Effects of Temperature Adaptation on the Metabolism and Physiological Properties of Sturgeon Fish Larvae Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Temperature Adaption and Cell Growth and Size
2.3. Staining of Cytoskeleton
2.4. Quantitative Real-Time PCR
2.5. Seahorse Experiments
2.6. Measurement of Protein Content and Normalization
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Cells
3.2. Cytoskeleton
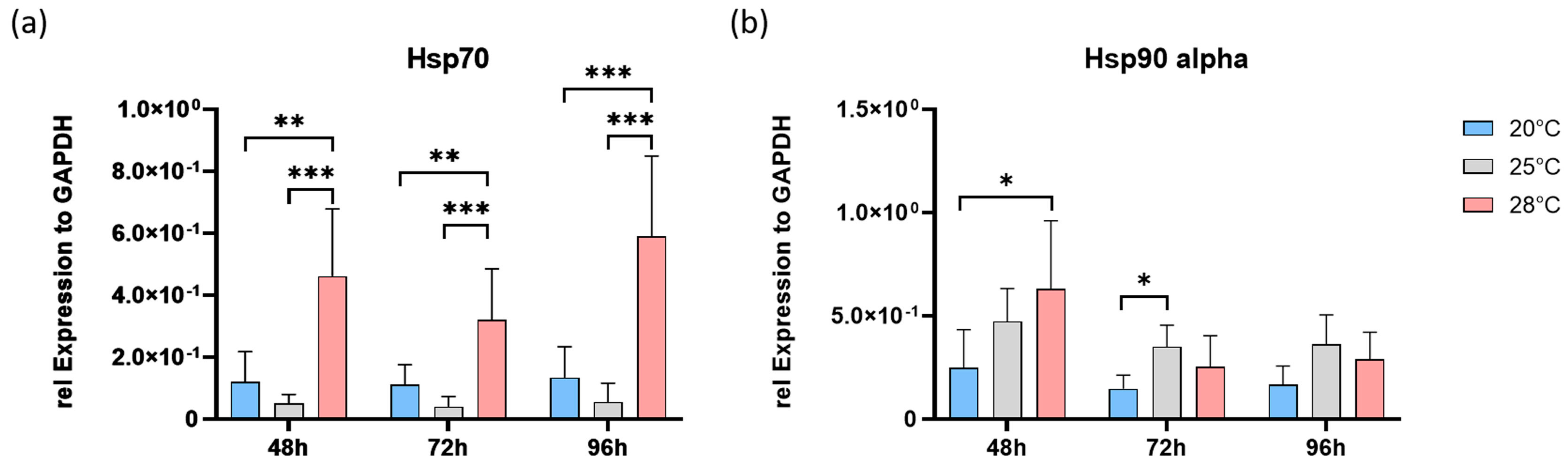
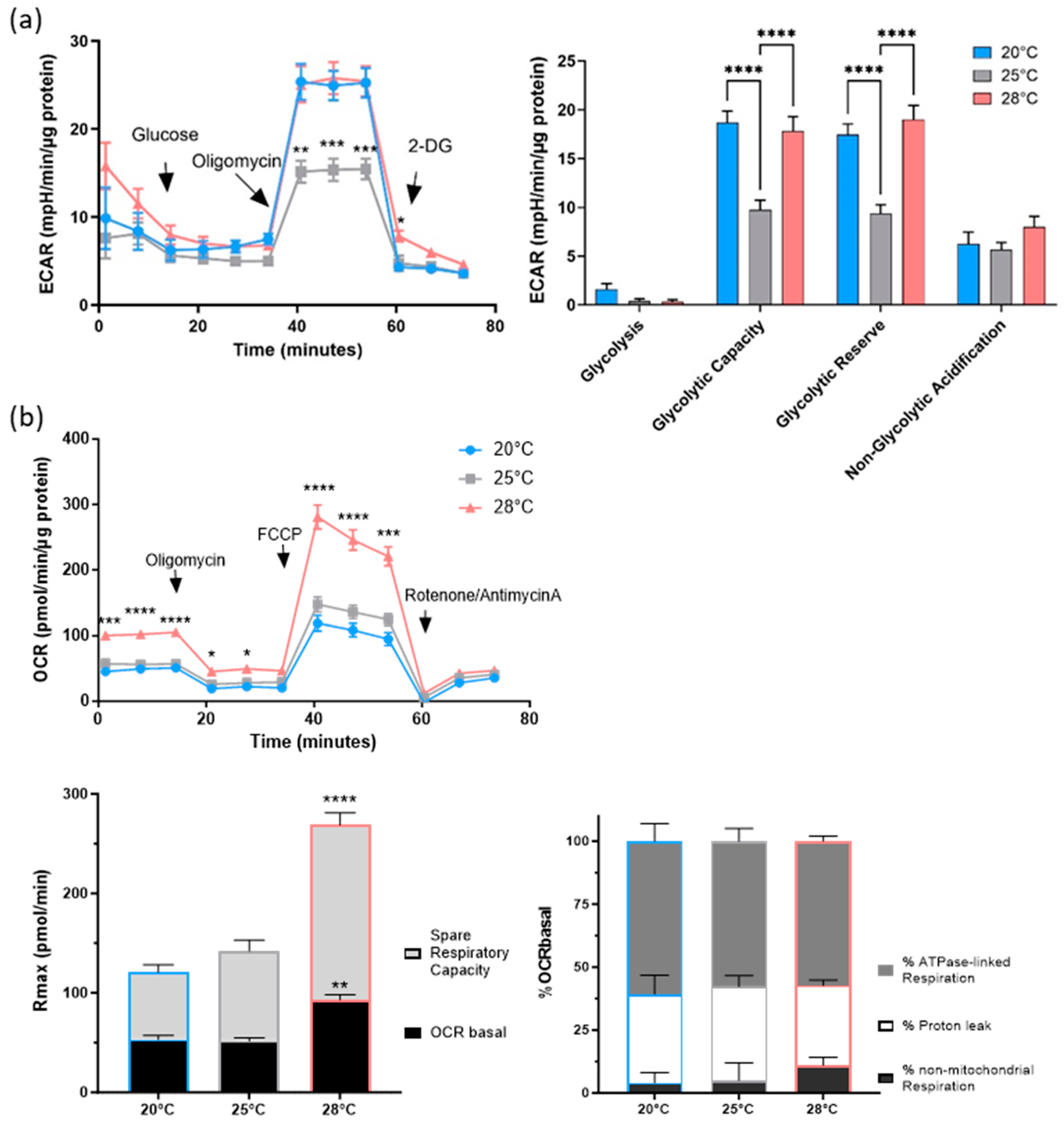
3.3. Stress Adaptation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland, 2007; p. 646. [Google Scholar]
- Available online: https://www.iucnredlist.org/ja/species/245/2785934#geographic-range (accessed on 12 June 2023).
- Chebanov, M.S.; Galich, E.V. Sturgeon Hatchery Manual; FAO Fisheries Technical Paper 2013; No. 558; Food and Agriculture Organization of the United Nations: Ankara, Turkey, 2013; p. 303. [Google Scholar]
- Available online: https://www.fisheries.noaa.gov/species/atlantic-sturgeon (accessed on 12 June 2023).
