Quality Control of Stem Cell-Based Cultured Meat According to Specific Differentiation Abilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Livestock Tissues
2.2. Cell Preparation
2.3. Antibody Staining and Flow Cytometry
2.4. Cell Culture
2.5. Cell Differentiation
2.6. Oil Red O Staining
2.7. Differentiation of CD29+ Cells to Meat Bud Spheroid
2.8. Immunofluorescent Staining
2.9. Whole-Mount Immunofluorescent Staining
2.10. Colony-Forming Unit Fibroblast Assay
2.11. RNA Isolation and Quantitative Real-Time PCR
2.12. Electron Microscopy
2.13. Ststistical Analysis
3. Results
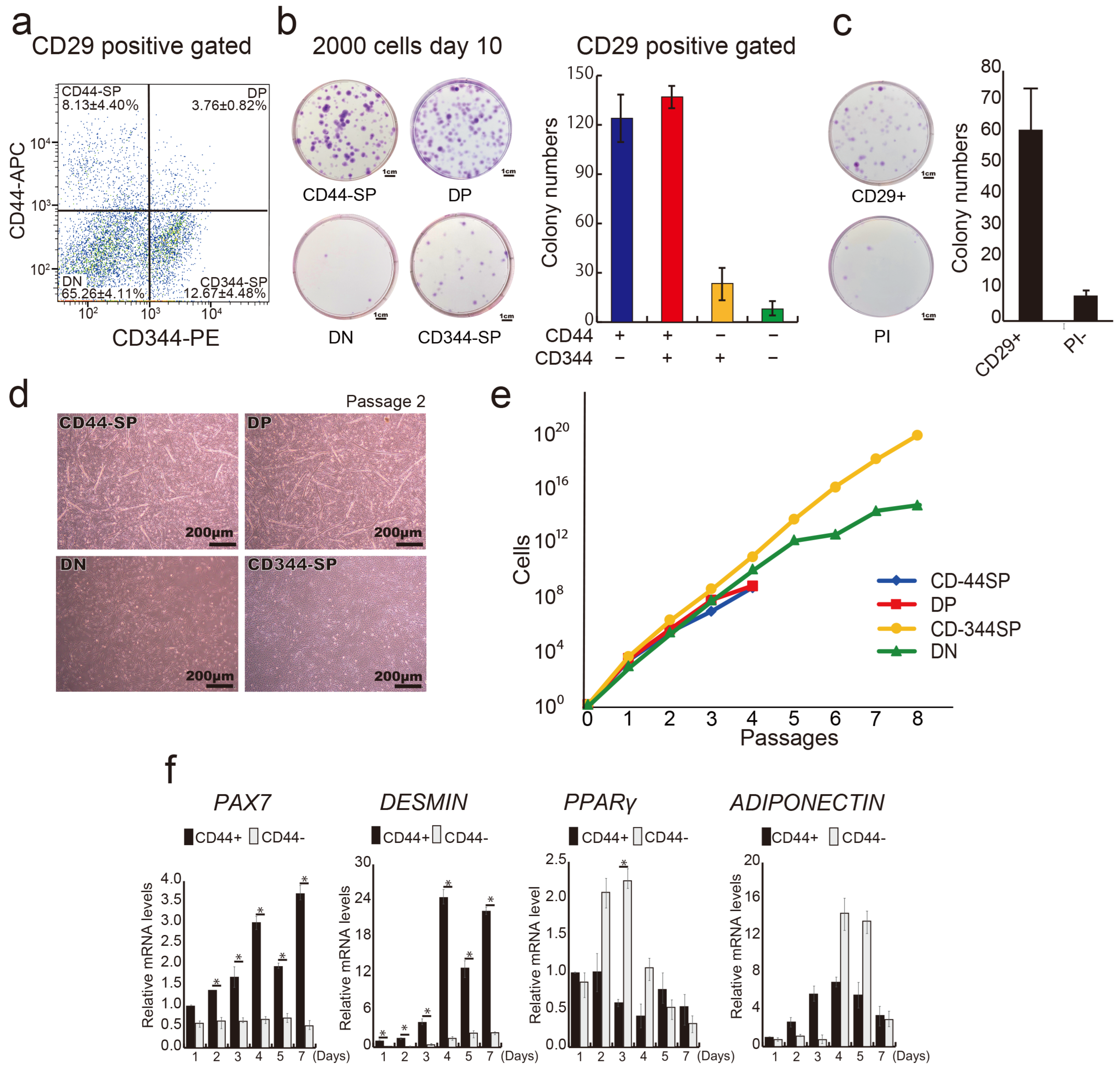
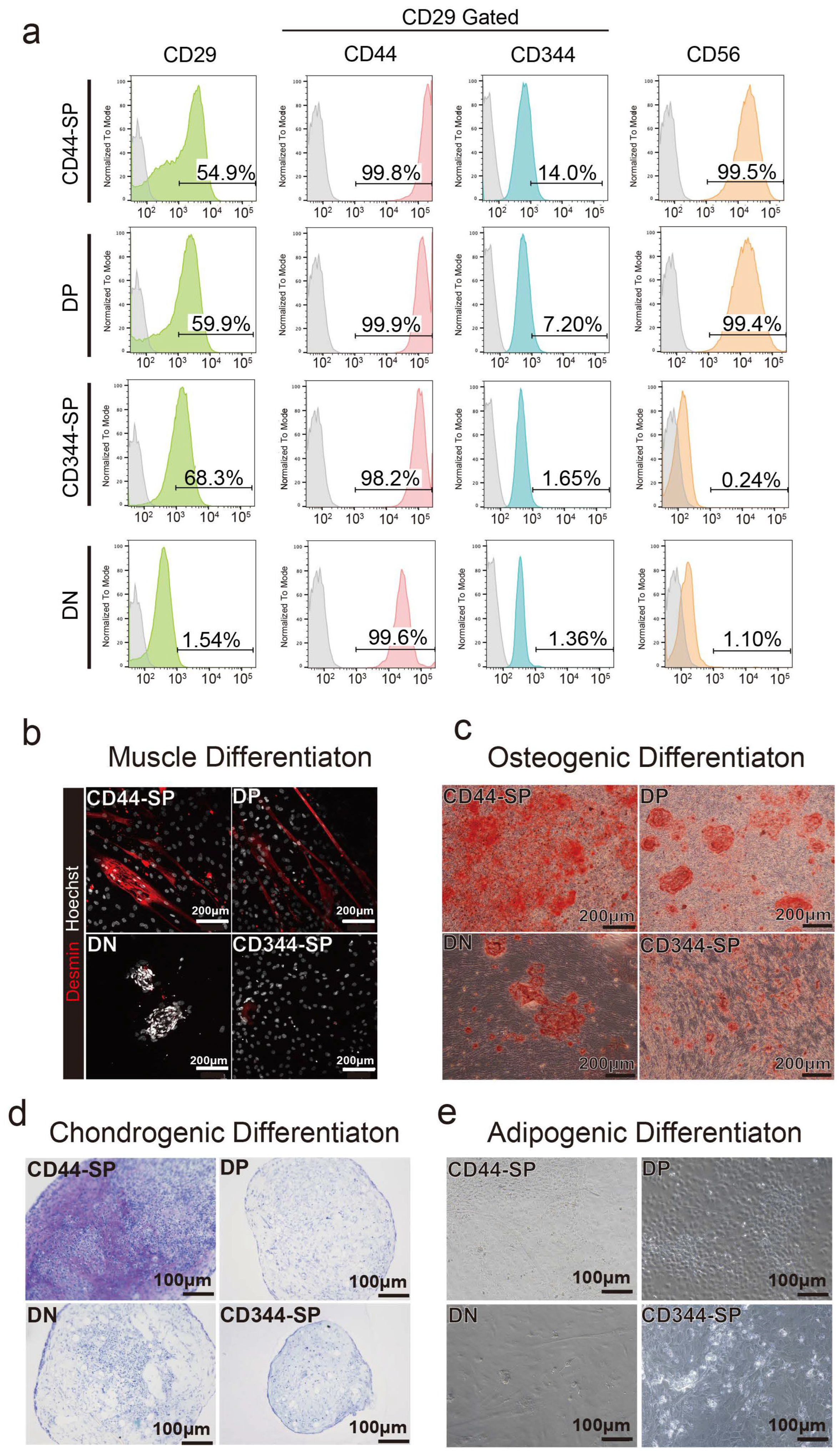
3.1. CD29+ Cell Fractions Are Composed of Heterogeneous Populations
3.2. Distinct Functional Populations Based on CD44 Expression
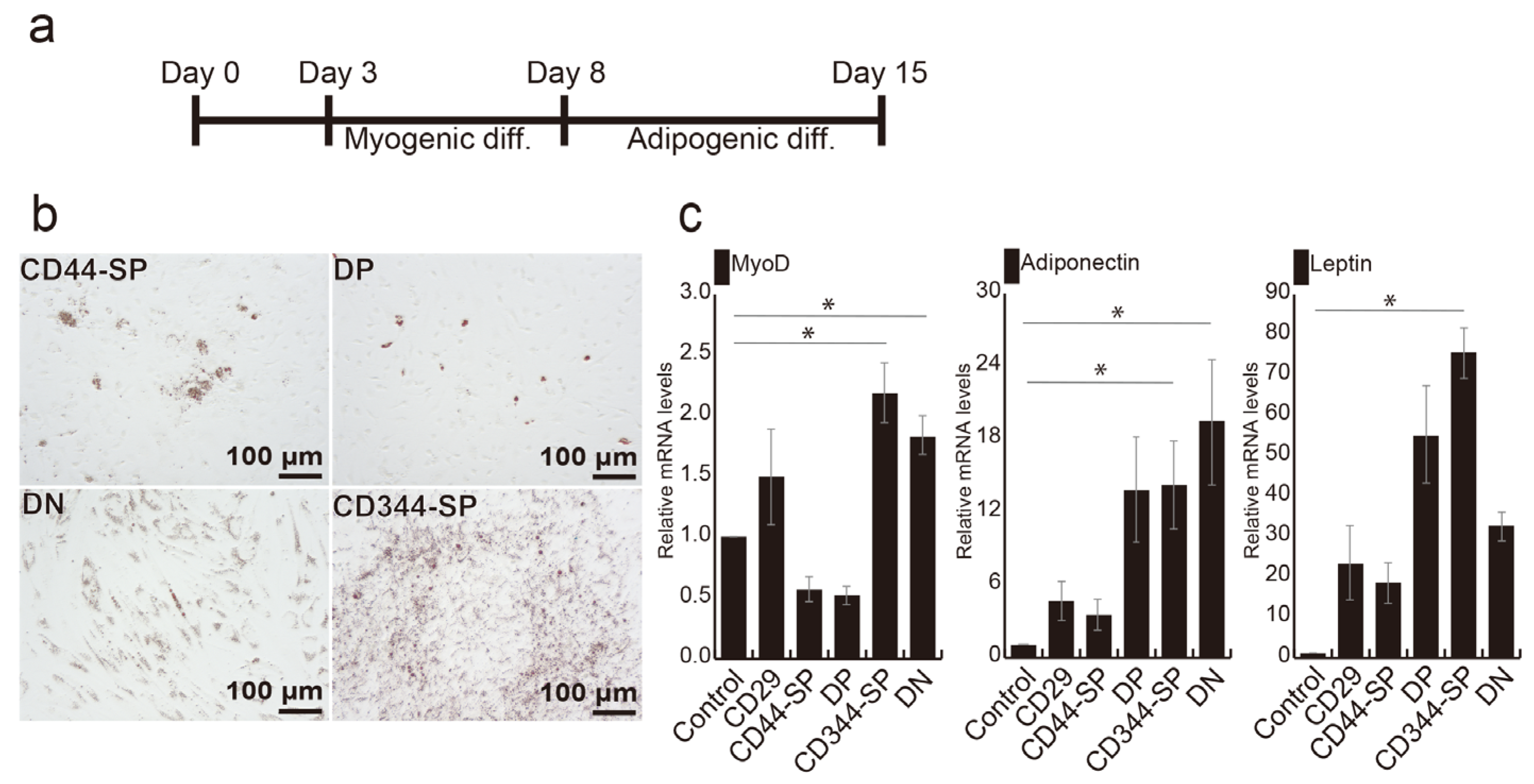
3.3. Induction of Adipocyte Differentiation Using Bovine Skeletal Muscle-Derived CD29+CD44− Cells
3.4. Meat-Like Structure Design Via Constitutive Cell Regulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2019. In Data Booklet; United Nations: New York, NY, USA, 2019; pp. 1–25. [Google Scholar]
- United Nations; FAO. World Livestock 2011. Livestock in Food Security; Food and Agriculture Organization: Roma, Italy, 2011. [Google Scholar]
- Sugii, S.; Wong, C.Y.Q.; Lwin, A.K.O.; Chew, L.J.M. Reassessment of adipocyte technology for cellular agriculture of alternative fat. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4146–4163. [Google Scholar] [CrossRef]
- Stout, A.J.; Mirliani, A.B.; Rittenberg, M.L.; Shub, M.; White, E.C.; Yuen, J.S.K., Jr.; Kaplan, D.L. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 2022, 5, 466. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.N.; Cosenza, Z.A.; Baar, K.; Block, D.E. Considerations for the development of cost-effective cell culture media for cultivated meat production. Compr. Rev. Food Sci. Food Saf. 2021, 20, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Bahmid, N.A.; Karim, I.; Mehany, T.; Gvozdenko, A.A.; Blinov, A.V.; Nagdalian, A.A.; Arsyad, M.; Lorenzo, J.M. Cultured meat: Processing, packaging, shelf life, and consumer acceptance. LWT 2022, 172, 114192. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khan, S.; Murid, M.; Asif, Z.; Oboturova, N.P.; Nagdalian, A.A.; Blinov, A.V.; Ibrahim, S.A.; Jafari, S.M. Marketing Strategies for Cultured Meat: A Review. Appl. Sci. 2022, 12, 8795. [Google Scholar] [CrossRef]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations Throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. [Google Scholar] [CrossRef] [PubMed]
- Kurt, E.; Klont, E.; Ergun, O.; Klont, R. White Paper Cell Cultured Meat. Austin Food Sci. 2021, 6, 1041–1046. [Google Scholar]
- Benny, A.; Pandi, K.; Upadhyay, R. Techniques, Challenges and Future Prospects for Cell-Based Meat. Food Sci. Biotechnol. 2022, 31, 1225–1242. [Google Scholar] [CrossRef]
- Choi, K.H.; Yoon, J.W.; Kim, M.; Lee, H.J.; Jeong, J.; Ryu, M.; Jo, C.; Lee, C.K. Muscle Stem Cell Isolation and In Vitro Culture for Meat Production: A Methodological Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 429–457. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, M.; Yoon, J.W.; Jeong, J.; Ryu, M.; Jo, C.; Lee, C.K. Purification of Pig Muscle Stem Cells Using Magnetic-Activated Cell Sorting (MACS) Based on the Expression of Cluster of Differentiation 29 (CD29). Food Sci. Anim. Resour. 2020, 40, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Swennen, G.N.M.; Messmer, T.; Gagliardi, M.; Molin, D.G.M.; Li, C.; Zhou, G.; Post, M.J. Maintaining Bovine Satellite Cells Stemness Through p38 Pathway. Sci. Rep. 2018, 8, 10808. [Google Scholar] [CrossRef]
- Le Grand, F.; Jones, A.E.; Seale, V.; Scimè, A.; Rudnicki, M.A. Wnt7a Activates the Planar Cell Polarity Pathway to Drive the Symmetric Expansion of Satellite Stem Cells. Cell Stem Cell 2009, 4, 535–547. [Google Scholar] [CrossRef] [PubMed]
- von Maltzahn, J.; Bentzinger, C.F.; Rudnicki, M.A. Wnt7a-Fzd7 Signalling Directly Activates the Akt/mTOR Anabolic Growth Pathway in Skeletal Muscle. Nat. Cell Biol. 2011, 14, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; von Maltzahn, J.; Soleimani, V.D.; Yin, H.; Rudnicki, M.A. Fibronectin Regulates Wnt7a Signaling and Satellite Cell Expansion. Cell Stem Cell 2013, 12, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.D.; Rubio, N.R.; Stout, A.J.; Yuen, J.S.K.; Kaplan, D.L. Prospects and Challenges for Cell-Cultured Fat as a Novel Food Ingredient. Trends Food Sci. Technol. 2020, 98, 53–67. [Google Scholar] [CrossRef]
