A Mathematical Model for Predicting Patient Responses to Combined Radiotherapy with CTLA-4 Immune Checkpoint Inhibitors
Abstract
:1. Introduction
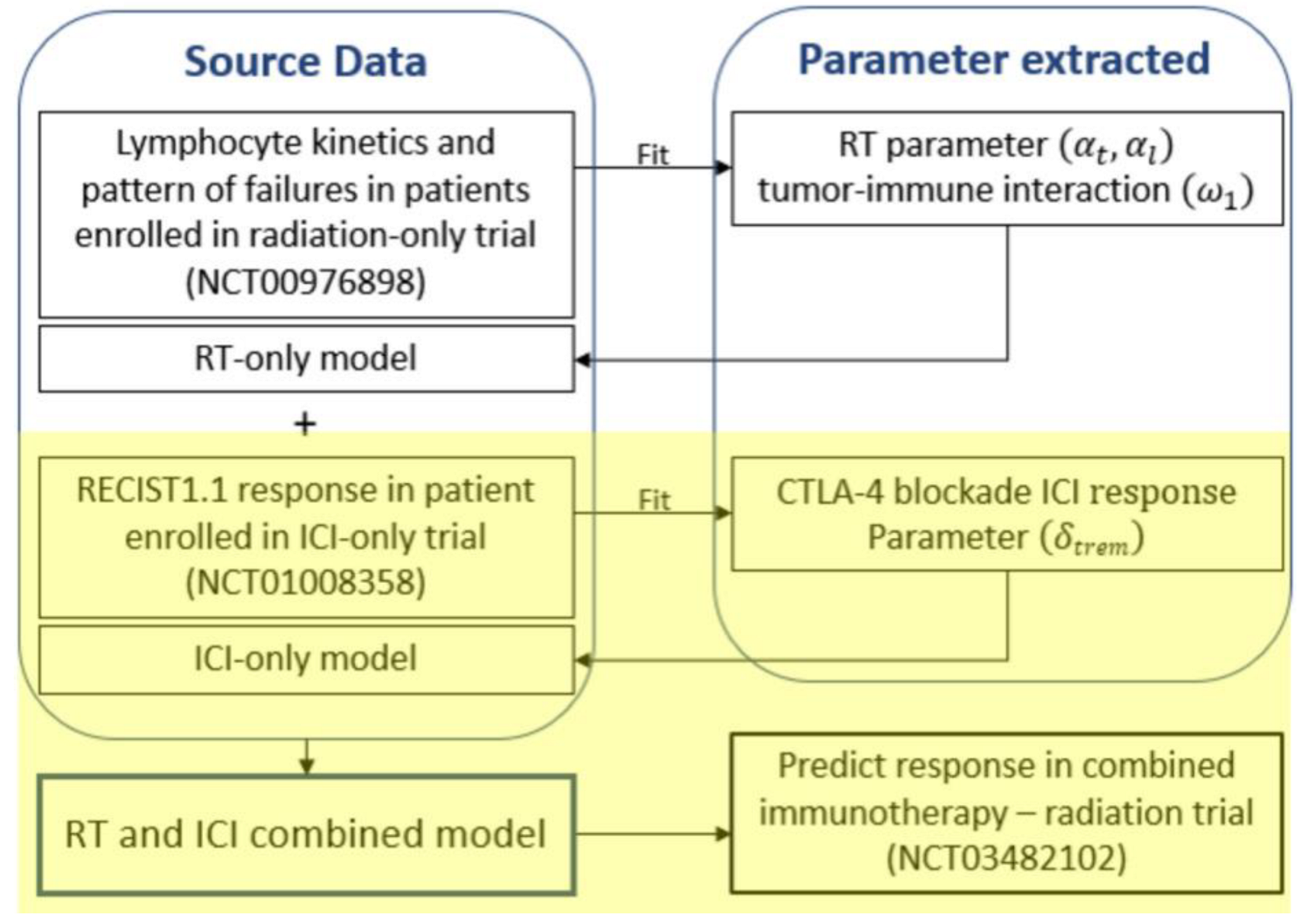
2. Materials and Methods
2.1. Model Equations
2.2. Patient Cohort
2.3. Model Fitting
3. Results
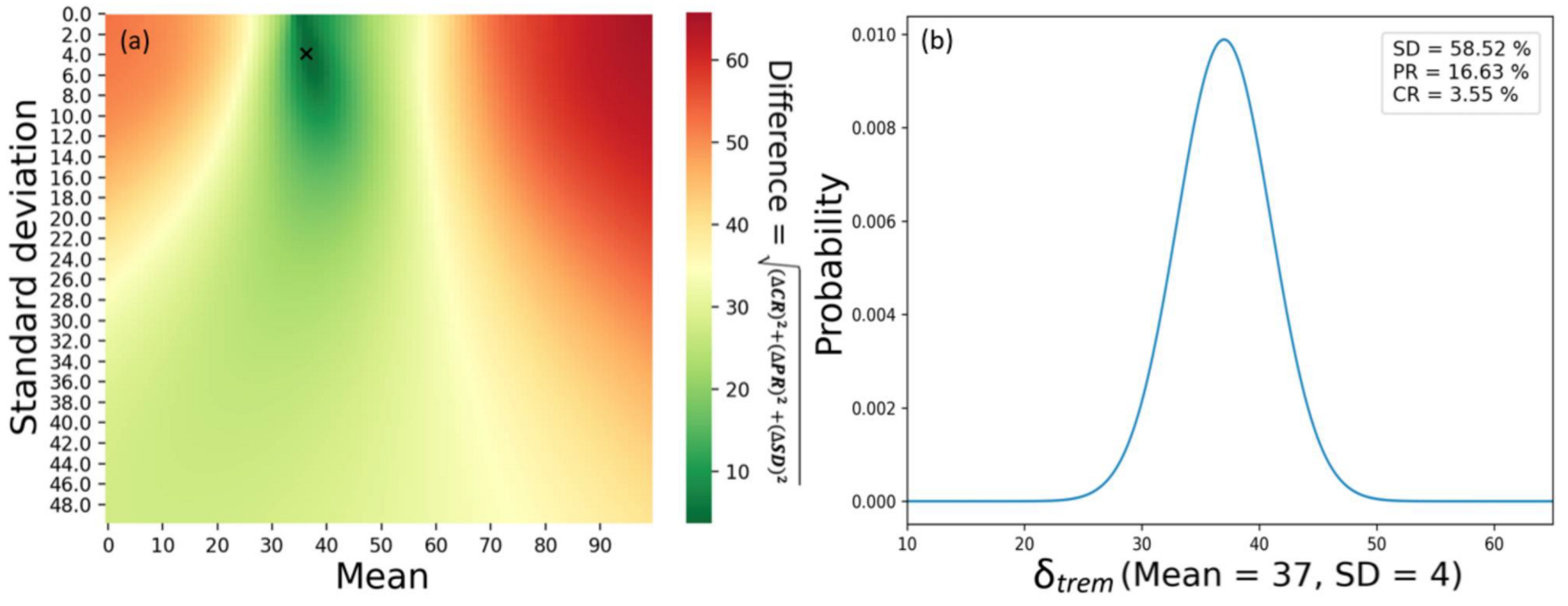
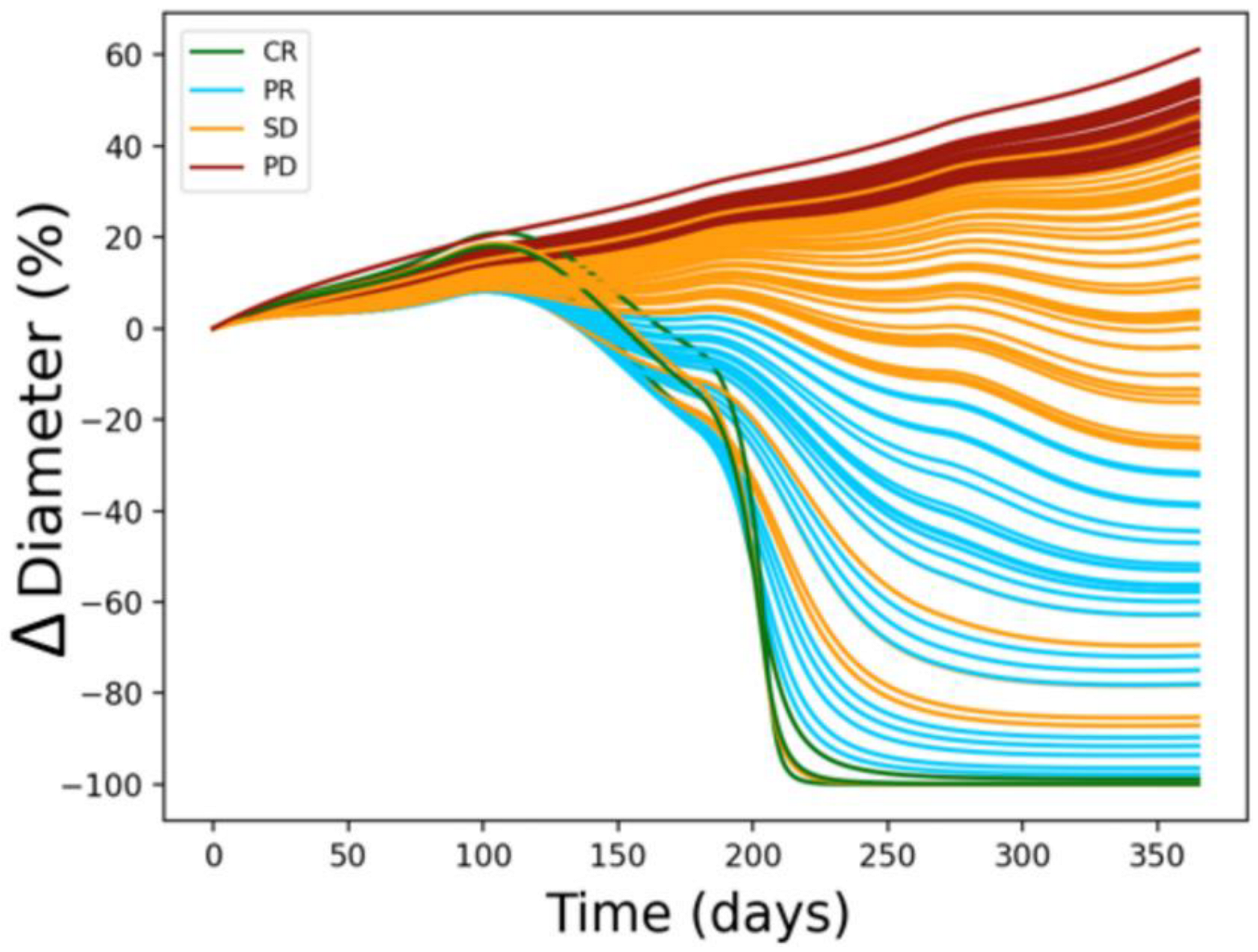
3.1. Calibration
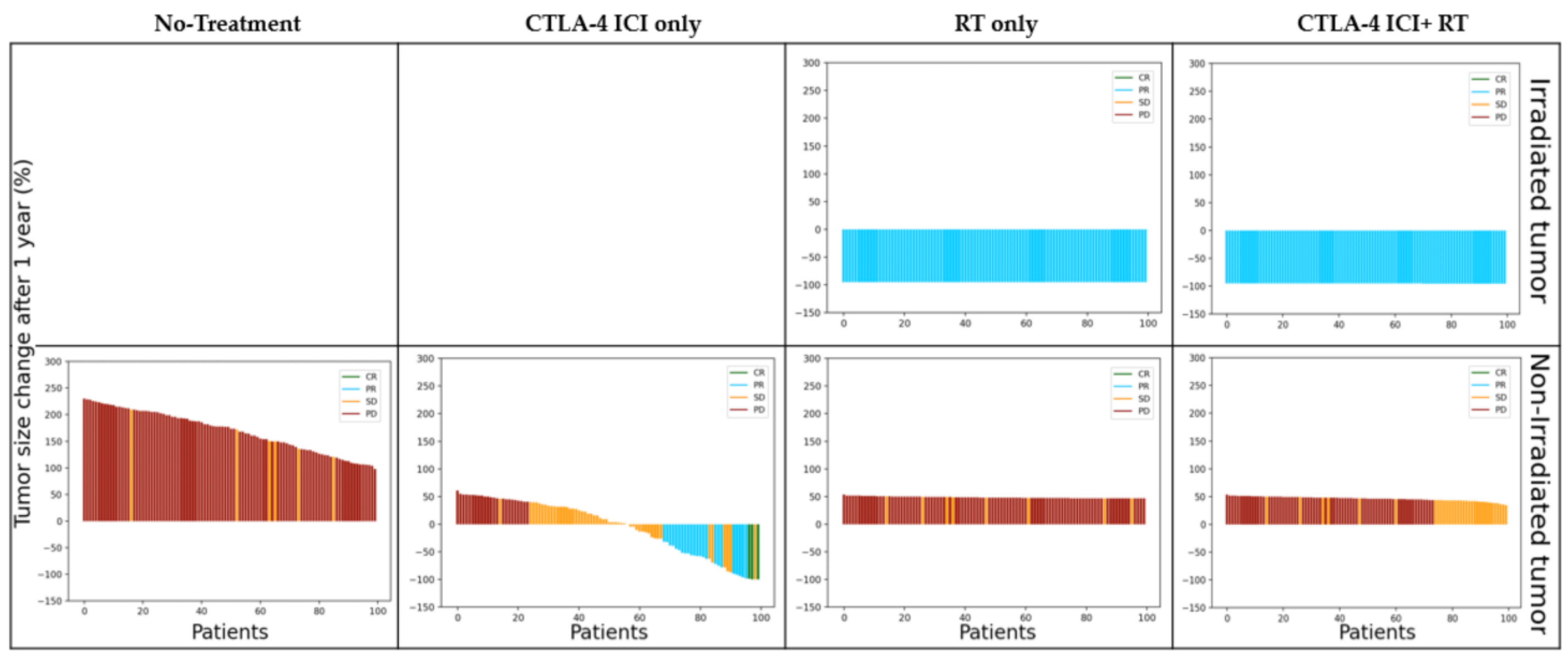
3.2. Simulation
3.2.1. Irradiated Tumor Burden
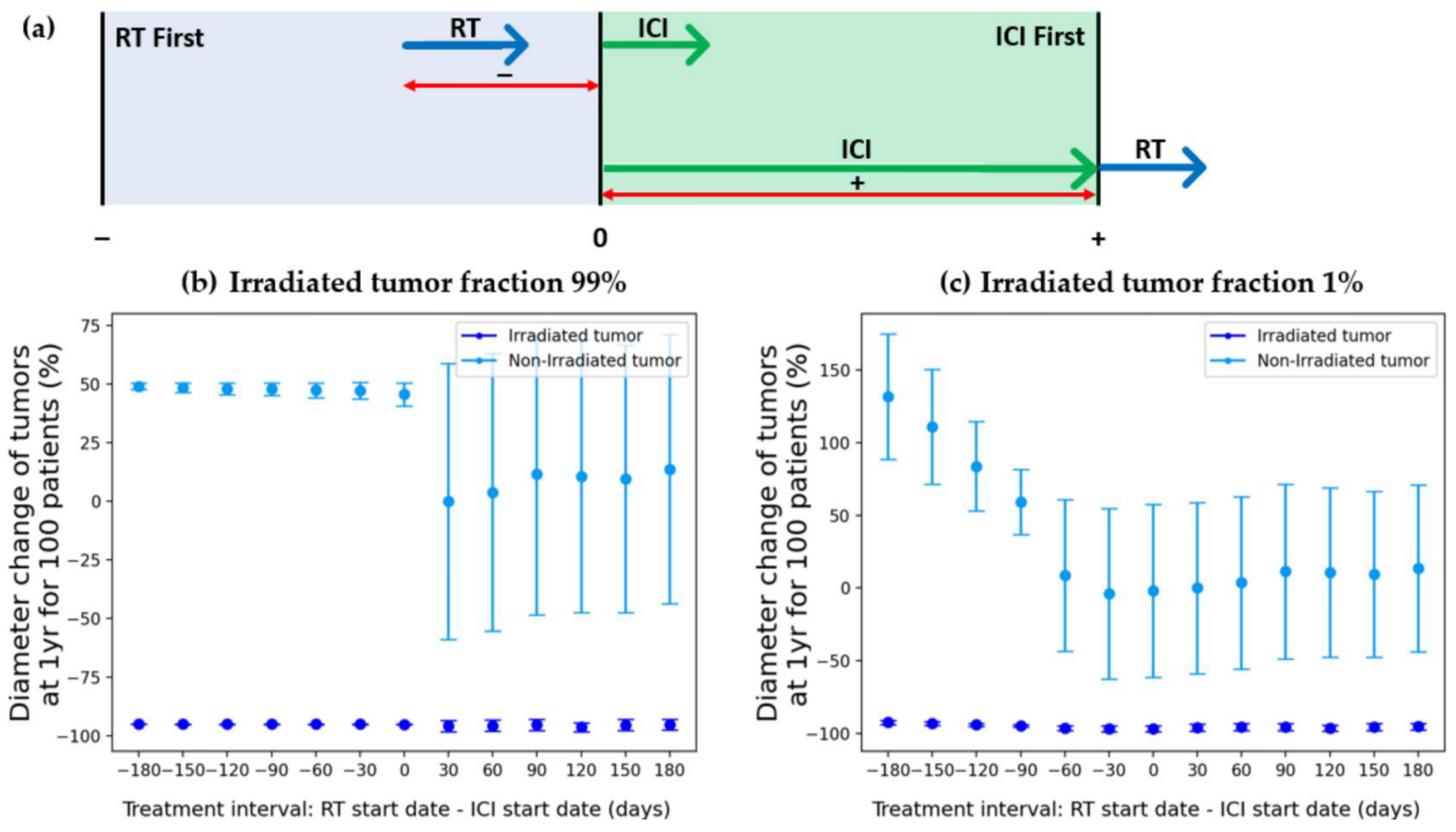
3.2.2. Treatment Sequence
4. Discussion
4.1. Treatment Modality
4.2. Irradiated Tumor Burden
4.3. Treatment Sequence
4.4. Further Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Sáfrány, G. The impact of radiation therapy on the antitumor immunity: Local effects and systemic consequences. Cancer Lett. 2015, 356, 114–125. [Google Scholar] [CrossRef]
- Pike, L.R.; Bang, A.; Mahal, B.A.; Taylor, A.; Krishnan, M.; Spektor, A.; Cagney, D.N.; Aizer, A.A.; Alexander, B.M.; Rahma, O. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Harrington, N.; Chambers, K.; Ross, W.; Filion, L. Radiation damage and immune suppression in splenic mononuclear cell populations. Clin. Exp. Immunol. 