MuSK Myasthenia Gravis—Potential Pathomechanisms and Treatment Directed against Specific Targets
Abstract
:1. Introduction
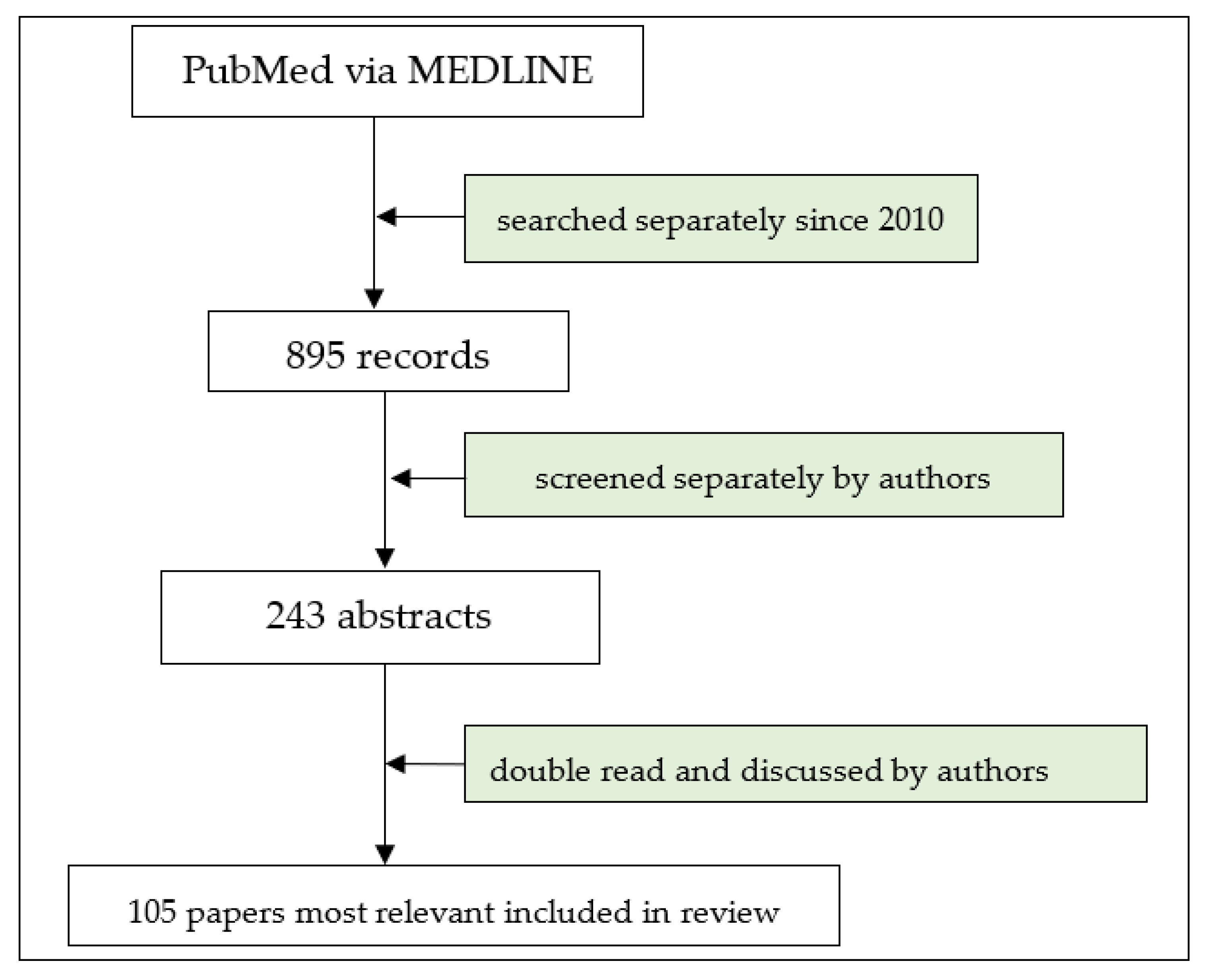
2. Methods
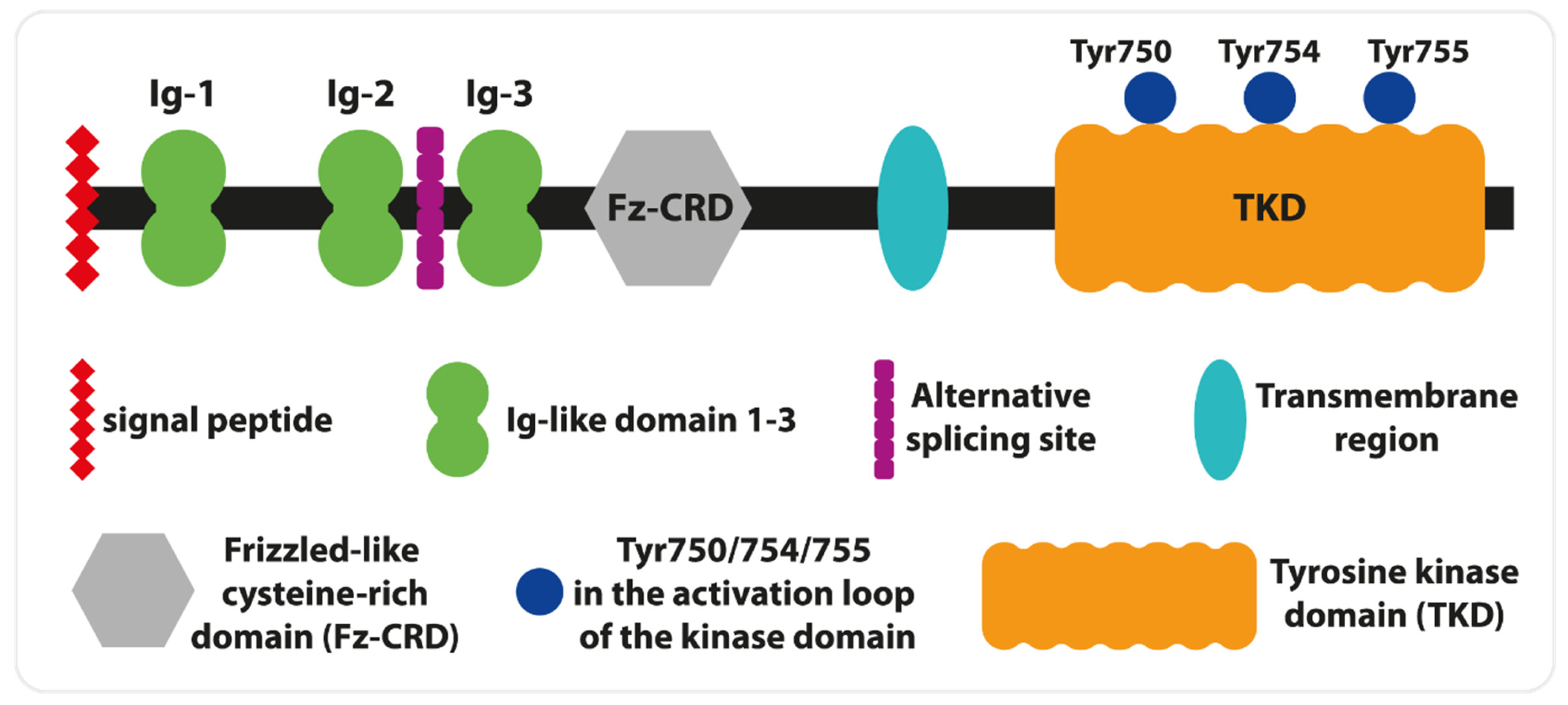
3. MUSK: From Gene to Functions
- The presynaptic part, including the motoneuron endings;
- The synaptic gap into which synaptic vesicles are secreted from the motoneuron axon and from which the neurotransmitter, acetylcholine, is released;
MuSK: From Gene to Disease
4. Specificity of Neurophysiological Diagnostic Tests
5. Non-Neurological Manifestations of MuSK-MG
6. Molecular Commonalities between MuSK-MG and Other Autoimmune Diseases of the Nervous System
7. Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AchE-Is | acetylcholinesterase inhibitors |
| AP | action potential |
| Caspr1 | contactin-associated protein 1 |
| CMS | congenital myasthenic syndromes |
| 3,4-DAP | 3,4-diaminopyridine |
| Dok7 | downstream of kinase-7 |
| EDC | extensor digitorum communis |
| EGF | epidermal growth factor |
| IgG4-RD | IgG4-related diseases |
| LRP4 | low-density lipoprotein receptor-related protein 4 |
| MASC | myotube-associated specificity component |
| MG | myasthenia gravis |
| MuSK | muscle-specific tyrosine kinase |
| NF155 | neurofascin-155 |
| NMJ | neuromuscular junction |
| QoL | quality of life |
| RNS | Repetitive Nerve Stimulation |
| RTX | Rituximab |
| SFAPs | single muscle fibers |
References
- Fichtner, M.L.; Jiang, R.; Bourke, A.; Nowak, R.J.; O’Connor, K.C. Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front. Immunol. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- Dziadkowiak, E.; Waliszewska-Prosół, M.; Wieczorek, M.; Bladowska, J.; Budrewicz, S.; Ejma, M. Myasthenia Gravis-An Analysis of Multimodal Evoked Potentials. Brain Sci. 2021, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- El-Salem, K.; Yassin, A.; Al-Hayk, K.; Yahya, S.; Al-Shorafat, D.; Dahbour, S.S. Treatment of MuSK-Associated Myasthenia Gravis. Curr. Treat. Options Neurol. 2014, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Rodolico, C.; Bonanno, C.; Toscano, A.; Vita, G. MuSK-Associated Myasthenia Gravis: Clinical Features and Management. Front. Neurol. 2020, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Evoli, A.; Alboini, P.E.; Damato, V.; Iorio, R.; Provenzano, C.; Bartoccioni, E.; Marino, M. Myasthenia gravis with antibodies to MuSK: An update. Ann. N. Y. Acad. Sci. 2018, 1412, 82–89. [Google Scholar] [CrossRef]
- Leite, M.I.; Ströbel, P.; Jones, M.; Micklem, K.; Moritz, R.; Gold, R.; Niks, E.H.; Berrih-Aknin, S.; Scaravilli, F.; Canelhas, A.; et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann. Neurol. 2005, 57, 444–448. [Google Scholar] [CrossRef]
- Clifford, K.M.; Hobson-Webb, L.D.; Benatar, M.; Burns, T.M.; Barnett, C.; Silvestri, N.J.; Howard, J.F., Jr.; Visser, A.; Crum, B.A.; Nowak, R.; et al. Thymectomy may not be associated with clinical improvement in MuSK myasthenia gravis. Muscle Nerve 2019, 59, 404–410. [Google Scholar] [CrossRef]
- Borges, L.S.; Richman, D.P. Muscle-Specific Kinase Myasthenia Gravis. Front. Immunol. 2020, 11, 707. [Google Scholar] [CrossRef]