- Zhu, S.; Luo, Y.; Graf, R.; Wrzesiński, D.; Sojka, M.; Sun, B.; Kong, L.; Ji, Q.; Luo, W. Reconstruction of long-term water temperature indicates significant warming in Polish rivers during 1966–2020. J. Hydrol. Reg. Stud. 2022, 44, 101281. [Google Scholar] [CrossRef]
- Brett, J.R. Energetic Responses of Salmon to Temperature. A Study of Some Thermal Relations in the Physiology and Freshwater Ecology of Sockeye Salmon (Oncorhynchus nerkd). Am. Zool. 1971, 11, 99–113. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, P.; Guderley, H. Time course of the response of mitochondria from oxidative muscle during thermal acclimation of rainbow trout, Oncorhynchus mykiss. J. Exp. Biol. 2003, 206, 3455–3465. [Google Scholar] [CrossRef] [PubMed]
- Remen, M.; Nederlof, M.A.J.; Folkedal, O.; Thorsheim, G.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J.; Oppedal, F.; Olsen, R.E. Effect of temperature on the metabolism, behaviour and oxygen requirements of Sparus aurata. Aquac. Environ. Interact. 2015, 7, 115–123. [Google Scholar] [CrossRef]
- Sandblom, E.; Gräns, A.; Axelsson, M.; Seth, H. Temperature acclimation rate of aerobic scope and feeding metabolism in fishes: Implications in a thermally extreme future. Proc. Biol. Sci. 2014, 281, 20141490. [Google Scholar] [CrossRef]
- Prystay, T.S.; Eliason, E.J.; Lawrence, M.J.; Dick, M.; Brownscombe, J.W.; Patterson, D.A.; Crossin, G.T.; Hinch, S.G.; Cooke, S.J. The influence of water temperature on sockeye salmon heart rate recovery following simulated fisheries interactions. Conserv. Physiol. 2017, 5, cox050. [Google Scholar] [CrossRef]
- Christensen, E.A.F.; Norin, T.; Tabak, I.; van Deurs, M.; Behrens, J.W. Effects of temperature on physiological performance and behavioral thermoregulation in an invasive fish, the round goby. J. Exp. Biol. 2021, 224, jeb237669. [Google Scholar] [CrossRef]
- Li, S.; Guo, H.; Du, C.-Y.; Tao, Y.-X.; Feng, J.-Y.; Xu, H.; Pang, X.; Li, Y. Effect of temperature on exercise metabolism, hypoxia tolerance, and RNA-seq analysis in Sinilabeo rendahli from the Yangtze River, China. Front. Ecol. Evol. 2023, 11, 1159161. [Google Scholar] [CrossRef]
- Guderley, H. Metabolic responses to low temperature in fish muscle. Biol. Rev. Camb. Philos. Soc. 2004, 79, 409–427. [Google Scholar] [CrossRef]
- Tirsgaard, B.; Behrens, J.W.; Steffensen, J.F. The effect of temperature and body size on metabolic scope of activity in juvenile Atlantic cod Gadus morhua L. Comp. Biochem. Physiol. Part A Mol. Integrat. Physiol. 2015, 179, 89–94. [Google Scholar] [CrossRef]
- Available online: https://www.britannica.com/animal/sturgeon-fish (accessed on 3 July 2023).
- Available online: https://en.wikipedia.org/wiki/Paleoclimatology (accessed on 3 July 2023).
