Fusobacterium nucleatum: An Overview of Evidence, Demi-Decadal Trends, and Its Role in Adverse Pregnancy Outcomes and Various Gynecological Diseases, including Cancers
Abstract
:1. Introduction
2. Evidence Acquisition and Synthesis
3. F. nucleatum in Adverse Pregnancy Outcomes
 definition, etiologies, and symptoms.
definition, etiologies, and symptoms.4. F. nucleatum in Gynecological Diseases
 definition, histopathologies, etiologies, and symptoms.
definition, histopathologies, etiologies, and symptoms.- Salpingitis is particularly defined as an infection and inflammation in the oviducts (fallopian tubes). These tubes are responsible for transporting oocytes and sperm, as well as facilitating fertilization and early embryonic development. The inflammation can be acute or chronic and can range in severity from mild to severe. It is typically caused by an infection that spreads from the lower tract to the upper genital tract [108]. It is also referred to as salpingitis isthmica nodosa (SIN) and is believed to be a part of the chronic pelvic inflammatory disease (PID) spectrum in some patients. Histopathologically, SIN is characterized by nodular thickening of the muscularis layer of the fallopian tube and the formation of inclusion cysts or diverticula due to overgrown epithelium. It is strongly associated with both infertility and ectopic pregnancies [109]. The current known causes of salpingitis include infection, cellular invasion, and congenital malformations [110]. Clinically, it is often manifested by edema, congestion of the fallopian tubes, and inflammation of the peritoneal structures [109].
- Perihepatitis, or Fitz–Hugh–Curtis syndrome, is a rare and chronic complication of PID that primarily affects premenopausal women. It is characterized by inflammation of the liver capsule and adhesion of the peritoneum, resulting in right upper quadrant pain [111]. The condition can be caused by various factors, including spontaneous ascending infection where microbes from the cervix or vagina travel to the endometrium, through the fallopian tubes, and into the peritoneal cavity; lymphatic spread, such as infection of the parametrium from an intrauterine device; and hematogenous spread, such as with tuberculosis. Common symptoms include acute pain and/or chronic tenderness in the right upper abdomen [112].
- Endometritis is an infectious inflammation of the endometrium, which is the innermost uterine layer. When the inflammation spreads into the muscular layer, the process is termed endomyometritis, and when it extends through to the parametrium, it is called endoparametritis. Histopathologically, acute endometritis is usually characterized by microabscesses and neutrophil invasion of the superficial endometrial epithelium, glandular lumens, and endometrial cavity. However, chronic endometritis is characterized by the infiltration of endometrial stromal plasmacytes (ESPCs), micropolyposis, edematous changes in the proliferative phase, and dissociated maturation between the stroma and epithelium. Additionally, B cells can accumulate in the endometrial stroma and glands. This condition is caused by the migration of normal bacterial flora from the cervix and vagina into the uterine cavity but can also be caused by bacteria from outside the genital tract. Symptoms of endometritis typically include irregular bleeding, pelvic discomfort, and leukorrhea [113].
- Oophoritis is a condition in which the ovaries become inflamed due to certain infections, potentially leading to impaired ovarian function. This inflammation can result in atrophic and fibrotic ovaries and, in rare cases, the replacement of ovarian stroma by foamy macrophages and histiocytes [114,115]. Various factors can contribute to the development of oophoritis, including sexual transmission, infection during pregnancy, and peripubertal infection, depending on the specific virus or pathogenic agent. Common clinical symptoms of oophoritis include anorexia, fever, suprapubic pain, menorrhagia, vaginal bleeding, adnexal tenderness, and/or a pelvic mass [114].
- Peritonitis is a medical condition characterized by inflammation of the peritoneum, which is the membrane that lines the abdominal cavity [116]. This inflammation can be caused by various factors, such as underlying health conditions or the presence of infectious agents. In some cases, it may also present as granulomas with central caseous necrosis, although this is rare. Some of the known causes of peritonitis include gastroduodenal perforations, intestinal volvulus, ruptured abscesses, traumatic bowel perforation, perforated peptic ulcers, tubo-ovarian abscesses, and amoebic colonic perforations [117]. The classic symptoms of peritonitis include severe abdominal pain, tenderness and rigidity, fever, chills, and altered mental status [118].
- Tubo-ovarian abscess (TOA) is a complex infectious mass that forms in the adnexa as a result of PID. It is often caused by bacteria from the lower genital tract that travel up to the fallopian tube, ovary, and potentially other nearby pelvic organs. Common risk factors include being of reproductive age, having an intrauterine device (IUD) inserted, having multiple sexual partners, and having a previous episode of PID. TOAs are typically polymicrobial and often contain a high proportion of anaerobic bacteria. Symptoms may include an adnexal mass, fever, elevated white blood cell count (WBC), lower abdominal or pelvic pain, and/or vaginal discharge [119].
5. F. nucleatum in Gynecological Cancers
 definition, types, histopathologies, etiologies, symptoms, and staging.
definition, types, histopathologies, etiologies, symptoms, and staging.6. Conclusions, Future Challenges, and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Young, P.; Ron, E.; Pecetta, S.; Pizza, M. Save the microbes to save the planet. A call to action of the International Union of the Microbiological Societies (IUMS). One Health Outlook 2023, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.A.R. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Kouzu, K.; Tsujimoto, H.; Kishi, Y.; Ueno, H.; Shinomiya, N. Role of Microbial Infection-Induced Inflammation in the Development of Gastrointestinal Cancers. Medicines 2021, 8, 45. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Muhammad, J.S.; Siddiqui, R. The role of gut microbiome in cancer genesis and cancer prevention. Health Sciences Review 2022, 2, 100010. [Google Scholar] [CrossRef]
- Xu, S.; Xiong, Y.; Fu, B.; Guo, D.; Sha, Z.; Lin, X.; Wu, H. Bacteria and macrophages in the tumor microenvironment. Front. Microbiol. 2023, 14, 1115556. [Google Scholar] [CrossRef]
- Sipos, A.; Ujlaki, G.; Miko, E.; Maka, E.; Szabo, J.; Uray, K.; Krasznai, Z.; Bai, P. The role of the microbiome in ovarian cancer: Mechanistic insights into oncobiosis and to bacterial metabolite signaling. Mol. Med. 2021, 27, 33. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, N.; Han, W.; Ban, M.; Sun, T.; Xu, J. Gut Microbes in Gynecologic Cancers: Causes or Biomarkers and Therapeutic Potential. Front. Oncol. 2022, 12, 902695. [Google Scholar] [CrossRef] [PubMed]
- Ventolini, G.; Vieira-Baptista, P.; De Seta, F.; Verstraelen, H.; Lonnee-Hoffmann, R.; Lev-Sagie, A. The Vaginal Microbiome: IV. The Role of Vaginal Microbiome in Reproduction and in Gynecologic Cancers. J. Low. Genit. Tract. Dis. 2022, 26, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef] [PubMed]