- Mori, S.; Shigemoto, K. Mechanisms associated with the pathogenicity of antibodies against muscle-specific kinase in myasthenia gravis. Autoimmun. Rev. 2013, 12, 912–917. [Google Scholar] [CrossRef]
- Lepore, E.; Casola, I.; Dobrowolny, G.; Musarò, A. Neuromuscular Junction as an Entity of Nerve-Muscle Communication. Cells 2019, 8, 906. [Google Scholar] [CrossRef]
- Rodríguez Cruz, P.M.; Cossins, J.; Beeson, D.; Vincent, A. The Neuromuscular Junction in Health and Disease: Molecular Mechanisms Governing Synaptic Formation and Homeostasis. Front. Mol. Neurosci. 2020, 13, 610964. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Gnanasambandan, K. Structure and activation of MuSK, a receptor tyrosine kinase central to neuromuscular junction formation. Biochim. Biophys. Acta 2013, 1834, 2166–2169. [Google Scholar] [CrossRef]
- Burden, S.J. SnapShot: Neuromuscular Junction. Cell 2011, 144, 826.e1. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, S.; Wang, Q.; Suzuki, T.; Xiong, W.C.; Mei, L. LRP4 serves as a coreceptor of agrin. Neuron 2008, 60, 285–297. [Google Scholar] [CrossRef]
- Kim, N.; Stiegler, A.L.; Cameron, T.O.; Hallock, P.T.; Gomez, A.M.; Huang, J.H.; Hubbard, S.R.; Dustin, M.L.; Burden, S.J. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 2008, 135, 334–342. [Google Scholar] [CrossRef]
- Koneczny, I.; Herbst, R. Myasthenia Gravis: Pathogenic Effects of Autoantibodies on Neuromuscular Architecture. Cells 2019, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R. MuSk function during health and disease. Neurosci. Lett. 2020, 716, 134676. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, F.; Rahman, M.A.; Masuda, A.; Ohe, K.; Takeda, J.; Ohno, K. HnRNP C, YB-1 and hnRNP L coordinately enhance skipping of human MUSK exon 10 to generate a Wnt-insensitive MuSK isoform. Sci. Rep. 2014, 4, 6841. [Google Scholar] [CrossRef] [PubMed]
- Vergoossen, D.L.E.; Keo, A.; Mahfouz, A.; Huijbers, M.G. Timing and localization of myasthenia gravis-related gene expression. Eur. J. Neurosci. 2021, 54, 5574–5585. [Google Scholar] [CrossRef]
- Valenzuela, D.M.; Stitt, T.N.; DiStefano, P.S.; Rojas, E.; Mattsson, K.; Compton, D.L.; Nunez, L.; Park, J.S.; Stark, J.L.; Gies, D.R.; et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: Expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 1995, 15, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, V.D.; Jech, R.; Bures, J.; Nadel, L.; Růzicka, E. Spatial and nonspatial memory involvement in myasthenia gravis. J. Neurol. 1997, 244, 529–532. [Google Scholar] [CrossRef]
- Zhang, W.; Coldefy, A.S.; Hubbard, S.R.; Burden, S.J. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK). J. Biol. Chem. 2011, 286, 40624–40630. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, A.L.; Burden, S.J.; Hubbard, S.R. Crystal structure of the agrin-responsive immunoglobulin-like domains 1 and 2 of the receptor tyrosine kinase MuSK. J. Mol. Biol. 2006, 364, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Inoue, A.; Okada, M.; Murata, Y.; Kakuta, S.; Jigami, T.; Kubo, S.; Shiraishi, H.; Eguchi, K.; Motomura, M.; et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 2006, 312, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, E.; Hallock, P.T.; Burden, S.J.; Hubbard, S.R. The cytoplasmic adaptor protein Dok7 activates the receptor tyrosine kinase MuSK via dimerization. Mol. Cell 2010, 39, 100–109. [Google Scholar] [CrossRef]