1997, 107, 417–424. [Google Scholar] [CrossRef]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Iivanainen, S.; Koivunen, J.P. Possibilities of improving the clinical value of immune checkpoint inhibitor therapies in cancer care by optimizing patient selection. Int. J. Mol. Sci. 2020, 21, 556. [Google Scholar] [CrossRef] [PubMed]
- Karwacki, J.; Kiełbik, A.; Szlasa, W.; Sauer, N.; Kowalczyk, K.; Krajewski, W.; Saczko, J.; Kulbacka, J.; Szydełko, T.; Małkiewicz, B. Boosting the Immune Response—Combining Local and Immune Therapy for Prostate Cancer Treatment. Cells 2022, 11, 2793. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Ding, W.; Cheng, Z.; Feng, Y.; Kang, Z.; Qiu, R.; Zhao, S.; Hu, W.; Zhou, F.; Wu, D. Preclinical Study of Plasmodium Immunotherapy Combined with Radiotherapy for Solid Tumors. Cells 2022, 11, 3600. [Google Scholar] [CrossRef] [PubMed]
- Keisari, Y.; Kelson, I. The potentiation of anti-tumor immunity by tumor abolition with alpha particles, protons, or carbon ion radiation and its enforcement by combination with immunoadjuvants or inhibitors of immune suppressor cells and checkpoint molecules. Cells 2021, 10, 228. [Google Scholar] [CrossRef]