- Tefiku, U.; Popovska, M.; Cana, A.; Zendeli-Bedxeti, L.; Recica, B.; Spasovska-Gjorgovska, A.; Spasovski, S. Determination of the Role of Fusobacterium nucleatum in the Pathogenesis in and Out the Mouth. Pril 2020, 41, 87–99. [Google Scholar] [CrossRef]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium nucleatum and its associated systemic diseases: Epidemiologic studies and possible mechanisms. J. Oral. Microbiol. 2023, 15, 2145729. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Waterboer, T.; Idahl, A.; Brenner, N.; Brinton, L.A.; Butt, J.; Coburn, S.B.; Hartge, P.; Hufnagel, K.; Inturrisi, F.; et al. Antibodies Against Chlamydia trachomatis and Ovarian Cancer Risk in Two Independent Populations. J. Natl. Cancer Inst. 2019, 111, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jeraldo, P.; Jang, J.S.; Eckloff, B.; Jen, J.; Walther-Antonio, M. Bacterial Single Cell Whole Transcriptome Amplification in Microfluidic Platform Shows Putative Gene Expression Heterogeneity. Anal. Chem. 2019, 91, 8036–8044. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. Microbial Alterations and Risk Factors of Breast Cancer: Connections and Mechanistic Insights. Cells 2020, 9, 1091. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Gober, H.J.; Leung, W.T.; Huang, Z.; Pan, X.; Li, C.; Zhang, N.; Wang, L. Alterations in the intestinal microbiome associated with PCOS affect the clinical phenotype. Biomed. Pharmacother. 2021, 133, 110958. [Google Scholar] [CrossRef]
- Boutriq, S.; Gonzalez-Gonzalez, A.; Plaza-Andrades, I.; Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Peralta-Linero, J.; Dominguez-Recio, M.E.; Bermejo-Perez, M.J.; Lavado-Valenzuela, R.; Alba, E.; et al. Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer. J. Pers. Med. 2021, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.P.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef]
- Kumar, M.; Saadaoui, M.; Al Khodor, S. Infections and Pregnancy: Effects on Maternal and Child Health. Front. Cell Infect. Microbiol. 2022, 12, 873253. [Google Scholar] [CrossRef] [PubMed]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and cancer. Periodontology 2000 2022, 89, 166–180. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, X.; Fu, L.; Yang, K. Fusobacterium nucleatum: A new player in regulation of cancer development and therapeutic response. Cancer Drug Resist. 2022, 5, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Chanomethaporn, A.; Chayasadom, A.; Wara-Aswapati, N.; Kongwattanakul, K.; Suwannarong, W.; Tangwanichgapong, K.; Sumanonta, G.; Matangkasombut, O.; Dasanayake, A.P.; Pitiphat, W. Association between periodontitis and spontaneous abortion: A case-control study. J. Periodontol. 2019, 90, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, T.S.; Noh, E.J.; Jang, A.R.; Ahn, J.H.; Kim, D.Y.; Jung, D.H.; Song, E.J.; Lee, Y.J.; Lee, Y.J.; et al. Receptor-interacting protein kinase 2 contributes to host innate immune responses against Fusobacterium nucleatum in macrophages and decidual stromal cells. Am. J. Reprod. Immunol. 2021, 86, e13403. [Google Scholar] [CrossRef]
- Parhi, L.; Abed, J.; Shhadeh, A.; Alon-Maimon, T.; Udi, S.; Ben-Arye, S.L.; Tam, J.; Parnas, O.; Padler-Karavani, V.; Goldman-Wohl, D.; et al. Placental colonization by Fusobacterium nucleatum is mediated by binding of the Fap2 lectin to placentally displayed Gal-GalNAc. Cell Reports 2022, 38, 110537. [Google Scholar] [CrossRef]
- Muraoka, A.; Suzuki, M.; Hamaguchi, T.; Watanabe, S.; Iijima, K.; Murofushi, Y.; Shinjo, K.; Osuka, S.; Hariyama, Y.; Ito, M.; et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci. Transl. Med. 2023, 15, eadd1531. [Google Scholar] [CrossRef]
- Achu Joseph, R.; Ajitkumar, S.; Kanakasabapathy Balaji, S.; Santhanakrishnan, M. Evaluation of Microbial Profile in Patients with Polycystic Ovary Syndrome and Periodontal Disease: A Case-Control Study. Int. J. Fertil. Steril. 2023, 17, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Rokos, T.; Holubekova, V.; Kolkova, Z.; Hornakova, A.; Pribulova, T.; Kozubik, E.; Biringer, K.; Kudela, E. Is the Physiological Composition of the Vaginal Microbiome Altered in High-Risk HPV Infection of the Uterine Cervix? Viruses 2022, 14, 2130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Ziebolz, D.; Schmalz, G.; Jia, B.; Zhao, J. More Than Just a Periodontal Pathogen -the Research Progress on Fusobacterium nucleatum. Front. Cell Infect. Microbiol. 2022, 12, 815318. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Han, Y.W. Oral bacteria, oral health, and adverse pregnancy outcomes. Periodontology 2000 2022, 89, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, T.; Li, Y.; Huang, L.; Yin, D. Fusobacterium nucleatum: The Opportunistic Pathogen of Periodontal and Peri-Implant Diseases. Front. Microbiol. 2022, 13, 860149. [Google Scholar] [CrossRef] [PubMed]
- Britton, T.A.; Wu, C.; Chen, Y.W.; Franklin, D.; Chen, Y.; Camacho, M.I.; Luong, T.T.; Das, A.; Ton-That, H. The respiratory enzyme complex Rnf is vital for metabolic adaptation and virulence in Fusobacterium nucleatum. mBio 2024, 15, e0175123. [Google Scholar] [CrossRef] [PubMed]
- Stokowa-Sołtys, K.; Wojtkowiak, K.; Jagiełło, K. Fusobacterium nucleatum—Friend or foe? J. Inorg. Biochem. 2021, 224, 111586. [Google Scholar] [CrossRef] [PubMed]
- Umana, A.; Sanders, B.E.; Yoo, C.C.; Casasanta, M.A.; Udayasuryan, B.; Verbridge, S.S.; Slade, D.J. Utilizing Whole Fusobacterium Genomes to Identify, Correct, and Characterize Potential Virulence Protein Families. J. Bacteriol. 2019, 201, e00273-19. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.H.; Li, B.B.; Wang, B.; Zhao, J.; Zhang, X.Y.; Li, T.T.; Li, W.B.; Tang, D.; Qiu, M.J.; Wang, X.C.; et al. The role of Fusobacterium nucleatum in colorectal cancer: From carcinogenesis to clinical management. Chronic Dis. Transl. Med. 2019, 5, 178–187. [Google Scholar] [CrossRef]
- Cochrane, K.; Robinson, A.V.; Holt, R.A.; Allen-Vercoe, E. A survey of Fusobacterium nucleatum genes modulated by host cell infection. Microb. Genom. 2020, 6, e000300. [Google Scholar] [CrossRef]
- Boehm, E.T.; Thon, C.; Kupcinskas, J.; Steponaitiene, R.; Skieceviciene, J.; Canbay, A.; Malfertheiner, P.; Link, A. Fusobacterium nucleatum is associated with worse prognosis in Lauren’s diffuse type gastric cancer patients. Sci. Rep. 2020, 10, 16240. [Google Scholar] [CrossRef]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and oral cancer: A critical review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontology 2000 2020, 83, 213–233. [Google Scholar] [CrossRef]
- Tadese, M.; Dagne, K.; Wubetu, A.D.; Abeway, S.; Bekele, A.; Misganaw Kebede, W.; Baye Mulu, G. Assessment of the adverse pregnancy outcomes and its associated factors among deliveries at Debre Berhan Comprehensive Specialized Hospital, Northeast Ethiopia. PLoS ONE 2022, 17, e0271287. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Tarrazo, M.; Garcia-Mantrana, I.; Gomez-Gallego, C.; Salminen, S.; Collado, M.C. Shaping Microbiota During the First 1000 Days of Life. Adv. Exp. Med. Biol. 2019, 1125, 3–24. [Google Scholar] [CrossRef]
- Schoenmakers, S.; Steegers-Theunissen, R.; Faas, M. The matter of the reproductive microbiome. Obstet. Med. 2019, 12, 107–115. [Google Scholar] [CrossRef]
- Figuero, E.; Han, Y.W.; Furuichi, Y. Periodontal diseases and adverse pregnancy outcomes: Mechanisms. Periodontology 2000 2020, 83, 175–188. [Google Scholar] [CrossRef]