- Till, J.H.; Becerra, M.; Watty, A.; Lu, Y.; Ma, Y.; Neubert, T.A.; Burden, S.J.; Hubbard, S.R. Crystal structure of the MuSK tyrosine kinase: Insights into receptor autoregulation. Structure 2002, 10, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Hallock, P.T.; Xu, C.F.; Park, T.J.; Neubert, T.A.; Curran, T.; Burden, S.J. Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L. Genes Dev. 2010, 24, 2451–2461. [Google Scholar] [CrossRef]
- Rødgaard, A.; Nielsen, F.C.; Djurup, R.; Somnier, F.; Gammeltoft, S. Acetylcholine receptor antibody in myasthenia gravis: Predominance of IgG subclasses 1 and 3. Clin. Exp. Immunol. 1987, 67, 82–88. [Google Scholar]
- Otsuka, K.; Ito, M.; Ohkawara, B.; Masuda, A.; Kawakami, Y.; Sahashi, K.; Nishida, H.; Mabuchi, N.; Takano, A.; Engel, A.G.; et al. Collagen Q and anti-MuSK autoantibody competitively suppress agrin/LRP4/MuSK signaling. Sci. Rep. 2015, 5, 13928. [Google Scholar] [CrossRef]
- Cao, M.; Liu, W.W.; Maxwell, S.; Huda, S.; Webster, R.; Evoli, A.; Beeson, D.; Cossins, J.A.; Vincent, A. IgG1-3 MuSK Antibodies Inhibit AChR Cluster Formation, Restored by SHP2 Inhibitor, Despite Normal MuSK, DOK7, or AChR Subunit Phosphorylation. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200147. [Google Scholar] [CrossRef]
- Koneczny, I.; Cossins, J.; Waters, P.; Beeson, D.; Vincent, A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS ONE 2013, 8, e80695. [Google Scholar] [CrossRef]
- Vergoossen, D.L.E.; Plomp, J.J.; Gstöttner, C.; Fillié-Grijpma, Y.E.; Augustinus, R.; Verpalen, R.; Wuhrer, M.; Parren, P.W.H.I.; Dominguez-Vega, E.; van der Maarel, S.M.; et al. Functional monovalency amplifies the pathogenicity of anti-MuSK IgG4 in myasthenia gravis. Proc. Natl. Acad. Sci. USA 2021, 118, e2020635118. [Google Scholar] [CrossRef]
- Hajdukovic, L.; Palibrk, A.; Peric, S.; Basta, I.; Minic, R.; Jankovic, M.; Lavrnic, D. Galactosylation of serum immunoglobulin G in myasthenia gravis with different autoantibodies. Scand. J. Clin. Lab. Investig. 2023, 83, 348–355. [Google Scholar] [CrossRef]
- Hong, Y.; Li, H.F.; Romi, F.; Skeie, G.O.; Gilhus, N.E. HLA and MuSK-positive myasthenia gravis: A systemic review and meta-analysis. Acta Neurol. Scand. 2018, 138, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Alahgholi-Hajibehzad, M.; Yilmaz, V.; Gülsen-Parman, Y.; Aysal, F.; Oflazer, P.; Deymeer, F.; Saruhan-Direskeneli, G. Association of HLA-DRB1∗14, -DRB1∗16 and -DQB1∗05 with MuSK-myasthenia gravis in patients from Turkey. Hum. Immunol. 2013, 74, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Uzawa, A.; Kawaguchi, N.; Sakamaki, T.; Yoshiyama, Y.; Himuro, K.; Oda, F.; Kuwabara, S. HLA-DRB1*14 and DQB1*05 are associated with Japanese anti-MuSK antibody-positive myasthenia gravis patients. J. Neurol. Sci. 2016, 363, 116–118. [Google Scholar] [CrossRef]