- Grunow, B.; Noglick, S.; Kruse, C.; Gebert, M. Isolation of Cells from Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) and Optimization of Culture Conditions. Aquat. Biol. 2011, 14, 67–75. [Google Scholar] [CrossRef]
- Grunow, B.; Franz, G.P.; Tönißen, K. In Vitro Fish Models for the Analysis of Ecotoxins and Temperature Increase in the Context of Global Warming. Toxics 2021, 9, 286. [Google Scholar] [CrossRef]
- Yebra-Pimentel, E.S.; Gebert, M.; Jansen, H.J.; Jong-Raadsen, S.A.; Dirks, R.P.H. Deep transcriptome analysis of the heat shock response in an Atlantic sturgeon (Acipenser oxyrinchus) cell line. Fish Shellfish Immunol. 2019, 88, 508–517. [Google Scholar] [CrossRef]
- Wanka, H.; Lutze, P.; Staar, D.; Grunow, B.; Peters, B.S.; Peters, J. An alternative renin isoform is cardioprotective by modulating mitochondrial metabolism. J. Cell Mol. Med. 2018, 22, 5991–6001. [Google Scholar] [CrossRef] [PubMed]
- Efremov, Y.M.; Velay-Lizancos, M.; Weaver, C.J.; Athamneh, A.I.; Zavattieri, P.D.; Suter, D.M.; Raman, A. Anisotropy vs isotropy in living cell indentation with AFM. Sci. Rep. 2019, 9, 5757. [Google Scholar] [CrossRef] [PubMed]
- Rianna, C.; Ventre, M.; Cavalli, S.; Radmacher, M.; Netti, P.A. Micropatterned Azopolymer Surfaces Modulate Cell Mechanics and Cytoskeleton Structure. ACS Appl. Mater. Interfaces 2015, 7, 21503–21510. [Google Scholar] [CrossRef]
- Peck, L.S. A Cold Limit to Adaptation in the Sea. Trends Ecol. Evol. 2016, 31, 13–26. [Google Scholar] [CrossRef]
- Babakhanova, G.; Zimmerman, S.M.; Pierce, L.T.; Sarkar, S.; Schaub, N.J.; Simon, C.G., Jr. Quantitative, traceable determination of cell viability using absorbance microscopy. PLoS ONE 2022, 17, e0262119. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, W.; Liang, Z.; Yao, S.; Zhang, X.; Zheng, Y. Effect of F-Actin Organization in Lamellipodium on Viscoelasticity and Migration of Huh-7 Cells Under pH Microenvironments Using AM-FM Atomic Force Microscopy. Front. Phys. 2021, 9, 674958. [Google Scholar] [CrossRef]
- Lehtimäki, J.I.; Rajakylä, E.K.; Tojkander, S.; Lappalainen, P. Generation of stress fibers through myosin-driven reorganization of the actin cortex. eLife 2021, 10, e60710. [Google Scholar] [CrossRef]
- Helfand, B.T.; Mendez, M.G.; Murthy, S.N.; Shumaker, D.K.; Grin, B.; Mahammad, S.; Aebi, U.; Wedig, T.; Wu, Y.I.; Hahn, K.M.; et al. Vimentin organization modulates the formation of lamellipodia. Mol. Biol. Cell 2011, 22, 1274–1289. [Google Scholar] [CrossRef]
- Toivola, D.M.; Strnad, P.; Habtezion, A.; Omary, M.B. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010, 20, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, F.; Jeffery, N. A guided tour of large genome size in animals: What we know and where we are heading. Chromosome Res. 2011, 19, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Hessen, D.; Daufresne, M. Temperature-size relations from the cellular-genomic perspective. Biol. Rev. Camb. Phil. Soc. 2013, 88, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Alfsnes, K.; Leinaas, H.P.; Hessen, D.O. Genome size in arthropods; different roles of phylogeny, habitat and life history in insects and crustaceans. Ecol. Evol. 2017, 7, 5939–5947. [Google Scholar] [CrossRef] [PubMed]
- van de Pol, I.L.E.; Hermaniuk, A.; Verberk, W.C.E.P. Interacting Effects of Cell Size and Temperature on Gene Expression, Growth, Development and Swimming Performance in Larval Zebrafish. Front. Physiol. 2021, 12, 738804. [Google Scholar] [CrossRef] [PubMed]