- Siena, M.; Laterza, L.; Matteo, M.V.; Mignini, I.; Schepis, T.; Rizzatti, G.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Gasbarrini, A. Gut and Reproductive Tract Microbiota Adaptation during Pregnancy: New Insights for Pregnancy-Related Complications and Therapy. Microorganisms 2021, 9, 473. [Google Scholar] [CrossRef]
- Saadaoui, M.; Singh, P.; Al Khodor, S. Oral microbiome and pregnancy: A bidirectional relationship. J. Reprod. Immunol. 2021, 145, 103293. [Google Scholar] [CrossRef]
- Moreno, I.; Capalbo, A.; Mas, A.; Garrido-Gomez, T.; Roson, B.; Poli, M.; Dimitriadis, E.; Santamaria, X.; Vilella, F.; Simon, C. The human periconceptional maternal-embryonic space in health and disease. Physiol. Rev. 2023, 103, 1965–2038. [Google Scholar] [CrossRef]
- Blanco, E.; Marin, M.; Nunez, L.; Retamal, E.; Ossa, X.; Woolley, K.E.; Oludotun, T.; Bartington, S.E.; Delgado-Saborit, J.M.; Harrison, R.M.; et al. Adverse pregnancy and perinatal outcomes in Latin America and the Caribbean: Systematic review and meta-analysis. Rev. Panam. Salud Publica 2022, 46, e21. [Google Scholar] [CrossRef] [PubMed]
- Muluneh, A.G.; Asratie, M.H.; Gebremariam, T.; Adu, A.; Enyew, M.M.; Cherkos, E.A.; Melkamu, S.; Berta, M.; Mamo, W.; Kassahun, D.; et al. Lifetime adverse pregnancy outcomes and associated factors among antenatal care booked women in Central Gondar zone and Gondar city administration, Northwest Ethiopia. Front. Public. Health 2022, 10, 966055. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Gallagher, K.; Beck, C.; Kumar, R.; Gernand, A.D. Maternal-Fetal Inflammation in the Placenta and the Developmental Origins of Health and Disease. Front. Immunol. 2020, 11, 531543. [Google Scholar] [CrossRef]
- Lukanovic, D.; Batkoska, M.; Kavsek, G.; Druskovic, M. Clinical chorioamnionitis: Where do we stand now? Front. Med. 2023, 10, 1191254. [Google Scholar] [CrossRef]
- Jain, V.G.; Willis, K.A.; Jobe, A.; Ambalavanan, N. Chorioamnionitis and neonatal outcomes. Pediatr. Res. 2022, 91, 289–296. [Google Scholar] [CrossRef]
- Granese, R.; Gitto, E.; D’Angelo, G.; Falsaperla, R.; Corsello, G.; Amadore, D.; Calagna, G.; Fazzolari, I.; Grasso, R.; Triolo, O. Preterm birth: Seven-year retrospective study in a single centre population. Ital. J. Pediatr. 2019, 45, 45. [Google Scholar] [CrossRef]
- Suman, V.; Luther, E.E. Preterm Labor. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Alves, C.; Jenkins, S.M.; Rapp, A. Early Pregnancy Loss (Spontaneous Abortion). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kasa, G.A.; Woldemariam, A.Y.; Adella, A.; Alemu, B. The factors associated with stillbirths among sub-saharan African deliveries: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2023, 23, 835. [Google Scholar] [CrossRef]
- Maslovich, M.M.; Burke, L.M. Intrauterine Fetal Demise. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Singh, M.; Alsaleem, M.; Gray, C.P. Neonatal Sepsis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Karrar, S.A.; Hong, P.L. Preeclampsia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef]
- Quintanilla Rodriguez, B.S.; Mahdy, H. Gestational Diabetes. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Heusler, M.; Einenkel, R.; Ehrhardt, J.; Muzzio, D.O.; Zygmunt, M. Low Abundance Fusobacterium nucleatum Supports Early Pregnancy Development—An In Vitro Study. Front. Immunol. 2021, 12, 698045. [Google Scholar] [CrossRef]
- Payne, M.S.; Newnham, J.P.; Doherty, D.A.; Furfaro, L.L.; Pendal, N.L.; Loh, D.E.; Keelan, J.A. A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the Predict1000 study). Am. J. Obstet. Gynecol. 2021, 224, 206-e1. [Google Scholar] [CrossRef]
- Lima, K.M.; Alves, C.M.; Vidal, F.C.; Gomes-Filho, I.S.; Costa, J.C.; Coletta, R.D.; Rodrigues, V.P.; Lopes, F.F. Fusobacterium nucleatum and Prevotella in women with periodontitis and preterm birth. Med. Oral. Patol. Oral. Cir. Bucal 2023, 28, e450–e456. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Y.W.; Scheible, M.; Chang, C.; Wittchen, M.; Lee, J.H.; Luong, T.T.; Tiner, B.L.; Tauch, A.; Das, A.; et al. Genetic and molecular determinants of polymicrobial interactions in Fusobacterium nucleatum. Proc. Natl. Acad. Sci. USA 2021, 118, e2006482118. [Google Scholar] [CrossRef]
- Zhao, F.; Hu, X.; Ying, C. Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases. Microorganisms 2023, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The Interplay Between Reproductive Tract Microbiota and Immunological System in Human Reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Dessì, A.; Bosco, A.; Pintus, R.; Orrù, G.; Fanos, V. Fusobacterium nucleatum and alteration of the oral microbiome: From pregnancy to SARS-COV-2 infection. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4579–4596. [Google Scholar] [CrossRef] [PubMed]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a Neonate Is Born, So Is a Microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Lu, W.; Manikam, R.; Malarvili, M.B.; Ambati, R.R.; Gundamaraju, R. Can metagenomics unravel the impact of oral bacteriome in human diseases? Biotechnol. Genet. Eng. Rev. 2023, 39, 85–117. [Google Scholar] [CrossRef]
- Vander Haar, E.L.; Wu, G.; Gyamfi-Bannerman, C.; Thomas, C.; Wapner, R.J.; Reddy, U.M.; Zhao, L.; Silver, R.M.; Goldenberg, R.L.; Han, Y.W. Microbial Analysis of Umbilical Cord Blood Reveals Novel Pathogens Associated with Stillbirth and Early Preterm Birth. mBio 2022, 13, e0203622. [Google Scholar] [CrossRef]
- Bonasoni, M.P.; Comitini, G.; Pati, M.; Bardaro, M.; Russello, G.; Carretto, E.; Dalla Dea, G.; Palicelli, A.; Bernardelli, G.; Chesi, E.; et al. Fulminant Sepsis and Perinatal Death at 23 Weeks Due to Fusobacterium nucleatum. Fetal Pediatr. Pathol. 2023, 42, 456–463. [Google Scholar] [CrossRef]
- Chan, E.; Brundler, M.A.; Zhang, K. Identification of Fusobacterium nucleatum in formalin-fixed, paraffin-embedded placental tissues by 16S rRNA sequencing in a case of extremely preterm birth secondary to amniotic fluid infection. Pathology 2019, 51, 320–322. [Google Scholar] [CrossRef]
- Ye, C.; Katagiri, S.; Miyasaka, N.; Kobayashi, H.; Khemwong, T.; Nagasawa, T.; Izumi, Y. The periodontopathic bacteria in placenta, saliva and subgingival plaque of threatened preterm labor and preterm low birth weight cases: A longitudinal study in Japanese pregnant women. Clin. Oral. Investig. 2020, 24, 4261–4270. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Geng, H.; Bai, J.; Feng, J.; Xu, N.; Liu, Y.; Liu, X.; Liu, G. Characterization of cervical canal and vaginal bacteria in pregnant women with cervical incompetence. Front. Microbiol. 2022, 13, 986326. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Nugent, W.H.; Alam, S.M.K.; Washington, S.L.; Teves, M.; Jefferson, K.K.; Strauss, J.F., 3rd. Protease Amplification of the Inflammatory Response Induced by Commensal Bacteria: Implications for Racial Disparity in Term and Preterm Birth. Reprod. Sci. 2020, 27, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Surlin, P.; Nicolae, F.M.; Surlin, V.M.; Patrascu, S.; Ungureanu, B.S.; Didilescu, A.C.; Gheonea, D.I. Could Periodontal Disease through Periopathogen Fusobacterium nucleatum be an Aggravating Factor for Gastric Cancer? J. Clin. Med. 2020, 9, 3885. [Google Scholar] [CrossRef] [PubMed]
- Beckers, K.F.; Sones, J.L. Maternal microbiome and the hypertensive disorder of pregnancy, preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1–H10. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Brown, J.A.; Rager, S.L.; Sanidad, K.Z.; Ananthanarayanan, A.; Zeng, M.Y. Maternal Microbiome and Infections in Pregnancy. Microorganisms 2020, 8, 1996. [Google Scholar] [CrossRef] [PubMed]