- Yi, J.S.; Guidon, A.; Sparks, S.; Osborne, R.; Juel, V.C.; Massey, J.M.; Sanders, D.B.; Weinhold, K.J.; Guptill, J.T. Characterization of CD4 and CD8 T cell responses in MuSK myasthenia gravis. J. Autoimmun. 2014, 52, 130–138. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. MicroRNA expression profiles of peripheral blood and mononuclear cells in myasthenia gravis: A systematic review. Int. Immunopharmacol. 2022, 112, 109205. [Google Scholar] [CrossRef] [PubMed]
- Beretta, F.; Huang, Y.F.; Punga, A.R. Towards Personalized Medicine in Myasthenia Gravis: Role of Circulating microRNAs miR-30e-5p, miR-150-5p and miR-21-5p. Cells 2022, 11, 740. [Google Scholar] [CrossRef]
- Punga, A.R.; Andersson, M.; Alimohammadi, M.; Punga, T. Disease specific signature of circulating miR-150-5p and miR-21-5p in myasthenia gravis patients. J. Neurol. Sci. 2015, 356, 90–96. [Google Scholar] [CrossRef]
- Punga, T.; Bartoccioni, E.; Lewandowska, M.; Damato, V.; Evoli, A.; Punga, A.R. Disease specific enrichment of circulating let-7 family microRNA in MuSK+ myasthenia gravis. J. Neuroimmunol. 2016, 292, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chevessier, F.; Girard, E.; Molgó, J.; Bartling, S.; Koenig, J.; Hantaï, D.; Witzemann, V. A mouse model for congenital myasthenic syndrome due to MuSK mutations reveals defects in structure and function of neuromuscular junctions. Hum. Mol. Genet. 2008, 17, 3577–3595. [Google Scholar] [CrossRef] [PubMed]
- Chevessier, F.; Faraut, B.; Ravel-Chapuis, A.; Richard, P.; Gaudon, K.; Bauché, S.; Prioleau, C.; Herbst, R.; Goillot, E.; Ioos, C.; et al. MUSK, a new target for mutations causing congenital myasthenic syndrome. Hum. Mol. Genet. 2004, 13, 3229–3240. [Google Scholar] [CrossRef] [PubMed]
- Maselli, R.A.; Arredondo, J.; Cagney, O.; Ng, J.J.; Anderson, J.A.; Williams, C.; Gerke, B.J.; Soliven, B.; Wollmann, R.L. Mutations in MUSK causing congenital myasthenic syndrome impair MuSK-Dok-7 interaction. Hum. Mol. Genet. 2010, 19, 2370–2379. [Google Scholar] [CrossRef]
- Ekstedt, J. Human single muscle fiber action potentials. Extracellular recording during voluntary and chemical activation. With some comments on end-plate physiology and on the fiber arrangement of the motor unit. Acta Physiol. Scand. Suppl. 1964, (Suppl. 226:1+). [Google Scholar] [PubMed]
- Stålberg, E.; Ekstedt, J.; Broman, A. Neuromuscular transmission in myasthenia gravis studied with single fibre electromyography. J. Neurol. Neurosurg. Psychiatry 1974, 37, 540–547. [Google Scholar] [CrossRef]
- Buchthal, F.; Guld, C.; Rosenfalck, F. Multielectrode study of the territory of a motor unit. Acta Physiol. Scand. 1957, 39, 83–104. [Google Scholar] [CrossRef]
- Sanders, D.B.; Arimura, K.; Cui, L.; Ertaş, M.; Farrugia, M.E.; Gilchrist, J.; Kouyoumdjian, J.A.; Padua, L.; Pitt, M.; Stålberg, E. Guidelines for single fiber EMG. Clin. Neurophysiol. 2019, 130, 1417–1439. [Google Scholar] [CrossRef]
- Oh, S.J.; Ohira, M. Single-fiber EMG and clinical correlation in Lambert-Eaton myasthenic syndrome. Muscle Nerve 2013, 47, 664–667. [Google Scholar] [CrossRef]
- Emeryk-Szajewska, B. Electrophysiological investigations in diagnosis and evaluation of ALS progress. Neurol. Neurochir. Pol. 2001, 35 (Suppl. S1), 11–24. [Google Scholar] [PubMed]
- Cui, L.Y.; Liu, M.S.; Tang, X.F. Single fiber electromyography in 78 patients with amyotrophic lateral sclerosis. Chin. Med. J. 2004, 117, 1830–1833. [Google Scholar] [PubMed]
