. Collagen Matrix Promotes Reorganization of Pancreatic Endocrine Cell Monolayers into Islet-like Organoids. J. Cell Biol. 1983, 97, 935–939. [Google Scholar] [CrossRef]
- Jakab, K.; Norotte, C.; Damon, B.; Marga, F.; Neagu, A.; Besch-Williford, C.L.; Kachurin, A.; Church, K.H.; Park, H.; Mironov, V.; et al. Tissue Engineering by Self-Assembly of Cells Printed into Topologically Defined Structures. Tissue Eng. Part A 2008, 14, 413–421. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesisin a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-Chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Woodfield, T.B.F.; Mironov, V.; Groll, J. Advances in Hybrid Fabrication toward Hierarchical Tissue Constructs. Adv. Sci. 2020, 7, 1902953. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; McDevitt, T.C. Mouse Gastruloids Take Heart. Nat. Rev. Cardiol. 2021, 18, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Rawal, P.; Tripathi, D.M.; Ramakrishna, S.; Kaur, S. Prospects for 3D Bioprinting of Organoids. Biodes. Manuf. 2021, 4, 627–640. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Chen, J. Modeling Better In Vitro Models for the Prediction of Nanoparticle Toxicity: A Review. Toxicol. Mech. Methods 2021, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.I.; Sei, Y.J.; Park, H.J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.J.; MacDonald, T.J.; Levey, A.I.; Kim, Y.T. Microengineered Human Blood–Brain Barrier Platform for Understanding Nanoparticle Transport Mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Weindl, G. Immunocompetent Human Intestinal Models in Preclinical Drug Development. Handb. Exp. Pharmacol. 2021, 265, 219–233. [Google Scholar] [CrossRef]
- Driehuis, E.; Oosterom, N.; Heil, S.G.; Muller, I.B.; Lin, M.; Kolders, S.; Jansen, G.; De Jonge, R.; Pieters, R.; Clevers, H.; et al. Patient-Derived Oral Mucosa Organoids as an In Vitro Model for Methotrexate Induced Toxicity in Pediatric Acute Lymphoblastic Leukemia. PLoS ONE 2020, 15, e0231588. [Google Scholar] [CrossRef]
- Kasagi, Y.; Chandramouleeswaran, P.M.; Whelan, K.A.; Tanaka, K.; Giroux, V.; Sharma, M.; Wang, J.; Benitez, A.J.; DeMarshall, M.; Tobias, J.W.; et al. The Esophageal Organoid System Reveals Functional Interplay Between Notch and Cytokines in Reactive Epithelial Changes. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.A.; Muir, A.B.; Nakagawa, H. Esophageal 3D Culture Systems as Modeling Tools in Esophageal Epithelial Pathobiology and Personalized Medicine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Chandramouleeswaran, P.M.; Guha, M.; Shimonosono, M.; Whelan, K.A.; Maekawa, H.; Sachdeva, U.M.; Ruthel, G.; Mukherjee, S.; Engel, N.; Gonzalez, M.V.; et al. Autophagy Mitigates Ethanol-Induced Mitochondrial Dysfunction and Oxidative Stress in Esophageal Keratinocytes. PLoS ONE 2020, 15, e0239625. [Google Scholar] [CrossRef]
- O’Rourke, K.; Ackerman, S.; Dow, L.; Lowe, S. Isolation, Culture, and Maintenance of Mouse Intestinal Stem Cells. Bio. Protoc. 2016, 6, e1733. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, W.D.; Panzica-Kelly, J.M.; Chen, S.-J.; Strassle, B.; Hasson, C.; Lecureux, L.; Wang, L.; Chen, W.; Sherry, T.; Gan, J.; et al. Development and Characterization of Rat Duodenal Organoids for ADME and Toxicology Applications. Toxicology 2020, 446, 152614. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Nanki, K.; Sato, T. Efficient Genetic Engineering of Human Intestinal Organoids Using Electroporation. Nat. Protoc. 2015, 10, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures In Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Chen, S.; Liang, L.; Li, X.; Tang, P.; Rao, X.; Pan, M.; Xu, X.; Li, Y.; Yao, Y.; et al. SIRT1 Inhibitors Mitigate Radiation-Induced GI Syndrome by Enhancing Intestinal-Stem-Cell Survival. Cancer Lett. 2021, 501, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jung, I.K.; Lee, Y.; Jin, S.; Yun, H.J.; Kim, B.W.; Kwon, H.J. Alcohol Stimulates the Proliferation of Mouse Small Intestinal Epithelial Cells via Wnt Signaling. Biochem. Biophys. Res. Commun. 2021, 534, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Belair, D.G.; Visconti, R.J.; Hong, M.; Marella, M.; Peters, M.F.; Scott, C.W.; Kolaja, K.L. Human Ileal Organoid Model Recapitulates Clinical Incidence of Diarrhea Associated with Small Molecule Drugs. Toxicol. In Vitro 2020, 68, 104928. [Google Scholar] [CrossRef]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef]
- Shinozawa, T.; Kimura, M.; Cai, Y.; Saiki, N.; Yoneyama, Y.; Ouchi, R.; Koike, H.; Maezawa, M.; Zhang, R.R.; Dunn, A.; et al. High-Fidelity Drug Induced Liver Injury Screen Using Human PSC-Derived Organoids. Gastroenterology 2020, 160, 831–846.e10. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed Differentiation of Human Pluripotent Stem Cells into Intestinal Tissue In Vitro. Nature 2011, 470, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered Human Pluripotent-Stem-Cell-Derived Intestinal Tissues with a Functional Enteric Nervous System. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Karve, S.S.; Weiss, A.A.; Hawkins, J.; Poling, H.M.; Helmrath, M.A.; Wells, J.M.; McCauley, H.A. Tissue Responses to Shiga Toxin in Human Intestinal Organoids. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.; Kim, W.J.; Choi, Y.H.; Yang, S.R.; Hong, S.H. Diesel Particulate Matter 2.5 Induces Epithelial-to-Mesenchymal Transition and Upregulation of Sars-Cov-2 Receptor during Human Pluripotent Stem Cell-Derived Alveolar Organoid Development. Int. J. Environ. Res. Public Health 2020, 17, 8410. [Google Scholar] [CrossRef] [PubMed]
- Przepiorski, A.; Sander, V.; Tran, T.; Hollywood, J.A.; Sorrenson, B.; Shih, J.H.; Wolvetang, E.J.; McMahon, A.P.; Holm, T.M.; Davidson, A.J. A Simple Bioreactor-Based Method to Generate Kidney Organoids from Pluripotent Stem Cells. Stem Cell Rep. 2018, 11, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Digby, J.L.M.; Vanichapol, T.; Przepiorski, A.; Davidson, A.J.; Sander, V. Evaluation of Cisplatin-Induced Injury in Human Kidney Organoids. Am. J. Physiol. Renal. Physiol. 2020, 318, F971–F978. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Sun, G.; Liu, S.; Peng, E.; Wan, M.; Chen, L.; Jackson, J.; Atala, A. Three-Dimensional Renal Organoids from Whole Kidney Cells: Generation, Optimization, and Potential Application in Nephrotoxicology In Vitro. Cell Transpl. 2020, 29. [Google Scholar] [CrossRef]