- Bodurska, T.; Konova, E.; Pachkova, S.; Yordanov, A. Endometrial Microbiome and Women’s Reproductive Health—Review of the Problem Endometrial Microbiome and Reproductive Health. J. Pure Appl. Microbiol. 2021, 15, 1727–1734. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Dutta, S.; Sengupta, P.; Bagchi, S. Reproductive tract microbiome and therapeutics of infertility. Middle East Fertil. Soc. J. 2023, 28, 11. [Google Scholar] [CrossRef]
- Ye, C.; Kapila, Y. Oral microbiome shifts during pregnancy and adverse pregnancy outcomes: Hormonal and Immunologic changes at play. Periodontology 2000 2021, 87, 276–281. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Farhat, S.; Hemmatabadi, M.; Ejtahed, H.S.; Shirzad, N.; Larijani, B. Microbiome alterations in women with gestational diabetes mellitus and their offspring: A systematic review. Front. Endocrinol. 2022, 13, 1060488. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.L.; Levy, M.; Nissapatorn, V.; de Oliveira, G.L.V. Editorial: Women in microbiome in health and disease 2021. Front Cell Infect Microbiol 2022, 12, 1054190. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chung, S.W.; Auh, Q.S.; Hong, S.J.; Lee, Y.A.; Jung, J.; Lee, G.J.; Park, H.J.; Shin, S.I.; Hong, J.Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, S.; Ramachandran, R.; Ali, F.R.; Lipovich, L.; Ho, S.B. Dietary Patterns and Associated Microbiome Changes that Promote Oncogenesis. Front. Cell Dev. Biol. 2021, 9, 725821. [Google Scholar] [CrossRef] [PubMed]
- Akimbekov, N.S.; Digel, I.; Yerezhepov, A.Y.; Shardarbek, R.S.; Wu, X.; Zha, J. Nutritional factors influencing microbiota-mediated colonization resistance of the oral cavity: A literature review. Front. Nutr. 2022, 9, 1029324. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef] [PubMed]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Tonse, R.; Saxena, A.; McGranaghan, P.; Kaiser, A.; Kotecha, R. Emerging Evidence on the Effects of Dietary Factors on the Gut Microbiome in Colorectal Cancer. Front. Nutr. 2021, 8, 718389. [Google Scholar] [CrossRef] [PubMed]
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef]
- Merchant, A.T.; Gupta, R.D.; Akonde, M.; Reynolds, M.; Smith-Warner, S.; Liu, J.; Tarannum, F.; Beck, J.; Mattison, D. Association of Chlorhexidine Use and Scaling and Root Planing with Birth Outcomes in Pregnant Individuals with Periodontitis: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2247632. [Google Scholar] [CrossRef]
- Meng, Q.; Gao, Q.; Mehrazarin, S.; Tangwanichgapong, K.; Wang, Y.; Huang, Y.; Pan, Y.; Robinson, S.; Liu, Z.; Zangiabadi, A.; et al. Fusobacterium nucleatum secretes amyloid-like FadA to enhance pathogenicity. EMBO Rep. 2021, 22, e52891. [Google Scholar] [CrossRef]
- Scheible, M.; Nguyen, C.T.; Luong, T.T.; Lee, J.H.; Chen, Y.W.; Chang, C.; Wittchen, M.; Camacho, M.I.; Tiner, B.L.; Wu, C.; et al. The Fused Methionine Sulfoxide Reductase MsrAB Promotes Oxidative Stress Defense and Bacterial Virulence in Fusobacterium nucleatum. mBio 2022, 13, e0302221. [Google Scholar] [CrossRef] [PubMed]
- Garcia-So, J.; Zhang, X.; Yang, X.; Rubinstein, M.R.; Mao, Y.; Kitajewski, J.; Liu, K.; Han, Y.W. Omega-3 fatty acids suppress Fusobacterium nucleatum-induced placental inflammation originating from maternal endothelial cells. JCI Insight 2019, 4, e125436. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Wang, Y.H.; Yu, H.C.; Chang, Y.C. Increased Risk of Polycystic Ovary Syndrome in Taiwanese Women with Chronic Periodontitis: A Nationwide Population-Based Retrospective Cohort Study. J. Womens Health 2019, 28, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Rubino, C.; Barbati, F.; Regoli, M.; Bencini, E.; Mattei, A.; Fierro, F.; Brizzi, I.; Indolfi, G. Recurrent Bilateral Salpingitis in a Sexually Inactive Adolescent: Don’t Forget about the Appendix. J. Pediatr. Adolesc. Gynecol. 2021, 34, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Mercer, V.J.; Naseemuddin, A.; Webb, A. Monckeberg’s arteriosclerosis: A case report of chronic endometritis presenting as postmenopausal bleeding. Menopause 2021, 29, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Yin, M.M.; Gao, Y.L.; Shang, J.; Zheng, C.H. MSF-LRR: Multi-Similarity Information Fusion through Low-Rank Representation to Predict Disease-Associated Microbes. IEEE/ACM Trans. Comput. Biol. Bioinform. 2023, 20, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Robinson, L.S.; Aggarwal, S.; Foster, L.R.; Hernandez-Leyva, A.; Lin, H.; Tortelli, B.A.; O’Brien, V.P.; Miller, L.; Kau, A.L.; et al. Glycan cross-feeding supports mutualism between Fusobacterium and the vaginal microbiota. PLoS Biol. 2020, 18, e3000788. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil Steril 2023, 120, 767–793. [Google Scholar] [CrossRef]
- Rasquin, L.I.; Anastasopoulou, C.; Mayrin, J.V. Polycystic Ovarian Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Greydanus, D.E.; Bacopoulou, F. Acute pelvic inflammatory disease: A narrative review. Pediatr. Med. 2019, 2, 36. [Google Scholar] [CrossRef]
- Owhor, L.E.; Reese, S.; Kolle, S. Salpingitis Impairs Bovine Tubal Function and Sperm-Oviduct Interaction. Sci. Rep. 2019, 9, 10893. [Google Scholar] [CrossRef] [PubMed]
- Revzin, M.V.; Moshiri, M.; Katz, D.S.; Pellerito, J.S.; Mankowski Gettle, L.; Menias, C.O. Imaging Evaluation of Fallopian Tubes and Related Disease: A Primer for Radiologists. Radiographics 2020, 40, 1473–1501. [Google Scholar] [CrossRef] [PubMed]
- Barkwill, D.; Tobler, K.J. Salpingitis Isthmica Nodosa. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kaga, M.; Ito, S.; Ueda, T. Two Cases of Perihepatitis with the Liver Capsule Irritation Sign: A New Physical Examination Technique. Cureus 2023, 15, e34327. [Google Scholar] [CrossRef] [PubMed]
- Basit, H.; Pop, A.; Malik, A.; Sharma, S. Fitz-Hugh-Curtis Syndrome. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Taylor, M.; Jenkins, S.M.; Pillarisetty, L.S. Endometritis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Giunta, I.; Zayat, N.; Muneyyirci-Delale, O. Histologic features, pathogenesis, and long-term effects of viral oophoritis. F&S Rev. 2021, 2, 342–352. [Google Scholar] [CrossRef]
- Dawande, P.; Wankhade, R.; Pande, M. Xanthogranulomatous Oophoritis: A Rare Case Report. Cureus 2023, 15, e43724. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, T.; Liu, J.K.; Ding, J.; Chen, L. Conservative management of multi-trauma induced peritonitis: Experience, outcomes, and indications. World J. Clin. Cases 2023, 11, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Garg, I.; Sarwar, A.H.; Kumar, L.; Kumar, V.; Ramrakhia, S.; Naz, S.; Jamil, A.; Iqbal, Z.Q.; Kumar, B. Causes of Acute Peritonitis and Its Complication. Cureus 2021, 13, e15301. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Razick, D.I.; Le, N.; Akhtar, M.; Jakobsen, J. Culture-Negative Fibrinous Peritonitis in a Postpartum Female. Cureus 2023, 15, e43339. [Google Scholar] [CrossRef] [PubMed]
- Kairys, N.; Roepke, C. Tubo-Ovarian Abscess. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Malvezzi, H.; Marengo, E.B.; Podgaec, S.; Piccinato, C.A. Endometriosis: Current challenges in modeling a multifactorial disease of unknown etiology. J. Transl. Med. 2020, 18, 311. [Google Scholar] [CrossRef]
- Ellis, K.; Munro, D.; Clarke, J. Endometriosis Is Undervalued: A Call to Action. Front. Glob. Womens Health 2022, 3, 902371. [Google Scholar] [CrossRef]