- Hatanaka, Y.; Oh, S.J. Single-fiber electromyography in sporadic inclusion body myopathy. Clin. Neurophysiol. 2007, 118, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Jian, F.; Cui, L.Y.; Li, B.H.; Du, H. Changes of single fiber electromyography in patients with inflammatory myopathies. Chin. Med. Sci. J. 2005, 20, 1–4. [Google Scholar] [PubMed]
- Padua, L.; Stalberg, E.; LoMonaco, M.; Evoli, A.; Batocchi, A.; Tonali, P. SFEMG in ocular myasthenia gravis diagnosis. Clin. Neurophysiol. 2000, 111, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Hatanaka, Y.; Hemmi, S.; Young, A.M.; Scheufele, M.L.; Nations, S.P.; Lu, L.; Claussen, G.C.; Wolfe, G.I. Repetitive nerve stimulation of facial muscles in MuSK antibody-positive myasthenia gravis. Muscle Nerve. 2006, 33, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Tonali, P.; Aprile, I.; Caliandro, P.; Bartoccioni, E.; Evoli, A. Seronegative myasthenia gravis: Comparison of neurophysiological picture in MuSK+ and MuSK- patients. Eur. J. Neurol. 2006, 13, 273–276. [Google Scholar] [CrossRef]
- Yoganathan, K.; Stevenson, A.; Tahir, A.; Sadler, R.; Radunovic, A.; Malek, N. Bedside and laboratory diagnostic testing in myasthenia. J. Neurol. 2022, 269, 3372–3384. [Google Scholar] [CrossRef]
- Abraham, A.; Alabdali, M.; Alsulaiman, A.; Breiner, A.; Barnett, C.; Katzberg, H.D.; Lovblom, L.E.; Bril, V. Repetitive nerve stimulation cutoff values for the diagnosis of myasthenia gravis. Muscle Nerve 2017, 55, 166–170. [Google Scholar] [CrossRef]
- Nemoto, Y.; Kuwabara, S.; Misawa, S.; Kawaguchi, N.; Hattori, T.; Takamori, M.; Vincent, A. Patterns and severity of neuromuscular transmission failure in seronegative myasthenia gravis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 714–718. [Google Scholar] [CrossRef]
- Nikolic, A.; Basta, I.; Stojanovic, V.R.; Stevic, Z.; Lavrnic, D. Electrophysiological profile of the patients with MuSK positive myasthenia gravis. Neurol. Res. 2014, 36, 945–949. [Google Scholar] [CrossRef]
- Kuwabara, S.; Nemoto, Y.; Misawa, S.; Takahashi, H.; Kawaguchi, N.; Hattori, T. Anti-MuSK-positive myasthenia gravis: Neuromuscular transmission failure in facial and limb muscles. Acta Neurol. Scand. 2007, 115, 126–128. [Google Scholar] [CrossRef]
- Farrugia, M.E.; Kennett, R.P.; Newsom-Davis, J.; Hilton-Jones, D.; Vincent, A. Single-fiber electromyography in limb and facial muscles in muscle-specific kinase antibody and acetylcholine receptor antibody myasthenia gravis. Muscle Nerve 2006, 33, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Rostedt Punga, A.; Ahlqvist, K.; Bartoccioni, E.; Scuderi, F.; Marino, M.; Suomalainen, A.; Kalimo, H.; Stålberg, E.V. Neurophysiological and mitochondrial abnormalities in MuSK antibody seropositive myasthenia gravis compared to other immunological subtypes. Clin. Neurophysiol. 2006, 117, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, M.; Peric, S.; Stojiljkovic Tamas, O.; Stankovic, T.; Nikolic, A.; Lavrnic, D.; Basta, I. Quality of life in patients with MuSK positive myasthenia gravis. Acta Neurol. Belg. 2018, 118, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, R.Y.; Ye, X.B.; Wang, N. Reduced quality of life in myasthenia gravis patients: A study on 185 patients from China. Front. Neurol. 2023, 13, 1072861. [Google Scholar] [CrossRef] [PubMed]