- Horsley, H.; Dharmasena, D.; Malone-Lee, J.; Rohn, J.L. A Urine-Dependent Human Urothelial Organoid Offers a Potential Alternative to Rodent Models of Infection. Sci. Rep. 2018, 8, 1238. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.K.; Dharmasena, D.; Horsley, H.; Jafari, N.V.; Malone-Lee, J.; Stride, E.; Edirisinghe, M.; Rohn, J.L. Novel Antibiotic-Loaded Particles Conferring Eradication of Deep Tissue Bacterial Reservoirs for the Treatment of Chronic Urinary Tract Infection. J. Control Release 2020, 328, 490–502. [Google Scholar] [CrossRef]
- Pendergraft, S.S.; Sadri-Ardekani, H.; Atala, A.; Bishop, C.E. Three-Dimensional Testicular Organoid: A Novel Tool for the Study of Human Spermatogenesis and Gonadotoxicity In Vitro. Biol. Reprod. 2017, 96, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Arzua, T.; Yan, Y.; Jiang, C.; Logan, S.; Allison, R.L.; Wells, C.; Kumar, S.N.; Schäfer, R.; Bai, X. Modeling Alcohol-Induced Neurotoxicity Using Human Induced Pluripotent Stem Cell-Derived Three-Dimensional Cerebral Organoids. Transl. Psychiatry 2020, 10, 347. [Google Scholar] [CrossRef]
- Bu, Q.; Huang, Y.; Li, M.; Dai, Y.; Fang, X.; Chen, K.; Liu, Q.; Xue, A.; Zhong, K.; Huang, Y.; et al. Acrylamide Exposure Represses Neuronal Differentiation, Induces Cell Apoptosis and Promotes Tau Hyperphosphorylation in HESC-Derived 3D Cerebral Organoids. Food Chem. Toxicol. 2020, 144, 111643. [Google Scholar] [CrossRef] [PubMed]
- Brüll, M.; Spreng, A.S.; Gutbier, S.; Loser, D.; Krebs, A.; Reich, M.; Kraushaar, U.; Britschgi, M.; Patsch, C.; Leist, M. Incorporation of Stem Cell-Derived Astrocytes into Neuronal Organoids to Allow Neuro-Glial Interactions in Toxicological Studies. ALTEX 2020, 37, 409–428. [Google Scholar] [CrossRef]
- Yin, F.; Zhu, Y.; Wang, Y.; Qin, J. Engineering Brain Organoids to Probe Impaired Neurogenesis Induced by Cadmium. ACS Biomater. Sci. Eng. 2018, 4, 1908–1915. [Google Scholar] [CrossRef]
- Eiraku, M.; Sasai, Y. Mouse Embryonic Stem Cell Culture for Generation of Three-Dimensional Retinal and Cortical Tissues. Nat. Protoc. 2012, 7, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.I.; Onishi, A.; Takahashi, M. Chemically-Induced Photoreceptor Degeneration and Protection in Mouse IPSC-Derived Three-Dimensional Retinal Organoids. Stem Cell Res. 2017, 24, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of Organoids from Mouse and Human Endometrium Showing Endometrial Epithelium Physiology and Long-Term Expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; van Zundert, I.; et al. Patient-Derived Organoids from Endometrial Disease Capture Clinical Heterogeneity and Are Amenable to Drug Screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Hill, D.R.; Huang, S.; Nagy, M.S.; Yadagiri, V.K.; Fields, C.; Mukherjee, D.; Bons, B.; Dedhia, P.H.; Chin, A.M.; Tsai, Y.-H.; et al. Bacterial Colonization Stimulates a Complex Physiological Response in the Immature Human Intestinal Epithelium. eLife 2017, 6, e29132. [Google Scholar] [CrossRef]
- Hanyu, H.; Yokoi, Y.; Nakamura, K.; Ayabe, T.; Tanaka, K.; Uno, K.; Miyajima, K.; Saito, Y.; Iwatsuki, K.; Shimizu, M.; et al. Mycotoxin Deoxynivalenol Has Different Impacts on Intestinal Barrier and Stem Cells by Its Route of Exposure. Toxins 2020, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.J.; Shin, T.H.; Kim, M.; Sung, C.O.; Jang, S.J.; Jeong, G.S. A One-Stop Microfluidic-Based Lung Cancer Organoid Culture Platform for Testing Drug Sensitivity. Lab. Chip. 2019, 19, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Gupta, K.; Harms, V.; Torr, E.; Evans, J.; Johnson, H.J.; Soref, C.; Acevedo-Acevedo, S.; Antosiewicz-Bourget, J.; Mamott, D.; et al. Engineered Perineural Vascular Plexus for Modeling Developmental Toxicity. Adv. Healthc. Mater. 2020, 9, 2000825. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Degirmenci, B.; Moor, A.E.; Herr, P.; Zimmerli, D.; Moor, M.B.; Hausmann, G.; Cantù, C.; Aguet, M.; Basler, K. Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell Rep. 2016, 15, 911–918. [Google Scholar] [CrossRef]
- Co, J.Y.; Margalef-Català, M.; Monack, D.M.; Amieva, M.R. Controlling the Polarity of Human Gastrointestinal Organoids to Investigate Epithelial Biology and Infectious Diseases. Nat. Protoc. 2021, 16, 5171–5192. [Google Scholar] [CrossRef] [PubMed]
- Kakni, P.; López-Iglesias, C.; Truckenmüller, R.; Habibović, P.; Giselbrecht, S. Reversing Epithelial Polarity in Pluripotent Stem Cell-Derived Intestinal Organoids. Front. Bioeng. Biotechnol. 2022, 10, 669. [Google Scholar] [CrossRef]
- Parrish, M.C.; Tan, Y.J.; Grimes, K.V.; Mochly-Rosen, D. Surviving in the Valley of Death: Opportunities and Challenges in Translating Academic Drug Discoveries. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Chrishtop, V.V.; Prilepskii, A.Y.; Nikonorova, V.G.; Mironov, V.A. Nanosafety vs. Nanotoxicology: Adequate Animal Models for Testing in Vivo Toxicity of Nanoparticles. Toxicology 2021, 462, 152952. [Google Scholar] [CrossRef]
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y.R.; Cho, C.F. Blood-Brain-Barrier Organoids for Investigating the Permeability of CNS Therapeutics. Nat. Protoc. 2018, 13, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Nzou, G.; Wicks, R.T.; VanOstrand, N.R.; Mekky, G.A.; Seale, S.A.; EL-Taibany, A.; Wicks, E.E.; Nechtman, C.M.; Marotte, E.J.; Makani, V.S.; et al. Multicellular 3D Neurovascular Unit Model for Assessing Hypoxia and Neuroinflammation Induced Blood-Brain Barrier Dysfunction. Sci. Rep. 2020, 10, 9766. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, S.; Naydenov, N.G.; Feygin, A.; Cruise, M.; Ervasti, J.M.; Ivanov, A.I. Loss of β-Cytoplasmic Actin in the Intestinal Epithelium Increases Gut Barrier Permeability in Vivo and Exaggerates the Severity of Experimental Colitis. Front. Cell Dev. Biol. 2020, 8, 588836. [Google Scholar] [CrossRef]