- Wu, S.; Hugerth, L.W.; Schuppe-Koistinen, I.; Du, J. The right bug in the right place: Opportunities for bacterial vaginosis treatment. NPJ Biofilms Microbiomes 2022, 8, 34. [Google Scholar] [CrossRef]
- Abou Chacra, L.; Fenollar, F.; Diop, K. Bacterial Vaginosis: What Do We Currently Know? Front. Cell Infect. Microbiol. 2021, 11, 672429. [Google Scholar] [CrossRef] [PubMed]
- Kairys, N.; Garg, M. Bacterial Vaginosis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Khedkar, R.; Pajai, S. Bacterial Vaginosis: A Comprehensive Narrative on the Etiology, Clinical Features, and Management Approach. Cureus 2022, 14, e31314. [Google Scholar] [CrossRef] [PubMed]
- Alan, S. Rare and Underappreciated Causes of Polycystic Ovarian Syndrome. In Polycystic Ovary Syndrome; Wang, Z., Ed.; IntechOpen: London, UK, 2022; Chapter 4. [Google Scholar]
- Kang, W.; Jia, Z.; Tang, D.; Zhang, Z.; Gao, H.; He, K.; Feng, Q. Fusobacterium nucleatum Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-kappaB Signaling Pathways in Human Gingival Fibroblasts. Oxid. Med. Cell Longev. 2019, 2019, 1681972. [Google Scholar] [CrossRef]
- Tawfeq, N.A.; Saleh, G.M. The Bacterium Fusobacterium sp. May Interfere with Conception. Iraqi J. Agric. Sci. 2020, 51, 903–915. [Google Scholar] [CrossRef]
- Chen, P.C.; Li, P.C.; Ding, D.C. Pelvic inflammatory disease and causative pathogens in older women in a medical center in eastern Taiwan: A retrospective cross-sectional study. PLoS ONE 2021, 16, e0257627. [Google Scholar] [CrossRef]
- Yan, C.; Diao, Q.; Zhao, Y.; Zhang, C.; He, X.; Huang, R.; Li, Y. Fusobacterium nucleatum infection-induced neurodegeneration and abnormal gut microbiota composition in Alzheimer’s disease-like rats. Front. Neurosci. 2022, 16, 884543. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Du, X.P.; Qiao, C.F. Successful laparoscopic resection of fallopian tube abscess caused by Escherichia coli in a 12-year-old adolescent virgin:a case report and review of the literature. BMC Pediatr. 2023, 23, 282. [Google Scholar] [CrossRef]
- Prem Kumar, R.; Sunith, R.; Rajanna, R. Bovine endometritis: A review article. Pharma Innov. 2020, 9, 55–58. [Google Scholar]
- Umar, T.; Yin, B.; Umer, S.; Ma, X.; Jiang, K.; Umar, Z.; Akhtar, M.; Shaukat, A.; Deng, G. MicroRNA: Could It Play a Role in Bovine Endometritis? Inflammation 2021, 44, 1683–1695. [Google Scholar] [CrossRef]
- Yin, B.; Umar, T.; Ma, X.; Chen, Y.; Umar, Z.; Umer, S.; Deng, G. Andrograpanin mitigates lipopolysaccharides induced endometritis via TLR4/NF-kappaB pathway. Reprod. Biol. 2022, 22, 100606. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Qu, Q.; Zhang, W.; Wang, X.; Fang, J.; Xue, J.; Liu, Z.; He, S. NRF2 Exerts Anti-Inflammatory Effects in LPS-Induced gEECs by Inhibiting the Activation of the NF-kappaB. Mediators Inflamm. 2021, 2021, 9960721. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, V.; Saini, S.; Ansari, S.; Ghai, S.; Thakur, A.; Chopra, S.; Verma, V.; Malakar, D. Allogenic adipose derived mesenchymal stem cells are effective than antibiotics in treating endometritis. Sci. Rep. 2023, 13, 11280. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Adnane, M.; Archunan, G. Significance of cervico-vaginal microbes in bovine reproduction and pheromone production—A hypothetical review. Res. Vet. Sci. 2021, 135, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Bannaga, A.; Armstrong, M.J.; Mehrzad, H.; Brown, R.M.; Tripathi, D. Small Intrahepatic Vein Budd-Chiari Syndrome Complicated by Fusobacterium nucleatum Peritonitis. ACG Case Rep. J. 2019, 6, e00121. [Google Scholar] [CrossRef] [PubMed]
- Morrall, A.; Schmidt, U. Fusobacterium Necrophorum Septicemia Secondary to an Ovarian Abscess: A Case Report. Cureus 2022, 14, e26047. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. Bacterial infection linked to endometriosis. Lancet Microbe 2023, 4, e768. [Google Scholar] [CrossRef] [PubMed]
- Graham, F. Daily briefing: Endometriosis could be linked to a bacterial infection. Nature 2023. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, J.D.; Laniewski, P.; Herbst-Kralovetz, M.M. Immunometabolic and potential tumor-promoting changes in 3D cervical cell models infected with bacterial vaginosis-associated bacteria. Commun. Biol. 2022, 5, 725. [Google Scholar] [CrossRef]
- Bernhard, V.R.; Faveri, M.; Santos, M.S.; Gomes, M.d.C.M.; Batitucci, R.G.; Tanaka, C.J.; Feres, M.; Feitosa, A. Subgingival microbial profile of women with breast cancer: A cross-sectional study. Appl. Cancer Res. 2019, 39, 13. [Google Scholar] [CrossRef]
- Almohaya, A.M.; Almutairy, T.S.; Alqahtani, A.; Binkhamis, K.; Almajid, F.M. Fusobacterium bloodstream infections: A literature review and hospital-based case series. Anaerobe 2020, 62, 102165. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Chen, J.; Lian, L.Y.; Cai, H.H.; Zeng, H.S.; Zheng, M.; Liu, M.B. Intratumoral levels and prognostic significance of Fusobacterium nucleatum in cervical carcinoma. Aging 2020, 12, 23337–23350. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yun, S.Y.; Lee, Y.; Lee, H.; Yong, D.; Lee, K. Clinical Differences in Patients Infected with Fusobacterium and Antimicrobial Susceptibility of Fusobacterium Isolates Recovered at a Tertiary-Care Hospital in Korea. Ann. Lab. Med. 2022, 42, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Asangba, A.E.; Chen, J.; Goergen, K.M.; Larson, M.C.; Oberg, A.L.; Casarin, J.; Multinu, F.; Kaufmann, S.H.; Mariani, A.; Chia, N.; et al. Diagnostic and prognostic potential of the microbiome in ovarian cancer treatment response. Sci. Rep. 2023, 13, 730. [Google Scholar] [CrossRef]
- Bernardo, G.; Le Noci, V.; Di Modica, M.; Montanari, E.; Triulzi, T.; Pupa, S.M.; Tagliabue, E.; Sommariva, M.; Sfondrini, L. The Emerging Role of the Microbiota in Breast Cancer Progression. Cells 2023, 12, 1945. [Google Scholar] [CrossRef]
- Barczynski, B.; Fraszczak, K.; Grywalska, E.; Kotarski, J.; Korona-Glowniak, I. Vaginal and Cervical Microbiota Composition in Patients with Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 8266. [Google Scholar] [CrossRef] [PubMed]
- Alkabban, F.M.; Ferguson, T. Breast Cancer. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lukanović, D.; Kobal, B.; Černe, K. Ovarian Cancer: Treatment and Resistance to Pharmacotherapy. Reprod. Med. 2022, 3, 127–140. [Google Scholar] [CrossRef]
- Arora, T.; Mullangi, S.; Lekkala, M.R. Ovarian Cancer. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cheasley, D.; Wakefield, M.J.; Ryland, G.L.; Allan, P.E.; Alsop, K.; Amarasinghe, K.C.; Ananda, S.; Anglesio, M.S.; Au-Yeung, G.; Bohm, M.; et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat. Commun. 2019, 10, 3935. [Google Scholar] [CrossRef]
- Tong, A.; Di, X.; Zhao, X.; Liang, X. Review the progression of ovarian clear cell carcinoma from the perspective of genomics and epigenomics. Front. Genet. 2023, 14, 952379. [Google Scholar] [CrossRef]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef]
- Mahdy, H.; Casey, M.J.; Crotzer, D. Endometrial Cancer. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ma, X.; Cao, D.; Zhou, H.; Wang, T.; Wang, J.; Zhang, Y.; Yu, M.; Cheng, N.; Peng, P.; Yang, J.; et al. Survival outcomes and the prognostic significance of clinicopathological features in patients with endometrial clear cell carcinoma: A 35-year single-center retrospective study. World J. Surg. Oncol. 2023, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Davidson, B.; Fadare, O.; Carlson, J.A.; Crum, C.P.; Gilks, C.B.; Irving, J.A.; Malpica, A.; Matias-Guiu, X.; McCluggage, W.G.; et al. High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S40–S63. [Google Scholar] [CrossRef] [PubMed]