- Lehnerer, S.; Jacobi, J.; Schilling, R.; Grittner, U.; Marbin, D.; Gerischer, L.; Stascheit, F.; Krause, M.; Hoffmann, S.; Meisel, A. Burden of disease in myasthenia gravis: Taking the patient’s perspective. J. Neurol. 2022, 269, 3050–3063. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, D.; Parvin-Nejad, S.; Phillips, G.; Cole, C.; Hughes, T.; Silvestri, N.; Govindarajan, R.; Jefferson, M.; Campbell, J.; Burnett, H. The humanistic burden of myasthenia gravis: A systematic literature review. J. Neurol. Sci. 2022, 437, 120268. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosół, M.; Ejma, M. Hashimoto Encephalopathy-Still More Questions than Answers. Cells 2022, 11, 2873. [Google Scholar] [CrossRef]
- Dziadkowiak, E.; Waliszewska-Prosół, M.; Nowakowska-Kotas, M.; Budrewicz, S.; Koszewicz, Z.; Koszewicz, M. Pathophysiology of the Different Clinical Phenotypes of Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP). Int. J. Mol. Sci. 2021, 23, 179. [Google Scholar] [CrossRef]
- Dalakas, M.C. Autoimmune Neurological Disorders with IgG4 Antibodies: A Distinct Disease Spectrum with Unique IgG4 Functions Responding to Anti-B Cell Therapies. Neurotherapeutics 2022, 19, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, P.; Alexopoulos, H.; Dalakas, M.C. Autoimmune antigenic targets at the node of Ranvier in demyelinating disorders. Nat. Rev. Neurol. 2015, 11, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. IgG4-Mediated Neurologic Autoimmunities: Understanding the Pathogenicity of IgG4, Ineffectiveness of IVIg, and Long-Lasting Benefits of Anti-B Cell Therapies. Neurol. Neuroimmunol. Neuroinflamm. 2021, 9, e1116. [Google Scholar] [CrossRef] [PubMed]
- Sabater, L.; Gaig, C.; Gelpi, E.; Bataller, L.; Lewerenz, J.; Torres-Vega, E.; Contreras, A.; Giometto, B.; Compta, Y.; Embid, C.; et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: A case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014, 13, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Deng, Z.; Wang, S.; Wang, Y. Basic Research and Clinical Reports Associated with Low Serum IgG4 Concentrations. Int. Arch. Allergy Immunol. 2020, 181, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Modoni, A.; Mastrorosa, A.; Spagni, G.; Evoli, A. Cholinergic hyperactivity in patients with myasthenia gravis with MuSK antibodies: A neurophysiological study. Clin. Neurophysiol. 2021, 132, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Huda, S.; Waters, P.; Woodhall, M.; Leite, M.I.; Jacobson, L.; De Rosa, A.; Maestri, M.; Ricciardi, R.; Heckmann, J.M.; Maniaol, A.; et al. IgG-specific cell-based assay detects potentially pathogenic MuSK-Abs in seronegative MG. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e357. [Google Scholar] [CrossRef]
- Evoli, A.; Alboini, P.E.; Damato, V.; Iorio, R. 3,4-Diaminopyridine may improve myasthenia gravis with MuSK antibodies. Neurology 2016, 86, 1070–1071. [Google Scholar] [CrossRef]