- Šuligoj, T.; Vigsnæs, L.K.; Van den Abbeele, P.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef]
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS Barrier-Forming Organoids with Cerebrospinal Fluid Production. Science 2020, 369, eaaz5626. [Google Scholar] [CrossRef]
- Bardenbacher, M.; Ruder, B.; Britzen-Laurent, N.; Naschberger, E.; Becker, C.; Palmisano, R.; Stürzl, M.; Tripal, P. Investigating Intestinal Barrier Breakdown in Living Organoids. J. Vis. Exp. 2020, 2020, e60546. [Google Scholar] [CrossRef]
- Spencer, C.E.; Flint, L.E.; Duckett, C.J.; Cole, L.M.; Cross, N.; Smith, D.P.; Clench, M.R. Role of MALDI-MSI in Combination with 3D Tissue Models for Early Stage Efficacy and Safety Testing of Drugs and Toxicants. Expert Rev. Proteom. 2020, 17, 827–841. [Google Scholar] [CrossRef]
- Park, E.; Kim, H.K.; Jee, J.H.; Hahn, S.; Jeong, S.; Yoo, J. Development of Organoid-Based Drug Metabolism Model. Toxicol. Appl. Pharmacol. 2019, 385, 114790. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.J.; Cook, R.S.; Sanders, M.E.; Aurisicchio, L.; Ciliberto, G.; Arteaga, C.L.; Skala, M.C. Quantitative Optical Imaging of Primary Tumor Organoid Metabolism Predicts Drug Response in Breast Cancer. Cancer Res. 2014, 74, 5184–5194. [Google Scholar] [CrossRef]
- Lu, W.; Rettenmeier, E.; Paszek, M.; Yueh, M.F.; Tukey, R.H.; Trottier, J.; Barbier, O.; Chen, S. Crypt Organoid Culture as an In Vitro Model in Drug Metabolism and Cytotoxicity Studies. Drug Metab. Dispos. 2017, 45, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Saenz-De-Santa-Maria, I.; Chastagner, P.; Perthame, E.; Delmas, C.; Toulas, C.; Moyal-Jonathan-Cohen, E.; Brou, C.; Zurzolo, C. Patient-Derived Glioblastoma Stem Cells Transfer Mitochondria through Tunneling Nanotubes in Tumor Organoids. Biochem. J. 2021, 478, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.; Lépine, P.; Han, C.; Lacalle-Aurioles, M.; Chen, C.X.Q.; Haag, R.; Durcan, T.M.; Maysinger, D. Nanotherapeutic Modulation of Human Neural Cells and Glioblastoma in Organoids and Monocultures. Cells 2020, 9, 2434. [Google Scholar] [CrossRef] [PubMed]
- Oszvald, Á.; Szvicsek, Z.; Pápai, M.; Kelemen, A.; Varga, Z.; Tölgyes, T.; Dede, K.; Bursics, A.; Buzás, E.I.; Wiener, Z. Fibroblast-Derived Extracellular Vesicles Induce Colorectal Cancer Progression by Transmitting Amphiregulin. Front. Cell Dev. Biol. 2020, 8, 558. [Google Scholar] [CrossRef] [PubMed]
- Oszvald, Á.; Szvicsek, Z.; Sándor, G.O.; Kelemen, A.; Soós, A.Á.; Pálóczi, K.; Bursics, A.; Dede, K.; Tölgyes, T.; Buzás, E.I.; et al. Extracellular Vesicles Transmit Epithelial Growth Factor Activity in the Intestinal Stem Cell Niche. Stem Cells 2020, 38, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Szvicsek, Z.; Oszvald, Á.; Szabó, L.; Sándor, G.O.; Kelemen, A.; Soós, A.Á.; Pálóczi, K.; Harsányi, L.; Tölgyes, T.; Dede, K.; et al. Extracellular Vesicle Release from Intestinal Organoids Is Modulated by Apc Mutation and Other Colorectal Cancer Progression Factors. Cell. Mol. Life Sci. 2019, 76, 2463–2476. [Google Scholar] [CrossRef]
- Takezawa, T.; Mori, Y.; Yonaha, T.; Yoshizato, K. Characterization of Morphology and Cellular Metabolism during the Spheroid Formation by Fibroblasts. Exp. Cell Res. 1993, 208, 430–441. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Koike, T.; Shiojiri, N. Immunohistochemical Analyses of Cell-Cell Interactions during Hepatic Organoid Formation from Fetal Mouse Liver Cells Cultured In Vitro. Histochem. Cell Biol. 2007, 128, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.B.; Roosens, T.; El Taghdouini, A.; Mannaerts, I.; Smout, A.J.; Najimi, M.; Sokal, E.; Noor, F.; Chesne, C.; van Grunsven, L.A. Novel Human Hepatic Organoid Model Enables Testing of Drug-Induced Liver Fibrosis In Vitro. Biomaterials 2016, 78, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hikage, F.; Atkins, S.; Kahana, A.; Smith, T.J.; Chun, T.H. HIF2A-LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology 2019, 160, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Kruk, P.A.; Auersperg, N. Human Ovarian Surface Epithelial Cells Are Capable of Physically Restructuring Extracellular Matrix. Am. J. Obstet. Gynecol. 1992, 167, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Moreno, K.; Chávez-García, D.; Hirata, G.; Vazquez-Duhalt, R. Monolayer (2D) or Spheroids (3D) Cell Cultures for Nanotoxicological Studies? Comparison of Cytotoxicity and Cell Internalization of Nanoparticles. Toxicol. In Vitro 2022, 85, 105461. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Sambale, F.; Lavrentieva, A.; Stahl, F.; Blume, C.; Stiesch, M.; Kasper, C.; Bahnemann, D.; Scheper, T. Three Dimensional Spheroid Cell Culture for Nanoparticle Safety Testing. J. Biotechnol. 2015, 205, 120–129. [Google Scholar] [CrossRef]
- Chia, S.L.; Tay, C.Y.; Setyawati, M.I.; Leong, D.T. Biomimicry 3D Gastrointestinal Spheroid Platform for the Assessment of Toxicity and Inflammatory Effects of Zinc Oxide Nanoparticles. Small 2015, 11, 702–712. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of Gold Nanoparticles Shape on Their Cytotoxicity against Human Osteoblast and Osteosarcoma in In Vitro Model. Evaluation of the Safety of Use and Anti-Cancer Potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Kladko, D.V.; Falchevskaya, A.S.; Serov, N.S.; Prilepskii, A.Y. Nanomaterial Shape Influence on Cell Behavior. Int. J. Mol. Sci. 2021, 22, 5266. [Google Scholar] [CrossRef]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and Shape-Dependent Cytotoxicity Profile of Gold Nanoparticles for Biomedical Applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Zhang, J.; Huang, C.; Yang, Z.; Cao, Y. The Cytotoxicity of Zinc Oxide Nanoparticles to 3D Brain Organoids Results from Excessive Intracellular Zinc Ions and Defective Autophagy. Cell Biol. Toxicol. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Simian, M.; Bissell, M.J. Organoids: A Historical Perspective of Thinking in Three Dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef]