- Toro-Wills, M.F.; Álvarez-Londoño, A.; Hernández-Blanquisett, A.; Marquez, F.S.; Martínez-Ávila, M.C. Endometrial carcinosarcoma: A poor prognosis debut with favourable therapeutic outcome. Ecancermedicalscience 2022, 16, 1472. [Google Scholar] [CrossRef]
- Boeckstaens, S.; Dewalheyns, S.; Heremans, R.; Vikram, R.; Timmerman, D.; Van den Bosch, T.; Verbakel, J.Y. Signs and symptoms associated with uterine cancer in pre- and postmenopausal women. Heliyon 2020, 6, e05372. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.R.; Maani, E.V.; Dunton, C.J.; Gasalberti, D.P.; Jack, B.W. Cervical Cancer. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gadkari, R.; Ravi, R.; Bhatia, J.K. Cervical Cancers: Varieties and the Lower Anogenital Squamous Terminology. Cytojournal 2022, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Shiraishi, K.; Kato, T. Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers. Cancers 2021, 13, 6351. [Google Scholar] [CrossRef]
- Chadha, J.; Nandi, D.; Atri, Y.; Nag, A. Significance of human microbiome in breast cancer: Tale of an invisible and an invincible. Semin. Cancer Biol. 2021, 70, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Alpuim Costa, D.; Nobre, J.G.; Batista, M.V.; Ribeiro, C.; Calle, C.; Cortes, A.; Marhold, M.; Negreiros, I.; Borralho, P.; Brito, M.; et al. Human Microbiota and Breast Cancer-Is There Any Relevant Link?—A Literature Review and New Horizons Toward Personalised Medicine. Front. Microbiol. 2021, 12, 584332. [Google Scholar] [CrossRef] [PubMed]
- Cullin, N.; Azevedo Antunes, C.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef]
- Despins, C.A.; Brown, S.D.; Robinson, A.V.; Mungall, A.J.; Allen-Vercoe, E.; Holt, R.A. Modulation of the Host Cell Transcriptome and Epigenome by Fusobacterium nucleatum. mBio 2021, 12, e0206221. [Google Scholar] [CrossRef]
- Issrani, R.; Reddy, R.J.; El-Metwally, T.H.; Prabhu, N. Periodontitis as a Risk Factor for Breast Cancer—What We Know Till Date? Asian Pac. J. Cancer Prev. 2021, 22, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Min, K.; Lee, E.; Kim, H.; Kim, S.; Kim, Y.; Kim, G.; Cho, B.; Jeong, C.; Kim, Y.; et al. Whole-Transcriptome Sequencing Reveals Characteristics of Cancer Microbiome in Korean Patients with GI Tract Cancer: Fusobacterium nucleatum as a Therapeutic Target. Microorganisms 2022, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Doocey, C.M.; Finn, K.; Murphy, C.; Guinane, C.M. The impact of the human microbiome in tumorigenesis, cancer progression, and biotherapeutic development. BMC Microbiol. 2022, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Gaba, F.I.; Gonzalez, R.C.; Martinez, R.G. The Role of Oral Fusobacterium nucleatum in Female Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Dent. 2022, 2022, 1876275. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tian, W.; Wei, Q.; Xu, J. Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Front. Immunol. 2022, 13, 968649. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, A.; Nuciforo, P.; Borrell, M.; Zamora, E.; Pimentel, I.; Saura, C.; Oliveira, M. The conundrum of breast cancer and microbiome—A comprehensive review of the current evidence. Cancer Treat. Rev. 2022, 111, 102470. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Byrd, D.A.; Wan, Y.; Ansong, D.; Clegg-Lamptey, J.N.; Wiafe-Addai, B.; Edusei, L.; Adjei, E.; Titiloye, N.; Dedey, F.; et al. The oral microbiome and breast cancer and nonmalignant breast disease, and its relationship with the fecal microbiome in the Ghana Breast Health Study. Int. J. Cancer 2022, 151, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- El Tekle, G.; Garrett, W.S. Bacteria in cancer initiation, promotion and progression. Nat. Rev. Cancer 2023, 23, 600–618. [Google Scholar] [CrossRef]
- Viswanathan, S.; Parida, S.; Lingipilli, B.T.; Krishnan, R.; Podipireddy, D.R.; Muniraj, N. Role of Gut Microbiota in Breast Cancer and Drug Resistance. Pathogens 2023, 12, 468. [Google Scholar] [CrossRef]
- Ponath, F.; Zhu, Y.; Cosi, V.; Vogel, J. Expanding the genetic toolkit helps dissect a global stress response in the early-branching species Fusobacterium nucleatum. Proc. Natl. Acad. Sci. USA 2022, 119, e2201460119. [Google Scholar] [CrossRef]
- Little, A.; Tangney, M.; Tunney, M.M.; Buckley, N.E. Fusobacterium nucleatum: A novel immune modulator in breast cancer? Expert. Rev. Mol. Med. 2023, 25, e15. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, Y.; Huang, Y.; Lian, J.; Wu, S.; Luo, D.; Gong, H. Fusobacterium nucleatum-derived small extracellular vesicles facilitate tumor growth and metastasis via TLR4 in breast cancer. BMC Cancer 2023, 23, 473. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Parida, S.; Sharma, D. The gut microbiota in breast cancer development and treatment: The good, the bad, and the useful! Gut Microbes 2023, 15, 2221452. [Google Scholar] [CrossRef] [PubMed]
- Nawab, S.; Bao, Q.; Ji, L.H.; Luo, Q.; Fu, X.; Fan, S.; Deng, Z.; Ma, W. The Pathogenicity of Fusobacterium nucleatum Modulated by Dietary Fibers-A Possible Missing Link between the Dietary Composition and the Risk of Colorectal Cancer. Microorganisms 2023, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Dharavath, B.; Manavalan, S.; Rane, A.; Redhu, A.K.; Sunder, R.; Butle, A.; Mishra, R.; Joshi, A.; Togar, T.; et al. Fusobacterium nucleatum is associated with inflammation and poor survival in early-stage HPV-negative tongue cancer. NAR Cancer 2022, 4, zcac006. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Chen, Q.; Yi, L.; Xu, Z.; Cai, M.; Qin, J.; Zhang, Y.; Du, G.; Hong, J.; et al. Isolation and characterization of novel Fusobacterium nucleatum bacteriophages. Front. Microbiol. 2022, 13, 945315. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Shhadeh, A.; Maalouf, N.; Alon-Maimon, T.; Scaiewicz, V.; Bachrach, G. Multiphoton Microscopy of FITC-labelled Fusobacterium nucleatum in a Mouse in vivo Model of Breast Cancer. Bio Protoc. 2023, 13, e4635. [Google Scholar] [CrossRef]
- Van der Merwe, M.; Van Niekerk, G.; Botha, A.; Engelbrecht, A.M. The onco-immunological implications of Fusobacterium nucleatum in breast cancer. Immunol. Lett. 2021, 232, 60–66. [Google Scholar] [CrossRef]
- Wang, X.; Sun, C.; Huang, X.; Li, J.; Fu, Z.; Li, W.; Yin, Y. The Advancing Roles of Exosomes in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 731062. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, J.; Chen, F.; Zeng, Q.; Liu, W.L.; Zhang, G. Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut 2021, 70, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, X.; Zhang, M.; Shang, Z.; Luo, Z.; Wang, Y.; Gui, X.; Liu, Q.; Li, T.; Zeng, S.; et al. Fusobacterium nucleatum-induced exosomal HOTTIP promotes gastric cancer progression through the microRNA-885-3p/EphB2 axis. Cancer Sci. 2023, 114, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S.H.; Morell-Perez, C.; Wyche, T.P.; Sana, T.R.; Lieberman, L.A.; Hett, E.C. Colorectal cancer-associated anaerobic bacteria proliferate in tumor spheroids and alter the microenvironment. Sci. Rep. 2020, 10, 5321. [Google Scholar] [CrossRef] [PubMed]