- Fraeye, I.; Kratka, M.; Vandenburgh, H.; Thorrez, L. Sensorial and Nutritional Aspects of Cultured Meat in Comparison to Traditional Meat: Much to Be Inferred. Front. Nutr. 2020, 7, 35. [Google Scholar] [CrossRef]
- Hausman, G.J.; Dodson, M.V.; Ajuwon, K.; Azain, M.; Barnes, K.M.; Guan, L.L.; Jiang, Z.; Poulos, S.P.; Sainz, R.D.; Smith, S.; et al. Board-Invited Review: The Biology and Regulation of Preadipocytes and Adipocytes in Meat Animals. J. Anim. Sci. 2009, 87, 1218–1246. [Google Scholar] [CrossRef]
- Zolocinska, A. The expression of marker genes during the differentiation of mesenchymal stromal cells. Adv. Clin. Exp. Med. 2018, 27, 717–723. [Google Scholar] [CrossRef]
- Mizuno, H. Adipose-derived stem cells for tissue repair and regeneration: Ten years of research and a literature review. J. Nippon Med. Sch. 2009, 76, 56–66. [Google Scholar] [CrossRef]
- Musina, R.A.; Bekchanova, E.S.; Belyavskii, A.V.; Sukhikh, G.T. Differentiation potential of mesenchymal stem cells of different origin. Bull. Exp. Biol. Med. 2006, 141, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Y.; Wang, G.; Li, H. Identification of microRNA and bioinformatics target gene analysis in beef cattle intramuscular fat and subcutaneous fat. Mol. Biosyst. 2013, 9, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Bong, J.J.; Cho, K.K.; Baik, M. Comparison of gene expression profiling between bovine subcutaneous and intramuscular adipose tissues by serial analysis of gene expression. Cell Biol. Int. 2009, 34, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Mabuchi, Y.; Naraoka, Y.; Hisamatsu, D.; Akazawa, C. Conservation of Markers and Stemness in Adipose Stem and Progenitor Cells between Cattle and Other Species. Int. J. Mol. Sci. 2023, 24, 11908. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liu, P.; Li, H.; Ding, S. Large-Scale Expansion of Porcine Adipose-Derived Stem Cells Based on Microcarriers System for Cultured Meat Production. Foods 2022, 11, 3364. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Louis, F.; Liu, H.; Shimoda, H.; Nishiyama, Y.; Nozawa, H.; Kakitani, M.; Takagi, D.; Kasa, D.; Nagamori, E.; et al. Engineered Whole Cut Meat-Like Tissue by the Assembly of Cell Fibers Using Tendon-Gel Integrated Bioprinting. Nat. Commun. 2021, 12, 5059. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef]