- Augustyniak, J.; Bertero, A.; Coccini, T.; Baderna, D.; Buzanska, L.; Caloni, F. Organoids Are Promising Tools for Species-Specific In Vitro Toxicological Studies. J. Appl. Toxicol. 2019, 39, 1610–1622. [Google Scholar] [CrossRef]
- Ogoke, O.; Maloy, M.; Parashurama, N. The Science and Engineering of Stem Cell-Derived Organoids-Examples from Hepatic, Biliary, and Pancreatic Tissues. Biol. Rev. 2021, 96, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Kihara, S.; Köper, I.; Mata, J.P.; McGillivray, D.J. Reviewing Nanoplastic Toxicology: It’s an Interface Problem. Adv. Colloid Interface Sci. 2021, 288, 102337. [Google Scholar] [CrossRef]
- Kopac, T. Protein Corona, Understanding the Nanoparticle–Protein Interactions and Future Perspectives: A Critical Review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle Interactions with Co-Existing Contaminants: Joint Toxicity, Bioaccumulation and Risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-Specific Toxicity of Magnetic Iron Oxide-Based Nanoparticles. Nanotoxicology 2021, 15, 167–204. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid Nanoparticles (SLN, NLC): Overcoming the Anatomical and Physiological Barriers of the Eye—Part I—Barriers and Determining Factors in Ocular Delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible Nanoparticles and Vesicular Systems in Transdermal Drug Delivery for Various Skin Diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-C.; Fan, J.; Wang, X.; Eacker, S.M.; Kam, T.-I.; Chen, L.; Yin, X.; Zhu, J.; Chi, Z.; Jiang, H.; et al. Cultured Networks of Excitatory Projection Neurons and Inhibitory Interneurons for Studying Human Cortical Neurotoxicity. Sci. Transl. Med. 2016, 8, 333ra48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, H.; Jiang, S.; She, C.; Cao, Y. Multi-Walled Carbon Nanotubes Decrease Neuronal NO Synthase in 3D Brain Organoids. Sci. Total Environ. 2020, 748, 141384. [Google Scholar] [CrossRef]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.W.; Seol, Y.J.; Zhang, Y.S.; Shin, S.R.; Zhao, L.; Aleman, J.; et al. Multi-Tissue Interactions in an Integrated Three-Tissue Organ-on-a-Chip Platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef] [PubMed]
- Mekky, G.; Seeds, M.; Diab, A.E.-A.A.; Shehata, A.M.; Ahmed-Farid, O.A.-H.; Alzebdeh, D.; Bishop, C.; Atala, A. The Potential Toxic Effects of Magnesium Oxide Nanoparticles and Valproate on Liver Tissue. J. Biochem. Mol. Toxicol. 2021, 35, e22676. [Google Scholar] [CrossRef]
- Park, S.B.; Jung, W.H.; Kim, K.Y.; Koh, B. Toxicity Assessment of SiO2 and TiO2 in Normal Colon Cells, in Vivo and in Human Colon Organoids. Molecules 2020, 25, 3594. [Google Scholar] [CrossRef] [PubMed]
- Boj, S.F.; Hwang, C.I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid Models of Human and Mouse Ductal Pancreatic Cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Palzer, J.; Mues, B.; Goerg, R.; Aberle, M.; Rensen, S.S.; Olde Damink, S.W.M.; Vaes, R.D.W.; Cramer, T.; Schmitz-Rode, T.; Neumann, U.P.; et al. Magnetic Fluid Hyperthermia as Treatment Option for Pancreatic Cancer Cells and Pancreatic Cancer Organoids. Int. J. Nanomed. 2021, 16, 2965–2981. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Ebel, J.; Kollenda, S.; Klein, K.; Kruse, B.; Veltkamp, C.; Lange, C.M.; Westendorf, A.M.; Epple, M. Uptake of Functional Ultrasmall Gold Nanoparticles in 3D Gut Cell Models. Small 2022, 18, 2201167. [Google Scholar] [CrossRef]
- Hou, Z.; Meng, R.; Chen, G.; Lai, T.; Qing, R.; Hao, S.; Deng, J.; Wang, B. Distinct Accumulation of Nanoplastics in Human Intestinal Organoids. Sci. Total Environ. 2022, 838, 155811. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, L.; Cao, C.; Ma, R.; Huang, Y.; Zhong, K.; Gao, H.; Huang, Y.; Bu, Q. Silver Nanoparticles Exposure Induces Developmental Neurotoxicity in HiPSC-Derived Cerebral Organoids. Sci. Total Environ. 2022, 845, 157047. [Google Scholar] [CrossRef] [PubMed]
- Han, S.I.; Lee, S.W.; Cho, M.G.; Yoo, J.M.; Oh, M.H.; Jeong, B.; Kim, D.; Park, O.K.; Kim, J.; Namkoong, E.; et al. Epitaxially Strained CeO2/Mn3O4 Nanocrystals as an Enhanced Antioxidant for Radioprotection. Adv. Mater. 2020, 32, 2001566. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tian, X.; Gao, D.; Lang, Y.; Zhang, X.X.; Yang, C.; Gu, M.M.; Shi, J.; Zhou, P.K.; Shang, Z.F. Oral Administration of Hydroxylated-Graphene Quantum Dots Induces Intestinal Injury Accompanying the Loss of Intestinal Stem Cells and Proliferative Progenitor Cells. Nanotoxicology 2019, 13, 1409–1421. [Google Scholar] [CrossRef]
- Peng, H.; Wang, C.; Xu, X.; Yu, C.; Wang, Q. An Intestinal Trojan Horse for Gene Delivery. Nanoscale 2015, 7, 4354–4360. [Google Scholar] [CrossRef]
- Qi, Y.; Shi, E.; Peroutka-Bigus, N.; Bellaire, B.; Wannemuehler, M.; Jergens, A.; Barrett, T.; Wu, Y.; Wang, Q. Ex Vivo Study of Telluride Nanowires in Minigut. J. Biomed. Nanotechnol. 2018, 14, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Astashkina, A.I.; Mann, B.K.; Prestwich, G.D.; Grainger, D.W. A 3-D Organoid Kidney Culture Model Engineered for High-Throughput Nephrotoxicity Assays. Biomaterials 2012, 33, 4700–4711. [Google Scholar] [CrossRef] [PubMed]
- Astashkina, A.I.; Mann, B.K.; Prestwich, G.D.; Grainger, D.W. Comparing Predictive Drug Nephrotoxicity Biomarkers in Kidney 3-D Primary Organoid Culture and Immortalized Cell Lines. Biomaterials 2012, 33, 4712–4721. [Google Scholar] [CrossRef]
- Astashkina, A.I.; Jones, C.F.; Thiagarajan, G.; Kurtzeborn, K.; Ghandehari, H.; Brooks, B.D.; Grainger, D.W. Nanoparticle Toxicity Assessment Using an In Vitro 3-D Kidney Organoid Culture Model. Biomaterials 2014, 35, 6323–6331. [Google Scholar] [CrossRef]
- He, C.; Ruan, F.; Jiang, S.; Zeng, J.; Yin, H.; Liu, R.; Zhang, Y.; Huang, L.; Wang, C.; Ma, S.; et al. Black Phosphorus Quantum Dots Cause Nephrotoxicity in Organoids, Mice, and Human Cells. Small 2020, 16, 2001371. [Google Scholar] [CrossRef]