- Mondini, M.; Levy, A.; Meziani, L.; Milliat, F.; Deutsch, E. Radiotherapy–immunotherapy combinations–perspectives and challenges. Mol. Oncol. 2020, 14, 1529–1537. [Google Scholar] [CrossRef]
- Kalbasi, A.; June, C.H.; Haas, N.; Vapiwala, N. Radiation and immunotherapy: A synergistic combination. J. Clin. Investig. 2013, 123, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Rajeev-Kumar, G.; Pitroda, S.P. Synergizing radiotherapy and immunotherapy: Current challenges and strategies for optimization. Neoplasia 2023, 36, 100867. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.; Hong, T.S.; Poznansky, M.C.; Paganetti, H.; Grassberger, C. Mathematical modeling to simulate the effect of adding radiotherapy to immunotherapy and application to hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 112, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, D.; Sotolongo-Grau, O.; Riquelme, R.E.; Sotolongo-Costa, O.; Miranda, J.A.S.; Antoranz, J. Assessment of cancer immunotherapy outcome in terms of the immune response time features. Math. Med. Biol. J. IMA 2007, 24, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Sotolongo-Grau, O.; Rodríguez-Pérez, D.; Santos-Miranda, J.; Sotolongo-Costa, O.; Antoranz, J. Immune system–tumour efficiency ratio as a new oncological index for radiotherapy treatment optimization. Math. Med. Biol. J. IMA 2009, 26, 297–307. [Google Scholar] [CrossRef]