- Naraoka, Y.; Mabuchi, Y.; Yoneyama, Y.; Suto, E.G.; Hisamatsu, D.; Ikeda, M.; Ito, R.; Nakamura, T.; Takebe, T.; Akazawa, C. Isolation and Characterization of Tissue Resident CD29-Positive Progenitor Cells in Livestock to Generate a Three-Dimensional Meat Bud. Cells 2021, 10, 2499. [Google Scholar] [CrossRef]
- Haynesworth, S.E.; Baber, M.A.; Caplan, A.I. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1 alpha. J. Cell. Physiol. 1996, 166, 585–592. [Google Scholar] [CrossRef]
- Conget, P.A.; Minguell, J.J. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J. Cell. Physiol. 1999, 181, 67–73. [Google Scholar] [CrossRef]
- Weng, X.; Maxwell-Warburton, S.; Hasib, A.; Ma, L.; Kang, L. The membrane receptor CD44: Novel insights into metabolism. Trends Endocrinol. Metab. 2022, 33, 318–332. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Suto, E.G.; Mabuchi, Y.; Toyota, S.; Taguchi, M.; Naraoka, Y.; Itakura, N.; Matsuoka, Y.; Fujii, Y.; Miyasaka, N.; Akazawa, C. Advantage of fat-derived CD73 positive cells from multiple human tissues, prospective isolated mesenchymal stromal cells. Sci. Rep. 2020, 10, 15073. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Sakurai, H.; Suzuki, N.; Mabuchi, Y.; Sekiya, I.; Sekiguchi, K.; Akazawa, C. Recapitulation of Extracellular LAMININ Environment Maintains Stemness of Satellite Cells In Vitro. Stem Cell Rep. 2018, 10, 568–582. [Google Scholar] [CrossRef]
- Suto, E.G.; Mabuchi, Y.; Suzuki, N.; Suzuki, K.; Ogata, Y.; Taguchi, M.; Muneta, T.; Sekiya, I.; Akazawa, C. Prospectively isolated mesenchymal stem/stromal cells are enriched in the CD73(+) population and exhibit efficacy after transplantation. Sci. Rep. 2017, 7, 4838. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chal, J.; Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Jankowski, M.; Mozdziak, P.; Petitte, J.; Kulus, M.; Kempisty, B. Avian Satellite Cell Plasticity. Animals 2020, 10, 1322. [Google Scholar] [CrossRef]