- Davoudi, Z.; Peroutka-Bigus, N.; Bellaire, B.; Wannemuehler, M.; Barrett, T.A.; Narasimhan, B.; Wang, Q. Intestinal Organoids Containing Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of Inflammatory Bowel Diseases. J. Biomed. Mater. Res. A 2018, 106, 876–886. [Google Scholar] [CrossRef]
- Pujara, N.; Giri, R.; Wong, K.Y.; Qu, Z.; Rewatkar, P.; Moniruzzaman, M.; Begun, J.; Ross, B.P.; McGuckin, M.; Popat, A. PH-Responsive Colloidal Carriers Assembled from β-Lactoglobulin and Epsilon Poly-L-Lysine for Oral Drug Delivery. J. Colloid Interface Sci. 2021, 589, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Oancea, I.; Movva, R.; Das, I.; Aguirre De Cárcer, D.; Schreiber, V.; Yang, Y.; Purdon, A.; Harrington, B.; Proctor, M.; Wang, R.; et al. Colonic Microbiota Can Promote Rapid Local Improvement of Murine Colitis by Thioguanine Independently of T Lymphocytes and Host Metabolism. Gut 2017, 66, 59–69. [Google Scholar] [CrossRef] [PubMed]
- VanDussen, K.L.; Marinshaw, J.M.; Shaikh, N.; Miyoshi, H.; Moon, C.; Tarr, P.I.; Ciorba, M.A.; Stappenbeck, T.S. Development of an Enhanced Human Gastrointestinal Epithelial Culture System to Facilitate Patient-Based Assays. Gut 2015, 64, 911–920. [Google Scholar] [CrossRef]
- Angireddy, R.; Chowdhury, A.R.; Zielonka, J.; Ruthel, G.; Kalyanaraman, B.; Avadhani, N.G. Alcohol-Induced CYP2E1, Mitochondrial Dynamics and Retrograde Signaling in Human Hepatic 3D Organoids. Free Radic. Biol. Med. 2020, 159, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.F.G.; Puigvert, L.F.; Messner, S.; Mortiz, W.; Ingelman-Sundberg, M. Hepatic 3D Spheroid Models for the Detection and Study of Compounds with Cholestatic Liability. Sci. Rep. 2016, 6, 35434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Q.; Li, J.; Yu, L.; Li, F.; Li, W.; Li, Y.; Peng, H.; Zhao, J.; Carmichael, P.L.; et al. Integration of In Vitro Data from Three Dimensionally Cultured HepaRG Cells and Physiologically Based Pharmacokinetic Modeling for Assessment of Acetaminophen Hepatotoxicity. Regul. Toxicol. Pharmacol. 2020, 114, 104661. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Xue, R.; Li, T.; Leng, L.; Wang, Y.; Wang, J.; Ma, J.; Yan, J.; Yan, F.; et al. Liver Extracellular Matrices Bioactivated Hepatic Spheroids as a Model System for Drug Hepatotoxicity Evaluations. Adv. Biosyst. 2018, 2, 1800110. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Grabinger, T.; Luks, L.; Kostadinova, F.; Zimberlin, C.; Medema, J.P.; Leist, M.; Brunner, T. Ex Vivo Culture of Intestinal Crypt Organoids as a Model System for Assessing Cell Death Induction in Intestinal Epithelial Cells and Enteropathy. Cell Death Dis. 2014, 5, e1228. [Google Scholar] [CrossRef]
- Lkhagvadorj, K.; Zeng, Z.; Song, J.; Reinders-Luinge, M.; Kooistra, W.; Song, S.; Krauss-Etschmann, S.; Melgert, X.B.N.; Cao, J.; Hylkema, X.M.H.N. Prenatal Smoke Exposure Dysregulates Lung Epithelial Cell Differentiation in Mouse Offspring: Role for AREG-Induced EGFR Signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L742–L751. [Google Scholar] [CrossRef]
- Clayton, N.P.; Burwell, A.; Jensen, H.; Williams, B.F.; Brown, Q.D.; Ovwigho, P.; Ramaiahgari, S.; Hermon, T.; Dixon, D. Preparation of Three-Dimensional (3-D) Human Liver (HepaRG) Cultures for Histochemical and Immunohistochemical Staining and Light Microscopic Evaluation. Toxicol. Pathol. 2018, 46, 653–659. [Google Scholar] [CrossRef]
- Piccinini, F.; Balassa, T.; Carbonaro, A.; Diosdi, A.; Toth, T.; Moshkov, N.; Tasnadi, E.A.; Horvath, P. Software Tools for 3D Nuclei Segmentation and Quantitative Analysis in Multicellular Aggregates. Comput. Struct. Biotechnol. J. 2020, 18, 1287–1300. [Google Scholar] [CrossRef]
- Schmuck, M.R.; Temme, T.; Dach, K.; de Boer, D.; Barenys, M.; Bendt, F.; Mosig, A.; Fritsche, E. Omnisphero: A High-Content Image Analysis (HCA) Approach for Phenotypic Developmental Neurotoxicity (DNT) Screenings of Organoid Neurosphere Cultures In Vitro. Arch. Toxicol. 2017, 91, 2017–2028. [Google Scholar] [CrossRef]
- Bode, K.J.; Mueller, S.; Schweinlin, M.; Metzger, M.; Brunner, T. A Fast and Simple Fluorometric Method to Detect Cell Death in 3D Intestinal Organoids. Biotechniques 2019, 67, 23–28. [Google Scholar] [CrossRef]
- Lawrence, M.L.; Elhendawi, M.; Davies, J.A. Investigating Aspects of Renal Physiology and Pharmacology in Organ and Organoid Culture. In Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 1926, pp. 127–142. [Google Scholar]
- Shah, A.T.; Heaster, T.M.; Skala, M.C. Metabolic Imaging of Head and Neck Cancer Organoids. PLoS ONE 2017, 12, e0170415. [Google Scholar] [CrossRef]
- Barrett, C.; Short, S.; Choksi, Y.; Williams, C. Whole-Mount Enteroid Proliferation Staining. Bio Protoc. 2016, 6, e1837. [Google Scholar] [CrossRef]
- Kaushik, G.; Ponnusamy, M.P.; Batra, S.K. Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused Cerebral Organoids Model Interactions between Brain Regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in Cancer Research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Qian, X.; Jacob, F.; Song, M.M.; Nguyen, H.N.; Song, H.; Ming, G. Generation of Human Brain Region–Specific Organoids Using a Miniaturized Spinning Bioreactor. Nat. Protoc. 2018, 13, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Seiler, M.J.; Tang, W.C.; Wang, J.Y.; Delgado, J.; McLelland, B.T.; Nistor, G.; Keirstead, H.S.; Browne, A.W. Retinal Organoids On-a-Chip: A Micro-Millifluidic Bioreactor for Long-Term Organoid Maintenance. Lab Chip 2021, 21, 3361–3377. [Google Scholar] [CrossRef]
- Cai, H.; Ao, Z.; Wu, Z.; Song, S.; Mackie, K.; Guo, F. Intelligent Acoustofluidics Enabled Mini-Bioreactors for Human Brain Organoids. Lab Chip 2021, 21, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-Enhanced Vascularization and Maturation of Kidney Organoids In Vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Magliaro, C.; Paczia, N.; Monzel, A.S.; Antony, P.; Linster, C.L.; Bolognin, S.; Ahluwalia, A.; Schwamborn, J.C. Millifluidic Culture Improves Human Midbrain Organoid Vitality and Differentiation. Lab Chip 2018, 18, 3172–3183. [Google Scholar] [CrossRef]