- Haran, M.; Schattner, A.; Mate, A.; Starobin, D.; Haran, G.; Shtalrid, M. Can a rare form of myasthenia gravis shed additional light on disease mechanisms? Clin. Neurol. Neurosurg. 2013, 115, 562–566. [Google Scholar] [CrossRef]
- König, N.; Stetefeld, H.R.; Dohmen, C.; Mergenthaler, P.; Kohler, S.; Schönenberger, S.; Bösel, J.; Lee, D.H.; Gerner, S.T.; Huttner, H.B.; et al. MuSK-antibodies are associated with worse outcome in myasthenic crisis requiring mechanical ventilation. J. Neurol. 2021, 268, 4824–4833. [Google Scholar] [CrossRef]
- Guptill, J.T.; Sanders, D.B. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr. Opin. Neurol. 2010, 23, 530–535. [Google Scholar] [CrossRef]
- Evoli, A.; Padua, L. Diagnosis and therapy of myasthenia gravis with antibodies to muscle-specific kinase. Autoimmun. Rev. 2013, 12, 931–935. [Google Scholar] [CrossRef]
- Marino, M.; Basile, U.; Spagni, G.; Napodano, C.; Iorio, R.; Gulli, F.; Todi, L.; Provenzano, C.; Bartoccioni, E.; Evoli, A. Long-Lasting Rituximab-Induced Reduction of Specific-But Not Total-IgG4 in MuSK-Positive Myasthenia Gravis. Front. Immunol. 2020, 11, 613. [Google Scholar] [CrossRef]
- Narayanaswami, P.; Sanders, D.B.; Wolfe, G.; Benatar, M.; Cea, G.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.L.; Massey, J.; et al. International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology 2021, 96, 114–122. [Google Scholar] [CrossRef]
- Sanders, D.B.; Wolfe, G.I.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef]
- Vesperinas-Castro, A.; Cortés-Vicente, E. Rituximab treatment in myasthenia gravis. Front. Neurol. 2023, 14, 1275533. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Clauder, A.K.; Manz, R.A. Targeting B Cells and Plasma Cells in Autoimmune Diseases. Front. Immunol. 2018, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.G.; Plomp, J.J.; van der Maarel, S.M.; Verschuuren, J.J. IgG4-mediated autoimmune diseases: A niche of antibody-mediated disorders. Ann. N. Y. Acad. Sci. 2018, 1413, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tejerina, D.; Sotoca, J.; Llaurado, A.; López-Diego, V.; Juntas-Morales, R.; Salvado, M. New Targeted Agents in Myasthenia Gravis and Future Therapeutic Strategies. J. Clin. Med. 2022, 11, 6394. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, R.; Bernasconi, P.; Cavalcante, P. Myasthenia gravis: From autoantibodies to therapy. Curr. Opin. Neurol. 2018, 31, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.; Putko, B.N.; Wagner, A.N.; Siddiqi, Z.A. Therapies Directed Against B-Cells and Downstream Effectors in Generalized Autoimmune Myasthenia Gravis: Current Status. Drugs 2019, 79, 353–364. [Google Scholar] [CrossRef]
- Lazaridis, K.; Tzartos, S.J. Autoantibody Specificities in Myasthenia Gravis; Implications for Improved Diagnostics and Therapeutics. Front. Immunol. 2020, 11, 212. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Karachaliou, E.; Chroni, E.; Zouvelou, V.; Tzanetakos, D.; Salakou, S.; Papadopoulou, M.; Tzartos, S.; Voumvourakis, K.; Kilidireas, C.; et al. Immunotherapies in MuSK-positive Myasthenia Gravis; an IgG4 antibody-mediated disease. Front. Immunol. 2023, 14, 1212757. [Google Scholar] [CrossRef]