- Tierney, M.T.; Sacco, A. Satellite Cell Heterogeneity in Skeletal Muscle Homeostasis. Trends Cell Biol. 2016, 26, 434–444. [Google Scholar] [CrossRef]
- Asakura, A.; Komaki, M.; Rudnicki, M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 2001, 68, 245–253. [Google Scholar] [CrossRef]
- Baroffio, A.; Bochaton-Piallat, M.L.; Gabbiani, G.; Bader, C.R. Heterogeneity in the progeny of single human muscle satellite cells. Differentiation 1995, 59, 259–268. [Google Scholar] [CrossRef]
- Dohmen, R.G.J.; Hubalek, S.; Melke, J.; Messmer, T.; Cantoni, F.; Mei, A.; Hueber, R.; Mitic, R.; Remmers, D.; Moutsatsou, P.; et al. Muscle-Derived Fibro-Adipogenic Progenitor Cells for Production of Cultured Bovine Adipose Tissue. NPJ Sci. Food 2022, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Heyman, E.; Meeremans, M.; Van Poucke, M.; Peelman, L.; Devriendt, B.; De Schauwer, C. Validation of multiparametric panels for bovine mesenchymal stromal cell phenotyping. Cytom. A 2023, 103, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Hou, L.; Ma, Y.; Chen, L.; Zhang, M.; Guan, W. Isolation and characterization of mesenchymal stem cells from chicken bone marrow. Cell Tissue Bank. 2013, 14, 437–451. [Google Scholar] [CrossRef]
- Adhikari, R.; Chen, C.; Waters, E.; West, F.D.; Kim, W.K. Isolation and Differentiation of Mesenchymal Stem Cells From Broiler Chicken Compact Bones. Front. Physiol. 2018, 9, 1892. [Google Scholar] [CrossRef]
- De Micheli, A.J.; Spector, J.A.; Elemento, O.; Cosgrove, B.D. A reference single-cell transcriptomic atlas of human skeletal muscle tissue reveals bifurcated muscle stem cell populations. Skelet. Muscle 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, P.; Li, C.; Liu, Q.; Yao, Z.; Li, Y.; Zhang, X.; Sun, J.; Simintiras, C.; Welborn, M.; et al. A single-cell atlas of bovine skeletal muscle reveals mechanisms regulating intramuscular adipogenesis and fibrogenesis. J. Cachexia Sarcopenia Muscle 2023, 14, 2152–2167. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Bats, M.L.; Peghaire, C.; Delobel, V.; Dufourcq, P.; Couffinhal, T.; Duplaa, C. Wnt/frizzled Signaling in Endothelium: A Major Player in Blood-Retinal- and Blood-Brain-Barrier Integrity. Cold Spring Harb. Perspect. Med. 2022, 12, a041219. [Google Scholar] [CrossRef]