- Jung, D.J.; Byeon, J.H.; Jeong, G.S. Flow Enhances Phenotypic and Maturation of Adult Rat Liver Organoids. Biofabrication 2020, 12, 045035. [Google Scholar] [CrossRef]
- Sekiya, S.; Kikuchi, T.; Shimizu, T. Perfusion Culture Maintained with an Air-Liquid Interface to Stimulate Epithelial Cell Organization in Renal Organoids In Vitro. BMC Biomed. Eng. 2019, 1, 15. [Google Scholar] [CrossRef]
- Tao, T.; Wang, Y.; Chen, W.; Li, Z.; Su, W.; Guo, Y.; Deng, P.; Qin, J. Engineering Human Islet Organoids from IPSCs Using an Organ-on-Chip Platform. Lab Chip 2019, 19, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.-N.; Jin, Y.; An, Y.; Kim, J.; Choi, Y.S.; Lee, J.S.; Kim, J.; Choi, W.-Y.; Koo, D.-J.; Yu, W.; et al. Microfluidic Device with Brain Extracellular Matrix Promotes Structural and Functional Maturation of Human Brain Organoids. Nat. Commun. 2021, 12, 4730. [Google Scholar] [CrossRef] [PubMed]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an In Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Eng. Part C Methods 2016, 22, 221–249. [Google Scholar] [CrossRef]
- Semertzidou, A.; Brosens, J.J.; McNeish, I.; Kyrgiou, M. Organoid Models in Gynaecological Oncology Research. Cancer Treat. Rev. 2020, 90, 102103. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.E.; Bouma, G.J.; Hollinshead, F.K. The Roles of Extracellular Vesicles and Organoid Models in Female Reproductive Physiology. Int. J. Mol. Sci. 2022, 23, 3186. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Khoei, H.; Esfandiari, F.; Hajari, M.A.; Ghorbaninejad, Z.; Piryaei, A.; Baharvand, H. Organoid Technology in Female Reproductive Biomedicine. Reprod. Biol. Endocrinol. 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Liu, D.; Dickson, K.-A.; Marsh, D.J.; Ford, C.E.; Stenzel, M.H. An Organotypic Model of High-Grade Serous Ovarian Cancer to Test the Anti-Metastatic Potential of ROR2 Targeted Polyion Complex Nanoparticles. J. Mater. Chem. B 2021, 9, 9123–9135. [Google Scholar] [CrossRef] [PubMed]
- Kopper, O.; de Witte, C.J.; Lõhmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An Organoid Platform for Ovarian Cancer Captures Intra- and Interpatient Heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Gasco, S.; Muñoz-Fernández, M.Á. A Review on the Current Knowledge on ZIKV Infection and the Interest of Organoids and Nanotechnology on Development of Effective Therapies against Zika Infection. Int. J. Mol. Sci. 2020, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Seleit, A.; Krämer, I.; Riebesehl, B.F.; Ambrosio, E.M.; Stolper, J.S.; Lischik, C.Q.; Dross, N.; Centanin, L. Neural Stem Cells Induce the Formation of Their Physical Niche during Organogenesis. eLife 2017, 6, e29173. [Google Scholar] [CrossRef]
- Daniel, E.; Cleaver, O. Vascularizing Organogenesis: Lessons from Developmental Biology and Implications for Regenerative Medicine. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, UK, 2019; Volume 132, pp. 177–220. ISBN 9780128104897. [Google Scholar]
- O’Connell, L.; Winter, D.C. Organoids: Past Learning and Future Directions. Stem Cells Dev. 2020, 29, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Achberger, K.; Probst, C.; Haderspeck, J.C.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging Organoid and Organ-on-a-Chip Technology to Generate Complex Multi-Layer Tissue Models in a Human Retina-on-a-Chip Platform. eLife 2019, 8, e46188. [Google Scholar] [CrossRef] [PubMed]
- Truskey, G.A. Human Microphysiological Systems and Organoids as In Vitro Models for Toxicological Studies. Front. Public Health. 2018, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Brassard, J.A.; Nikolaev, M.; Hübscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating Macro-Scale Tissue Self-Organization through Organoid Bioprinting. Nat. Mater. 2021, 20, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, V.A.; Khesuani, Y.D.; Petrov, S.V.; Karalkin, P.A.; Koudan, E.V.; Nezhurina, E.K.; Pereira, F.D.A.S.; Krokhmal, A.A.; Gryadunova, A.A.; Bulanova, E.A.; et al. Magnetic Levitational Bioassembly of 3D Tissue Construct in Space. Sci. Adv. 2020, 6, eaba4174. [Google Scholar] [CrossRef] [PubMed]

| Year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 * | ||
| Search queries | “organoid” “toxicity” “nanoparticles” | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 5 | 11 | 11 |
| “organoid” “toxicity” | 7 | 16 | 9 | 18 | 37 | 53 | 61 | 132 | 154 | 122 | |
| “organoid” | 151 | 189 | 277 | 466 | 761 | 1033 | 1360 | 2141 | 2839 | 2113 | |
| “toxicity” | 41,075 | 42,975 | 44,468 | 45,950 | 47,545 | 49,354 | 52,021 | 58,787 | 62,560 | 41,896 | |
| The share of toxicological studies in the number of articles devoted to organoids | 5% | 8% | 3% | 4% | 5% | 5% | 4% | 6% | 5% | 6% | |
| The share of organoids studies in the number of articles devoted to toxicity studies | 0.02% | 0.04% | 0.02% | 0.04% | 0.08% | 0.11% | 0.12% | 0.22% | 0.24% | 0.29% | |
| Organoid Type | Structures and Cell Diversity of Organoids | Routes of Administration of the Toxic Substances | Visualization and Assessment of Structures and Cell Types | Disadvantages of the Model | Organoid Formation Protocol | Reference |
|---|---|---|---|---|---|---|
| Patient-derived oral mucosa | Small proliferating epithelial cells were located outside, and larger ones with higher differentiation rates inside, the organoid | Incubation for 120 h | Immunohistochemistry (IHC) and hematoxylin-eosin staining | The inverted direction of the histological barrier. The outer layers are proliferating while the inner layers are highly differentiated | [13] | [13] |
| Human esophagus | Immortalized normal human esophageal keratinocyte cell lines with a differentiation gradient from periphery to center | Incubation for 24 h | High-resolution confocal microscopy (CLSM) and transmission electron microscopy (TEM). An increase in intracellular vacuolar structures has been qualitatively demonstrated | The inverted direction of the histological barrier | [14,15] | [16] |