- Geng, C.; Paganetti, H.; Grassberger, C. Prediction of treatment response for combined chemo-and radiation therapy for non-small cell lung cancer patients using a bio-mathematical model. Sci. Rep. 2017, 7, 13542. [Google Scholar] [CrossRef]
- Sung, W.; Grassberger, C.; McNamara, A.L.; Basler, L.; Ehrbar, S.; Tanadini-Lang, S.; Hong, T.S.; Paganetti, H. A tumor-immune interaction model for hepatocellular carcinoma based on measured lymphocyte counts in patients undergoing radiotherapy. Radiother. Oncol. 2020, 151, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Fecci, P.E.; Ochiai, H.; Mitchell, D.A.; Grossi, P.M.; Sweeney, A.E.; Archer, G.E.; Cummings, T.; Allison, J.P.; Bigner, D.D.; Sampson, J.H. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 2007, 13, 2158–2167. [Google Scholar] [CrossRef]
- Park, Y.; Choi, D.; Lim, H.K.; Rhim, H.; Kim, Y.-s.; Kim, S.H.; Lee, W.J. Growth rate of new hepatocellular carcinoma after percutaneous radiofrequency ablation: Evaluation with multiphase CT. Am. J. Roentgenol. 2008, 191, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Erickson, B.; Khater, K.A.; Li, X.A. Estimate of radiobiologic parameters from clinical data for biologically based treatment planning for liver irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V.A.; Makalkin, I.A.; Taylor, M.A.; Perelson, A.S. Nonlinear dynamics of immunogenic tumors: Parameter estimation and global bifurcation analysis. Bull. Math. Biol. 1994, 56, 295–321. [Google Scholar] [CrossRef]
- Serre, R.; Barlesi, F.; Muracciole, X.; Barbolosi, D. Immunologically effective dose: A practical model for immuno-radiotherapy. Oncotarget 2018, 9, 31812. [Google Scholar] [CrossRef] [PubMed]
- Sotolongo-Costa, O.; Molina, L.M.; Perez, D.R.; Antoranz, J.; Reyes, M.C. Behavior of tumors under nonstationary therapy. Phys. D Nonlinear Phenom. 2003, 178, 242–253. [Google Scholar] [CrossRef]
- Byun, H.K.; Kim, N.; Park, S.; Seong, J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther. Onkol. 2019, 195, 1007–1017. [Google Scholar] [CrossRef]
- Nakamura, N.; Kusunoki, Y.; Akiyama, M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat. Res. 1990, 123, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Poleszczuk, J.; Pilon-Thomas, S.; Kim, S.; Anderson, A.A.; Czerniecki, B.J.; Harrison, L.B.; Moros, E.G.; Enderling, H. Immune