- Qian, H.; Le Blanc, K.; Sigvardsson, M. Primary Mesenchymal Stem and Progenitor Cells from Bone Marrow Lack Expression of CD44 Protein. J. Biol. Chem. 2012, 287, 25795–25807. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhao, J.X.; Zhu, M.J.; Foretz, M.; Viollet, B.; Dodson, M.V.; Du, M. AMP-activated protein kinase alpha1 but not alpha2 catalytic subunit potentiates myogenin expression and myogenesis. Mol. Cell. Biol. 2013, 33, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Oh, S.; Shin, I. Ablation of CD44 induces glycolysis-to-oxidative phosphorylation transition via modulation of the c-Src-Akt-LKB1-AMPKalpha pathway. Biochem. J. 2016, 473, 3013–3030. [Google Scholar] [CrossRef] [PubMed]
- Jiwlawat, N.; Lynch, E.; Jeffrey, J.; Van Dyke, J.M.; Suzuki, M. Current Progress and Challenges for Skeletal Muscle Differentiation from Human Pluripotent Stem Cells Using Transgene-Free Approaches. Stem Cells Int. 2018, 2018, 6241681. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Borello, U.; Vivarelli, E.; Kelly, R.; Papkoff, J.; Duprez, D.; Buckingham, M.; Cossu, G. Differential Activation of Myf5 and MyoD by Different Wnts in Explants of Mouse Paraxial Mesoderm and the Later Activation of Myogenesis in the Absence of Myf5. Development 1998, 125, 4155–4162. [Google Scholar] [CrossRef]
- Bühring, H.J.; Treml, S.; Cerabona, F.; de Zwart, P.; Kanz, L.; Sobiesiak, M. Phenotypic Characterization of Distinct Human Bone Marrow-Derived MSC Subsets. Ann. N. Y. Acad. Sci. 2009, 1176, 124–134. [Google Scholar] [CrossRef]
- Wirths, S.; Malenke, E.; Kluba, T.; Rieger, S.; Müller, M.R.; Schleicher, S.; Hann von Weyhern, C.; Nagl, F.; Fend, F.; Vogel, W.; et al. Shared Cell Surface Marker Expression in Mesenchymal Stem Cells and Adult Sarcomas. Stem Cells Transl. Med. 2013, 2, 53–60. [Google Scholar] [CrossRef]
- Chen, A.E.; Ginty, D.D.; Fan, C.M. Protein Kinase A Signalling via CREB Controls Myogenesis Induced by WNT Proteins. Nature 2005, 433, 317–322. [Google Scholar] [CrossRef]
- Ikeya, M.; Takada, S. WNT Signaling from the Dorsal Neural Tube Is Required for the Formation of the Medial Dermomyotome. Development 1998, 125, 4969–4976. [Google Scholar] [CrossRef]