- Pani, G. Fusobacterium & Co. at the Stem of Cancer: Microbe-Cancer Stem Cell Interactions in Colorectal Carcinogenesis. Cancers 2023, 15, 2583. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jia, Q.; Wang, L.; Yang, D. A case report of severe Fusobacterium nucleatum sepsis secondary to nephrectomy. BMC Infect. Dis. 2022, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bajdak-Rusinek, K.; Kupnicka, P.; Kapczuk, P.; Siminska, D.; Chlubek, D.; Baranowska-Bosiacka, I. The Role of CXCL16 in the Pathogenesis of Cancer and Other Diseases. Int. J. Mol. Sci. 2021, 22, 3490. [Google Scholar] [CrossRef] [PubMed]
- Kenda Suster, N.; Virant-Klun, I. Presence and role of stem cells in ovarian cancer. World J. Stem Cells 2019, 11, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Chen, H.; Yan, J.; Cheng, H. Emodin exerts antitumor effects in ovarian cancer cell lines by preventing the development of cancer stem cells via epithelial mesenchymal transition. Oncol. Lett. 2022, 23, 95. [Google Scholar] [CrossRef]
- Loret, N.; Denys, H.; Tummers, P.; Berx, G. The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance. Cancers 2019, 11, 838. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, C.; Yue, C.; Liu, X. Fusobacterium nucleatum produces cancer stem cell characteristics via EMT-resembling variations. Int. J. Clin. Exp. Pathol. 2020, 13, 1819–1828. [Google Scholar] [PubMed]
- Chen, S.; Su, T.; Zhang, Y.; Lee, A.; He, J.; Ge, Q.; Wang, L.; Si, J.; Zhuo, W.; Wang, L. Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7. Gut Microbes 2020, 11, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, H.; Huang, Q.; Wu, J.; Zhang, M. A prognostic model based on immune-related long noncoding RNAs for patients with epithelial ovarian cancer. J. Ovarian Res. 2022, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Liu, T.; Wang, M.Y.; Yang, Y.J.; Zhang, Z.D.; Liu, Z.J.; Yang, B. KRT7 promotes epithelial-mesenchymal transition in ovarian cancer via the TGF-beta/Smad2/3 signaling pathway. Oncol. Rep. 2021, 45, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Motoyama, S.; Wakita, A.; Kawakita, Y.; Liu, J.; Nagaki, Y.; Nanjo, H.; Ito, S.; Terata, K.; Imai, K.; et al. High TLR4 expression predicts a poor prognosis after esophagectomy for advanced thoracic esophageal squamous cell carcinoma. Esophagus 2020, 17, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Liao, Q.; Zhao, Y. Pancreatic Cancer, Gut Microbiota, and Therapeutic Efficacy. J. Cancer 2020, 11, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; McAllister, M.; Nagaraju, R.; Badran, S.; Edwards, J.; McBain, A.J.; Barriuso, J.; Aziz, O. The intestinal microbiota in colorectal cancer metastasis—Passive observer or key player? Crit. Rev. Oncol. Hematol. 2022, 180, 103856. [Google Scholar] [CrossRef] [PubMed]
- Hakimjavadi, H.; George, S.H.; Taub, M.; Dodds, L.V.; Sanchez-Covarrubias, A.P.; Huang, M.; Pearson, J.M.; Slomovitz, B.M.; Kobetz, E.N.; Gharaibeh, R.; et al. The vaginal microbiome is associated with endometrial cancer grade and histology. Cancer Res. Commun. 2022, 2, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Imai, K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin. Oncol. 2019, 46, 261–270. [Google Scholar] [CrossRef]
- Singh Toor, S.; Kinra, P.; Kumar, A.; Hothi, O.S. Microsatellite Instability in Endometrial Carcinoma. Ann. Pathol. Lab. Med. 2020, 7, A545–A550. [Google Scholar] [CrossRef]
- Atjimakul, T.; Wattanapaisal, P.; Suwiwat, S.; Wanichsuwan, W.; Hanprasertpong, J. Microsatellite instability and oncological outcomes in Thai patients with endometrial cancer. J. Obstet. Gynaecol. 2022, 42, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Koi, M.; Takeda, K.; Ross, R.; Mukherjee, B.; Koeppe, E.; Stoffel, E.M.; Galanko, J.A.; McCoy, A.N.; Keku, T.O.; et al. Fusobacterium nucleatum infection correlates with two types of microsatellite alterations in colorectal cancer and triggers DNA damage. Gut Pathog. 2020, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, K.A.; Cho, H.Y.; Kim, D.; Kim, W.K.; Yong, D.; Lee, H.; Yoon, S.S.; Han, D.H.; Han, Y.D.; et al. Association between Fusobacterium nucleatum and patient prognosis in metastatic colon cancer. Sci. Rep. 2021, 11, 20263. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, C.Y.; Lau, H.C.; Huang, Q.; Gong, H.; Sun, J.; Cao, P.; Hu, C.; Zhang, M.; Tao, L.; Zhou, L. Fusobacterium nucleatum impairs DNA mismatch repair and stability in patients with squamous cell carcinoma of the head and neck. Cancer 2022, 128, 3170–3184. [Google Scholar] [CrossRef] [PubMed]
- Jian, F.; Che, X.; Zhang, J.; Liu, C.; Liu, G.; Tang, Y.; Feng, W. The long-noncoding RNA SOCS2-AS1 suppresses endometrial cancer progression by regulating AURKA degradation. Cell Death Dis. 2021, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wan, J.; Chu, J. Long non-coding RNAs and endometrial cancer. Biomed. Pharmacother. 2019, 119, 109396. [Google Scholar] [CrossRef] [PubMed]
- Sadłecki, P.; Jóźwicki, J.; Antosik, P.; Walentowicz-Sadłecka, M. Expression of Selected Epithelial-Mesenchymal Transition Transcription Factors in Endometrial Cancer. Biomed. Res. Int. 2020, 2020, 4584250. [Google Scholar] [CrossRef]
- Gelissen, J.H.; Huang, G.S. Intersections of endocrine pathways and the epithelial mesenchymal transition in endometrial cancer. Front. Oncol. 2022, 12, 914405. [Google Scholar] [CrossRef]
- Lu, X.; Xu, Q.; Tong, Y.; Zhang, Z.; Dun, G.; Feng, Y.; Tang, J.; Han, D.; Mao, Y.; Deng, L.; et al. Long non-coding RNA EVADR induced by Fusobacterium nucleatum infection promotes colorectal cancer metastasis. Cell Rep. 2022, 40, 111127. [Google Scholar] [CrossRef]



| SL. No. | Article Type | Country of Report | Year of Publication | Specific Findings/Main Highlights | References |
|---|---|---|---|---|---|
| 1 | Research article | Thailand | 2019 | Association between F. nucleatum and spontaneous abortions in individuals with periodontitis. | Chanomethaporn et al. [27] |
| 2 | Case report | Canada | 2019 | Association between F. nucleatum and a symptomatic case of acute chorioamnionitis and PROM; first report to use a specific molecular technique in neonatal death investigation. | Chan et al. [76] |
| 3 | Research article | USA | 2019 | Omega-3 fatty acid is a promising prophylactic therapy to protect against intrauterine infections, as it has been shown to suppress F. nucleatum-induced placental inflammation, both in pregnant mice and in vitro, using human umbilical cord endothelial cells. | Garcia-So et al. [98] |
| 4 | Research article | Japan | 2020 | A significant association between F. nucleatum and TPL in placental tissues. | Ye et al. [77] |
| 5 | Research article | USA | 2020 | A greater prevalence of F. nucleatum in the vaginal microbiome of African American women is capable of initiating the inflammatory response that might result in preterm birth. | Walsh et al. [79] |
| 6 | Research article | Korea | 2021 | F. nucleatum infection induces innate inflammatory responses in macrophages hDSCs and results in APOs by induction of aberrant production of cytokines and chemokines through NOD1/NOD2-Ripk2-mediated signaling, suggesting Ripk2 signaling as a potential preventive and therapeutic target against APOs. | Park et al. [28] |
| 7 | Research article | Australia | 2021 | Significant improvement in risk prediction for spontaneous preterm birth by inclusion of F. nucleatum in test algorithm. | Payne et al. [66] |
| 8 | Research article | USA | 2021 | The adhesin RadD, a major virulence factor of F. nucleatum, not only mediates polymicrobial interaction (or coaggregation) but is also critical in a mouse model of preterm birth. | Wu et al. [68] |
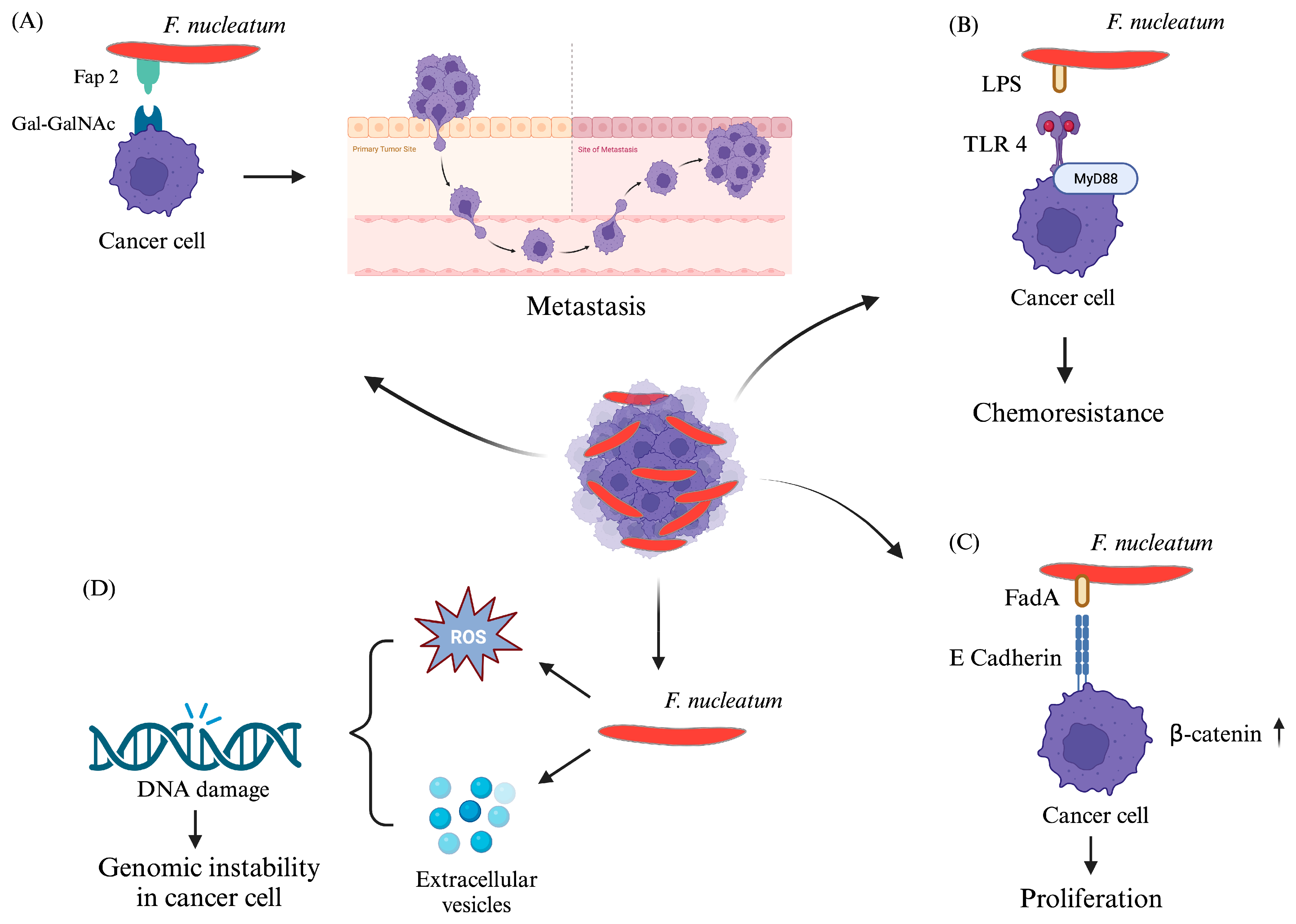