interconnectivity of anatomically distant tumors as a potential mediator of systemic responses to local therapy. Sci. Rep. 2018, 8, 9474. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; López-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, eaat7807. [Google Scholar] [CrossRef]
- Pilones, K.A.; Vanpouille-Box, C.; Demaria, S. Combination of radiotherapy and immune checkpoint inhibitors. Semin Radiat. Oncol. 2015, 25, 28–33. [Google Scholar] [CrossRef]
- Kang, J.; Demaria, S.; Formenti, S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J. Immunother. Cancer 2016, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Cushman, T.R.; Caetano, M.S.; Welsh, J.W.; Verma, V. Overview of ongoing clinical trials investigating combined radiotherapy and immunotherapy. Immunotherapy 2018, 10, 851-0. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Dahlman, E.L.; Leder, K.Z.; Hui, S.K. A mathematical model of tumor growth and its response to single irradiation. Theor. Biol. Med. Model. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Function | Value | Refs. |
|---|---|---|---|
| Tumor growth | [20] | ||
| Tumor—LQ cell death | [21] | ||
| Lymphocyte decay rate | [22] | ||
| Inactivated tumor cell decay rate | [23] | ||
| Tumor-directed lymphocyte efficiency | [16,24] | ||
| Tumor/inactivated tumor lymphocyte recruitment constant | [16,24] | ||
| Half-saturation constant | [16,24] | ||
| Lymphocyte regeneration | [25] | ||
| Tumor—LQ cell death | [21] | ||
| Lymphocytes—LQ cell death | [26,27] | ||
| Effectiveness of immune checkpoint inhibitor (tremelimumab) | Normally distributed (μ = 37 σ = 4) | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Choe, B.-Y.; Suh, T.S.; Sung, W. A Mathematical Model for Predicting Patient Responses to Combined Radiotherapy with CTLA-4 Immune Checkpoint Inhibitors. Cells 2023, 12, 1305. https://doi.org/10.3390/cells12091305
Kim Y, Choe B-Y, Suh TS, Sung W. A Mathematical Model for Predicting Patient Responses to Combined Radiotherapy with CTLA-4 Immune Checkpoint Inhibitors. Cells. 2023; 12(9):1305. https://doi.org/10.3390/cells12091305
Chicago/Turabian StyleKim, Yongjin, Bo-Young Choe, Tae Suk Suh, and Wonmo Sung. 2023. "A Mathematical Model for Predicting Patient Responses to Combined Radiotherapy with CTLA-4 Immune Checkpoint Inhibitors" Cells 12, no. 9: 1305. https://doi.org/10.3390/cells12091305