| Rat duodenum | Organoids had lobular morphology and formed microvilli lined with intestinal cells, mucus-secreting goblet cells, a small population of enteroendocrine cells, and Paneth cells | Incubation for 24 h | Phase-contrast microscopy was used to quantify the percentage of differentiated organoids over time | Lack of macrophages | [17] | [18] |
| Mouse/human intestines | Villus-like structures with stem cells, goblet cells, and endocrine cells at the base of the crypt. Cell apoptosis was observed at the tips of the villi | Incubation for 24 h | The percentage of surviving organoids was measured | Lack of macrophages | [19,20,21] | [22] |
| Mice intestines | Villus-like structures with stem and Paneth cells at the base of the crypt. Cell apoptosis was observed at the tips of the villi | Incubation for several days | Measurement of organoid area and number of buds per organoid | Lack of macrophages | [21] | [23] |
| Human intestines | Same as above | Incubation for 4 days | Quantification of organoid diameter. IHC staining of different cell types | Lack of macrophages | [21] | [24] |
| Human liver | Organoids have intraluminal structures; bile canaliculi and pericanalicular sheaths were formed | Incubation for several days | Gene expression analysis, histology examination and IHC staining | Lack of macrophages | [25] | [26] |
| Human intestines | The epithelial layer contains enterocytes, Paneth cells, enteroendocrine cells, and goblet cells. The epithelial layer is surrounded by mesenchymal cells. | Incubation for several days or injection into the lumen | Visualization and marker expression quantification in epithelial and mesenchymal cells | Deep crypt structures are not seen | [27,28] | [29] |
| Human alveoli | Organoids had a structure similar to an alveolar sac, with many alveoli and layers of epithelial and mesenchymal cells | Incubation for several days | The organoid diameter was measured | Has the level of fetal maturity | [30] | [30] |
| Human kidney | Organoids contained kidney tubules subdivided into proximal segments and distal portions, interstitial cells, regions resembling primitive glomeruli with podocytes, and proliferating cells | Incubation once for 24 or 48 h, or four times with a one-day interval | IHC staining | Organoids were composed of immature nephrons | [31] | [32] |
| Human kidney | The morphology was close to that of normal glomeruli. Extracellular matrix (ECM) was visible within the structure. | Incubation for 48 h | No toxic effects were visualized. The homogenate of organoids was investigated | The tubules of the nephron are missing | [33] | [33] |
| Human bladder | 5–7 cell layers with multiple layers of intermediate cells | Incubation in solution for 60 min | The penetration of particles labeled with a luminescent dye through all layers of the organoid is qualitatively shown | Non-homogenous differentiation with three discrete zones | [34] | [35] |
| Human testis | Leydig cells, Sertoli cells, spermatogonia, and peritubular cells | Incubation for 48 h | Polymerase chain reaction (PCR) and qualitative CLSM | The organization of cells into structures is not described, although the histological images show the peripheral distribution of Sertoli and Leydig cells | [36] | [36] |
| Human brain | Immature neurons and astrocytes formed in layers | Incubation for 6 h | TEM and qualitative IHC staining | Has the level of fetal maturity. Neurons migrate to the center of the organoid | [37] | [38] |
| Human brain | Same as above | Incubation for 24 h | IHC staining for apoptosis and cell proliferation. Assessment of tau and β-amyloid expression. | Same as above | [37] | [39] |
| Human brain | Dopaminergic neuron spheroid with incorporated astrocytes. Astrocytes were organized radially around the organoid, forming a glial corona | Incubation for 24 h | Quantitative fluorescence staining with calcein and propidium iodide (PI) | There are no data on the spatial distribution of subpopulations of Lund human mesencephalic cells in the organoid | [40] | [40] |
| Human brain | Large neuroepithelial buds containing fluid-filled cavities. A pool of neural progenitor cells was located near apical surface | Incubation for 10 days | IHC staining | Absence of macrogliocytes and microgliocytes | [41] | [41] |
| Mouse retina | Continuous epithelial structures with clear stratification, which contain all major neural retina components | Incubation in solution for 2 and 4 days | IHC staining | Absence of hematoneural barrier | [42] | [43] |
| Human endometrium | Lumen-bordering cell layers. Presence of secretory cells and mucus secretion. | Incubation for 72 h | XTT assay, ion channel activity, Ki67 expression assessment | Absence of endometrial stromal cells, low hormone responses | [44] | [45] |
| # | Organoid Possibilities | Organoid Feature | Challenges | Models that Lack These Features |
|---|---|---|---|---|
| 1 | Reproducing intercellular communication and paracrine effects | Organoids are heterogeneous (composed of several types of cells, including stem cells) | Difficulty in standardization and quantification | 2D cell cultures and spheroids |
| 2 | Investigating the penetration of NPs through tissue barriers | Reproduction of the structural organization of organs | Difficulty in visualizing the penetration process | 2D and 3D cell cultures and spheroids |
| 3 | Reproducing the reactions of human tissues [7] | Develops from stem cells | Requires the introduction of additional cells into the structure, which during embryogenesis penetrate the tissue by migration | Animal models |
| 4 | Reproducing biochemical gradients | Cell nutrition by diffusion | The size of the organoid is limited; the direction of cell differentiation is inverted to the center of the organoid | 2D cell cultures |
| 5 | The ability to obtain many quantitative data on different cell types and the state of organ structures and functions | Structural complexity | Lack of screening tools for imaging | 2D cell cultures and spheroids |
| Organoid Type | Organoid Structures and Cell Diversity | NP Delivery Route | NP Type | NP Dose | Visualization and Assessment of Structures and Cell Types | Organoid Formation Protocol Reference | Ref |