- Szarski, H. Cell size and the concept of wasteful and frugal evolutionary strategies. J. Theoret. Biol. 1983, 105, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Else, P.L. Mechanisms underlying the cost of living in animals. Annu. Rev. Physiol. 2000, 62, 207–235. [Google Scholar] [CrossRef]
- Maloyan, A.; Horowitz, M. Beta-Adrenergic signaling and thyroid hormones affect HSP72 expression during heat acclimation. J. Appl. Physiol. 2002, 93, 107–115. [Google Scholar] [CrossRef]
- Currie, S.; Reddin, K.; McGinn, P.; McConnell, T.; Perry, S.F. Beta-adrenergic stimulation enhances the heat-shock response in fish. Physiol. Biochem. Zool. 2008, 81, 414–425. [Google Scholar] [CrossRef]
- Margulis, B.A.; Antropova, O.; Kharazova, A.D. 70 kDa heat shock proteins from mollusc and human cells have common structural and functional domains. Comp. Biochem. Physiol. B 1989, 94, 621–623. [Google Scholar] [CrossRef]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef]
- Fangue, N.A.; Hofmeister, M.; Schulte, P.M. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 2006, 209, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Shatilina, Z.M.; Wolfgang Riss, H.; Protopopova, M.V.; Trippe, M.; Meyer, E.I.; Pavlichenko, V.V.; Bedulina, D.S.; Axenov-Gribanov, D.V.; Timofeyev, M.A. The role of the heat shock proteins (HSP70 and sHSP) in the thermotolerance of freshwater amphipods from contrasting habitats. J. Therm. Biol. 2011, 36, 142–149. [Google Scholar] [CrossRef]
- Feidantsis, K.; Antonopoulou, E.; Lazou, A.; Pörtner, H.O.; Michaelidis, B. Seasonal variations of cellular stress response of the gilthead sea bream (Sparus aurata). J. Comp. Physiol. B 2013, 183, 625–639. [Google Scholar] [CrossRef]
- Blair, S.; Glover, C. Acute exposure of larval rainbow trout (Oncorhynchus mykiss) to elevated temperature limits hsp70b expression and influences future thermotolerance. Hydrobiologia 2019, 836, 155–167. [Google Scholar] [CrossRef]
- Sekhar, A.; Rosenzweig, R.; Bouvignies, G.; Kay, L.E. Hsp70 biases the folding pathways of client proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E2794–E2801. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Luo, L. Genome-wide identification of Hsp70/110 genes in rainbow trout and their regulated expression in response to heat stress. PeerJ 2020, 8, e10022. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C. Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Gibson, O.R.; Tuttle, J.A.; Watt, P.W.; Maxwell, N.S.; Taylor, L. Hsp72 and Hsp90α mRNA transcription is characterised by large, sustained changes in core temperature during heat acclimation. Cell Stress Chaperones 2016, 21, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; Lagadari, M.; Toneatto, J.; Piwien-Pilipuk, G.; Galigniana, M.D. Regulatory role of the 90-kDa-heat-shock protein (Hsp90) and associated factors on gene expression. Biochim. Biophys. Acta 2014, 1839, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef]
- Duncan, R.F. Inhibition of Hsp90 function delays and impairs recovery from heat shock. FEBS J. 2005, 272, 5244–5256. [Google Scholar] [CrossRef]
- Espinosa-Ruiz, C.; Mayor-Lafuente, J.; Esteban, M.Á. Mitochondrial Metabolism Characterization of Four Different Fish Cell Lines. Fishes 2022, 7, 354. [Google Scholar] [CrossRef]
- Hendriks, K.D.W.; Lupi, E.; Hardenberg, M.C.; Hoogstra-Berends, F.; Deelman, L.E.; Henning, R.H. Differences in mitochondrial function and morphology during cooling and rewarming between hibernator and non-hibernator derived kidney epithelial cells. Sci. Rep. 2017, 7, 15482. [Google Scholar] [CrossRef]