- Borello, U.; Coletta, M.; Tajbakhsh, S.; Leyns, L.; De Robertis, E.M.; Buckingham, M.; Cossu, G. Transplacental Delivery of the Wnt Antagonist Frzb1 Inhibits Development of Caudal Paraxial Mesoderm and Skeletal Myogenesis in Mouse Embryos. Development 1999, 126, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, H.; Gay, S.; Fedon, Y.; Vernus, B.; Bonnieu, A.; Bacou, F. Wnt4 Activates the Canonical Beta-Catenin Pathway and Regulates Negatively Myostatin: Functional Implication in Myogenesis. Am. J. Physiol. Cell Physiol. 2011, 300, C1122–C1138. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef]
- Sunadome, K.; Suzuki, T.; Usui, M.; Ashida, Y.; Nishida, E. Antagonism Between the Master Regulators of Differentiation Ensures the Discreteness and Robustness of Cell Fates. Mol. Cell 2014, 54, 526–535. [Google Scholar] [CrossRef]
- Hisamatsu, D.; Itakura, N.; Mabuchi, Y.; Ozaki, R.; Suto, E.G.; Naraoka, Y.; Ikeda, A.; Ito, L.; Akazawa, C. CD73-Positive Cell Spheroid Transplantation Attenuates Colonic Atrophy. Pharmaceutics 2023, 15, 845. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naraoka, Y.; Mabuchi, Y.; Kiuchi, M.; Kumagai, K.; Hisamatsu, D.; Yoneyama, Y.; Takebe, T.; Akazawa, C. Quality Control of Stem Cell-Based Cultured Meat According to Specific Differentiation Abilities. Cells 2024, 13, 135. https://doi.org/10.3390/cells13020135
Naraoka Y, Mabuchi Y, Kiuchi M, Kumagai K, Hisamatsu D, Yoneyama Y, Takebe T, Akazawa C. Quality Control of Stem Cell-Based Cultured Meat According to Specific Differentiation Abilities. Cells. 2024; 13(2):135. https://doi.org/10.3390/cells13020135
Chicago/Turabian StyleNaraoka, Yuna, Yo Mabuchi, Mai Kiuchi, Kyoko Kumagai, Daisuke Hisamatsu, Yosuke Yoneyama, Takanori Takebe, and Chihiro Akazawa. 2024. "Quality Control of Stem Cell-Based Cultured Meat According to Specific Differentiation Abilities" Cells 13, no. 2: 135. https://doi.org/10.3390/cells13020135
APA StyleNaraoka, Y., Mabuchi, Y., Kiuchi, M., Kumagai, K., Hisamatsu, D., Yoneyama, Y., Takebe, T., & Akazawa, C. (2024). Quality Control of Stem Cell-Based Cultured Meat According to Specific Differentiation Abilities. Cells, 13(2), 135. https://doi.org/10.3390/cells13020135