|---|---|---|---|---|---|---|---|
| Human brain | Multi-layered, neurons expressed cortical layer I, V, and VI markers | Incubation for 24 h | Multi-walled carbon nanotubes (MWCNTs), diameter 5–15 nm, length 0.5–2 μm | 16 or 64 μg/mL | Organoids were dissociated into individual cells, stained with a NO probe, dihydroethidium (DHE) superoxide probe, and AO/DAPI to determine cytotoxicity. All results were quantified | [96] | [97] |
| Human liver | Primary hepatocytes, stellate and Kupffer cells | Incubation for 1 to 2 days | 20 nm MgO | 100 µg/mL | Quantification of ROS and ATP based on image analysis after IHC staining | [98] | [99] |
| Human colon | No structural characteristics provided | Incubation for 24 h | 10–20 nm SiO2 21 nm TiO2 | 0.8 mM 1.1 mM | Live/dead cell ratio was determined after fluorescent labeling | [100] | [100] |
| Human pancreatic cancer | No structural characteristics provided | Incubation for 2 or 24 h | Magnetoliposomes with SPION core and phospholipid bilayer. Size 11.1 ± 2.5 nm | 225 µg [Fe]/mL | IHC staining, CellTiter Glo Assay for cell viability assessment, apoptotic marker expression measurement | [101] | [102] |
| Human gut | Cystic structure, consisting of an epithelial cell layer that envelops a hollow lumen. Apical side was covered with mucus | Incubation for 24 h | 2 nm gold NPs conjugated with doxorubicin and AlexaFluor 647 | 50 µg [Au]/mL | Confocal microscopy with AlexaFluor 647, DAPI, and Actin-488 | [103] | [103] |
| Human intestines | Highly convoluted epithelial structures surrounded by mesenchyme | Incubation for 1, 2, and 14 days | 50 nm polystyrene | 10 and 100 µg/mL | IHC staining, assessment of inflammatory response, TUNEL assay, ROS generation, endocytosis inhibition | [104] | [104] |
| Human brain | No specific information about inner structure was provided. Neural progenitor cells, neurons, and astrocytes were presented | Incubation for 7 days | PVP-coated 20 nm Ag NPs | 0.1 and 0.5 µg/mL | RNA sequencing, IHC staining, TUNEL assay for assessment of apoptosis rates, cytoskeleton structure stability evaluation | STEMdiff Cerebral Organoid Kit | [105] |
| Mouse intestines | No structural characteristic provided | Animals were subjected to NP action, and organoids were formed from intestines of these animals | 10 nm CeO2/Mn3O4 nanocrystals | 0.55 mg/kg | The number of organoid crypts was qualitatively determined via light microscopy. Apoptotic cell percent and ROS were qualitatively determined by IHC staining | - | [106] |
| Mouse intestines | Villus-like structures with stem cells and Paneth cells mixed at the base of the crypt. Cell apoptosis was observed at the tips of the villi | Same as above | ~3 nm hydroxylated graphene quantum dots (QDs) | 5 mg/kg | The organoid size was determined using light microscopy | [21] | [107] |
| Mouse intestines | Crypt-like structures fed into luminal domains where apoptotic cells pinched off into the lumen. Epithelial cells formed a monolayer at the organoid-gel interface | Incubation for 3 days | >500 nm Bi2Te3 nanowires | 0, 50, 100, and 200 µg/mL | Quantitative measurements of organoid surface area. Cell viability were quantitatively analyzed based on a modified colorimetric MTT assay | [108] | [109] |
| Mouse kidney | Structures similar to the proximal tubules of the nephron | Incubations with NMs for 48 h | Gold NPs, size 5.2 ± 1.3 nm G5-OH PAMAM, size 2.6 ± 0.17 nm | 56.6 μg/mL and 3.5 μg/mL 0.675 mg/mL and 0.05 mg/mL | A qualitative investigation of IHC-stained sections with biomarkers for kidney toxicity, Kim-1, and TNFα | [110,111] | [112] |
| Mouse and human kidney | Glomerulus-like structures, podocytes, and proximal tubules had developed in the kidney organoids | Incubations with NMs for 24 h | QDs: CdTe, CdSe/ZnS, InP/ZnS, GO, BP | 0, 0.2, 1, 5, and 25 mg/mL | No quantitative assessment has been carried out. Sections stained with hematoxylin-eosin and IHC with antibodies against LTL, NPHS1 or KIM-1 were qualitatively assessed | [113] | [113] |
| Organization Level | Test Object | Toxic Effects | Nanotoxicity Mechanisms | Methods |
|---|---|---|---|---|
| Cell | 2D culture | Cytotoxic effects | Oxidative stress Mutagenic effect | In silico modeling Genetic methods Cytological methods |
| Tissue | Cell spheroid | Cytotoxic effects | Same as above | Same as above |
| Organ | Organoid | Influence on the intercellular substance. Permeability of histohematogenous barriers. Cytotoxic effects in disease modeling. Stem cell toxicity | Same as above + interaction with receptors, deposit effects, interaction with the extracellular substance | Same as above + morphological methods |
| Interorgan integrations | Organ-on-chip | Modifications of nanomaterials and formation of protein corona in the body. Pharmacokinetics parameters | Same as above + toxic effects associated with the formation of the protein corona, effects associated with a combination of toxic effects with diseases | Same as above + physiological methods |
| Intersystem interactions and reactions of the body | Animal model | Chronic effects and effects on offspring and higher nervous activity | Same as above + Chronic toxicity | Same as above + behavioral tests |
| Social aspects | Human | Social groups’ lifestyle influence | Same as above + social features of different population groups | Same as above + epidemiology and sociology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikonorova, V.G.; Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y. Advantages and Potential Benefits of Using Organoids in Nanotoxicology. Cells 2023, 12, 610. https://doi.org/10.3390/cells12040610
Nikonorova VG, Chrishtop VV, Mironov VA, Prilepskii AY. Advantages and Potential Benefits of Using Organoids in Nanotoxicology. Cells. 2023; 12(4):610. https://doi.org/10.3390/cells12040610
Chicago/Turabian StyleNikonorova, Varvara G., Vladimir V. Chrishtop, Vladimir A. Mironov, and Artur Y. Prilepskii. 2023. "Advantages and Potential Benefits of Using Organoids in Nanotoxicology" Cells 12, no. 4: 610. https://doi.org/10.3390/cells12040610