- Howard, J.F., Jr.; Bril, V.; Vu, T.; Karam, C.; Peric, S.; Margania, T.; Murai, H.; Bilinska, M.; Shakarishvili, R.; Smilowski, M.; et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): A multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021, 20, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, P.; Kumar, A.; Heiden, J.A.V.; Pascual-Goñi, E.; Nowak, R.J.; O’Connor, K.C. Mechanisms underlying B cell immune dysregulation and autoantibody production in MuSK myasthenia gravis. Ann. N. Y. Acad. Sci. 2018, 1412, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Matic, A.; Alfaidi, N.; Bril, V. An evaluation of rozanolixizumab-noli for the treatment of anti-AChR and anti-MuSK antibody-positive generalized myasthenia gravis. Expert Opin. Biol. Ther. 2023, 23, 1163–1171. [Google Scholar] [CrossRef]
- Keller, C.W.; Pawlitzki, M.; Wiendl, H.; Lünemann, J.D. Fc-Receptor Targeted Therapies for the Treatment of Myasthenia gravis. Int. J. Mol. Sci. 2021, 22, 5755. [Google Scholar] [CrossRef]
- Albazli, K.; Kaminski, H.J.; Howard, J.F., Jr. Complement Inhibitor Therapy for Myasthenia Gravis. Front. Immunol. 2020, 11, 917. [Google Scholar] [CrossRef]
- Dalakas, M.C. Role of complement, anti-complement therapeutics, and other targeted immunotherapies in myasthenia gravis. Expert Rev. Clin. Immunol. 2022, 18, 691–701. [Google Scholar] [CrossRef]
- Dhillon, S. Eculizumab: A Review in Generalized Myasthenia Gravis. Drugs 2018, 78, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.F., Jr.; Utsugisawa, K.; Benatar, M.; Murai, H.; Barohn, R.J.; Illa, I.; Jacob, S.; Vissing, J.; Burns, T.M.; Kissel, J.T.; et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017, 16, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Gold, C.; Reinacher-Schick, A.; Ellrichmann, G.; Gold, R. Bortezomib in severe MuSK-antibody positive myasthenia gravis: First clinical experience. Ther. Adv. Neurol. Disord. 2017, 10, 339–341. [Google Scholar] [CrossRef] [PubMed]
- DeHart-McCoyle, M.; Patel, S.; Du, X. New and emerging treatments for myasthenia gravis. BMJ Med. 2023, 2, e000241. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Andreetta, F.; Antozzi, C.; Confalonieri, P.; Cornelio, F.; Scaioli, V.; Mantegazza, R. Two cases of thymoma-associated myasthenia gravis without antibodies to the acetylcholine receptor. Neuromuscul. Disord. 2008, 18, 678–680. [Google Scholar] [CrossRef]
- Marx, A.; Yamada, Y.; Simon-Keller, K.; Schalke, B.; Willcox, N.; Ströbel, P.; Weis, C.A. Thymus and autoimmunity. Semin. Immunopathol. 2021, 43, 45–64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziadkowiak, E.; Baczyńska, D.; Waliszewska-Prosół, M. MuSK Myasthenia Gravis—Potential Pathomechanisms and Treatment Directed against Specific Targets. Cells 2024, 13, 556. https://doi.org/10.3390/cells13060556
Dziadkowiak E, Baczyńska D, Waliszewska-Prosół M. MuSK Myasthenia Gravis—Potential Pathomechanisms and Treatment Directed against Specific Targets. Cells. 2024; 13(6):556. https://doi.org/10.3390/cells13060556
Chicago/Turabian StyleDziadkowiak, Edyta, Dagmara Baczyńska, and Marta Waliszewska-Prosół. 2024. "MuSK Myasthenia Gravis—Potential Pathomechanisms and Treatment Directed against Specific Targets" Cells 13, no. 6: 556. https://doi.org/10.3390/cells13060556