| 9 | Research article | Israel | 2022 | Galactose-sensitive adhesin, Fap2 of F. nucleatum contributes to its virulence for successful colonization in the placenta by selectively binding to Gal-GalNAc, also called T antigen as this antigen is over-displayed during fetal development. | Parhi et al. [29] |
| 10 | Research article | USA | 2022 | F. nucleatum has been detected as the most prevalent species in cord blood in early preterm live birth cases. | Vander Haar et al. [74] |
| 11 | Research article | China | 2022 | F. nucleatum in the vagina can serve as a potential biomarker for APO. | Sun et al. [78] |
| 12 | Research article | USA | 2022 | F. nucleatum possesses a multigene locus encoding a fused MsrAB and the associated factors Trx/CcdA that help the bacteria colonize the placenta and spread to the amniotic fluid to induce preterm birth in a murine model. | Scheible et al. [97] |
| 13 | Research article | Brazil | 2023 | A significantly higher proportion of F. nucleatum in the subgingival biofilm of the women with gestational age < 37 weeks compared to those with gestational age ≥ 37 weeks. | Lima et al. [67] |
| 14 | Case report | Italy | 2023 | A minor dental procedure may contribute to the development of F. nucleatum-associated chorioamnionitis and PPROMwithout any prior symptoms in the mother. | Bonasoni et al. [75] |
| SL. No. | Article Type | Country of Report | Year of Publication | Specific Findings/Main Highlights | References |
|---|---|---|---|---|---|
| 1 | Case report | UK | 2019 | F. nucleatum-induced peritonitis can complicate a clinical case of small hepatic vein” BCS in a symptomatic Pakistani woman. | Bannaga et al. [138] |
| 2 | Research article | USA | 2020 | F. nucleatum has a mutualistic relationship with the BV-associated bacteria such as Gardnerella vaginalis as they are major sialidase producers, enabling F. nucleatum to consume sialic acids from the host-produced mucus, thereby supporting colonization and vaginal dysbiosis. | Agarwal et al. [103] |
| 3 | Case report | Canada | 2021 | Association between F. nucleatum and a symptomatic case of chronic endometritis. | Mercer et al. [101] |
| 4 | Research article | USA | 2022 | The use of a multi-omics approach with 3-D cervical epithelial cell culture model reveals pro-inflammatory and metabolic changes (hallmarks of cancer) elicited by BV-associated F. nucleatum. | Maarsingh et al. [142] |
| 5 | Research article | India | 2023 | Significantly higher levels of F. nucleatum in the subgingival plaque samples in patients with PCOS and periodontitis and patients with PCOS and gingivitis, compared to the healthy individuals are indicative of the association between PCOS and oral microflora. | Achu Joseph et al. [31] |
| 6 | Research article | Japan | 2023 | Identification of a novel pathogenic mechanism of endometriosis involving F. nucleatum infection in the endometrium and its eradication by specific antibiotics against this bacterium that can serve as an attractive option for the treatment of endometriosis. | Muraoka et al. [30] |
| SL. No. | Article Type | Country of Report | Year of Publication | Specific Findings/Main Highlights | References |
|---|---|---|---|---|---|
| 1 | Research article | Brazil | 2019 | F. nucleatum-induced chronic periodontitis, causing chronic inflammation may indirectly contribute to BC through different mechanisms. | Bernhard et al. [143] |
| 2 | Research article | Israel | 2020 | An analysis of more than 1000 tumor samples of seven cancer types, and adjacent noncancerous tissues, identifies tumor-type-specific microbiomes composed mostly of intracellular bacteria with F. nucleatum being concomitantly associated with BC. | Nejman et al. [182] |
| 3 | Research article | Israel | 2020 | High Gal-GalNAc level in BC tissues acts as an oncoantigen and plays a critical role by serving as a ligand to Fap2 adhesin of F. nucleatum, supporting colonization, promoting mammary tumor growth and progression and thereby indicating Fap2 as a potential drug target. | Parhi et al. [14] |
| 4 | Case series | Saudi Arabia | 2020 | F. nucleatum-associated bacteremia in a 72-year-old female patient with metastatic OC, dies within 30 days of detection, despite receiving 14 days of antibiotic treatment. | Almohaya et al. [144] |
| 5 | Research article | China | 2020 | Distinctively high levels of F. nucleatum in CC, especially in relapsed disease, CC cells with high burdens of F. nucleatum intratumoral infiltration exhibiting CSC characteristics and patients with high burdens of intratumorally infiltrated F. nucleatum displaying poor rates of both overall survival and PFS, thereby suggesting that F. nucleatum might be one potential CC diagnostic and prognostic biomarker. | Huang et al. [145] |
| 6 | Research article | India | 2022 | The use of a computational tool named IPD to identify infectious pathogens from heterogeneous NGS datasets does not reveal the enrichment of F. nucleatum in breast transcriptome samples. | Desai et al. [184] |
| 7 | Research article | Korea | 2022 | F. nucleatum infection in an OC patient, with isolates susceptible to all 10 antimicrobial agents tested. | Kim et al. [146] |
| 8 | Research article | Florida | 2022 | A significantly greater abundance of F. nucleatum in the vaginal samples of high-grade EC patients together with the non-significant increase in the low-grade EC patients, compared to those in benign individuals, suggest the bacterium’s role in tumor growth. | Hakimjavadi et al. [205] |
| 9 | Research article | USA | 2022 | The use of the multi-omics approach with 3-D cervical epithelial cell culture model reveals that F. nucleatum infection can promote HPV infection and persistence and consequently cervical neoplasia by generating pro-inflammatory responses and upregulating the metabolic hallmarks of CC. | Maarsingh et al. [142] |
| 10 | Research article | China | 2023 | F. nucleatum-derived small EVs can promote and enhance malignant manifestations of BC such as proliferation, migration, and invasion via TLR4. | Li et al. [179] |
| 11 | Research article | USA | 2023 | Enrichment of F. nucleatum, in patients with other OC histologies in comparison to the serous OC patients, within the whole OC cohort. | Asangba et al. [147] |
| 12 | Research article | Poland | 2023 | The vaginal and cervical microbiome of women with EC are enriched with F. nucleatum and this suggests that this bacterium is a potential endometrial cause/co-factor to promote/stimulate endometrial carcinogenesis. | Barczynski et al. [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, A.; Jaaback, K.; Boulton, A.; Wong-Brown, M.; Raymond, S.; Dutta, P.; Bowden, N.A.; Ghosh, A. Fusobacterium nucleatum: An Overview of Evidence, Demi-Decadal Trends, and Its Role in Adverse Pregnancy Outcomes and Various Gynecological Diseases, including Cancers. Cells 2024, 13, 717. https://doi.org/10.3390/cells13080717
Ghosh A, Jaaback K, Boulton A, Wong-Brown M, Raymond S, Dutta P, Bowden NA, Ghosh A. Fusobacterium nucleatum: An Overview of Evidence, Demi-Decadal Trends, and Its Role in Adverse Pregnancy Outcomes and Various Gynecological Diseases, including Cancers. Cells. 2024; 13(8):717. https://doi.org/10.3390/cells13080717
Chicago/Turabian StyleGhosh, Arunita, Ken Jaaback, Angela Boulton, Michelle Wong-Brown, Steve Raymond, Partha Dutta, Nikola A. Bowden, and Arnab Ghosh. 2024. "Fusobacterium nucleatum: An Overview of Evidence, Demi-Decadal Trends, and Its Role in Adverse Pregnancy Outcomes and Various Gynecological Diseases, including Cancers" Cells 13, no. 8: 717. https://doi.org/10.3390/cells13080717