- Mitov, M.I.; Harris, J.W.; Alstott, M.C.; Zaytseva, Y.Y.; Evers, B.M.; Butterfield, D.A. Temperature induces significant changes in both glycolytic reserve and mitochondrial spare respiratory capacity in colorectal cancer cell lines. Exp. Cell Res. 2017, 354, 112–121. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Paradyse, A.; Ferrick, D.A.; Murphy, A.N.; Jastroch, M. Chapter Sixteen—Analysis and Interpretation of Microplate-Based Oxygen Consumption and pH Data. In Methods Enzymol; Murphy, A.N., Chan, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 309–354. [Google Scholar]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Jastroch, M. A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements. Nat. Metab. 2022, 4, 978–994. [Google Scholar] [CrossRef]
- Horváthová, J.; Moravčík, R.; Matúšková, M.; Šišovský, V.; Boháč, A.; Zeman, M. Inhibition of Glycolysis Suppresses Cell Proliferation and Tumor Progression In Vivo: Perspectives for Chronotherapy. Int. J. Mol. Sci. 2021, 22, 4390. [Google Scholar] [CrossRef] [PubMed]



| Gene Symbol | Official Names | Genbank ID | NCBI Reference Sequence | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | qPCR Product (bp) |
|---|---|---|---|---|---|---|
| Housekeeping Gene | ||||||
| gapdh | glyceraldehyde-3-phosphate dehydrogenase | SCEB01214188.1.1 | XM_034042013.3 | ACACCCGCTCATCAATCTTT | AGGTCCACGACTCTGTTGCT | 80 |
| Target Gene | ||||||
| Proliferation | ||||||
| pcna | proliferating cell nuclear antigen | MK077520.1 | XM_059013649.1 | GCTGTGACGATCGAGATGAA | AACCAGAGCACACATGCTG | 215 |
| Cytoskeleton | ||||||
| vim | vimentin | AJ493266.1 | XM_034058632.3 | GATTTCGCCTTGTCCGATGC | TTGGTGGTGCGTTTTCCCTT | 350 |
| Stress-related genes | ||||||
| hsp70 | heat shock protein 70 | JN098420.1 | XM_033996031.3 | CCCGTGGAGAAGTCC | CCCGTTGAAGAAATCCTG | 123 |
| hsp90a | heat shock protein 90alpha | JN700180.1 | XM_034018059.3 | GGTCATCTTGCACCTGA | TTCTGCTTCATCATCGCTG | 250 |
| Index | Calculation of Index Values |
|---|---|
| Mito Stress Test | |
| Basal respiration | Baseline OCR—non-mitochondrial respiration |
| ATP production | Basal respiration—oligomycin OCR |
| Maximal respiration | FCCP respiration—non-mitochondrial respiration |
| Space capacity | FCCP respiration—basal respiration |
| Non-mitochondrial respiration | OCR with rotenone/antimycin A treatment |
| Glycolysis Stress Test | |
| Glycolysis | Glucose ECAR—starvation ECAR |
| Glycolytic Capacity | Oligomycin ECAR—starvation ECAR |
| Glycolytic Reserve | Glycolytic Capacity—Glycolysis |
| Non-Glycolytic Acidification | Starvation ECAR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutze, P.; Brenmoehl, J.; Tesenvitz, S.; Ohde, D.; Wanka, H.; Meyer, Z.; Grunow, B. Effects of Temperature Adaptation on the Metabolism and Physiological Properties of Sturgeon Fish Larvae Cell Line. Cells 2024, 13, 269. https://doi.org/10.3390/cells13030269
Lutze P, Brenmoehl J, Tesenvitz S, Ohde D, Wanka H, Meyer Z, Grunow B. Effects of Temperature Adaptation on the Metabolism and Physiological Properties of Sturgeon Fish Larvae Cell Line. Cells. 2024; 13(3):269. https://doi.org/10.3390/cells13030269
Chicago/Turabian StyleLutze, Philipp, Julia Brenmoehl, Stephanie Tesenvitz, Daniela Ohde, Heike Wanka, Zianka Meyer, and Bianka Grunow. 2024. "Effects of Temperature Adaptation on the Metabolism and Physiological Properties of Sturgeon Fish Larvae Cell Line" Cells 13, no. 3: 269. https://doi.org/10.3390/cells13030269






