AAV-Mediated Restoration of Dystrophin-Dp71 in the Brain of Dp71-Null Mice: Molecular, Cellular and Behavioral Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. AAV-Vector Administration and Experimental Groups
2.3. Real-Time Quantitative PCR
2.4. Western Blots
2.5. Immunochemistry
2.6. Confocal Microscopy
2.7. Behavioral Testing
2.8. Statistics
3. Results
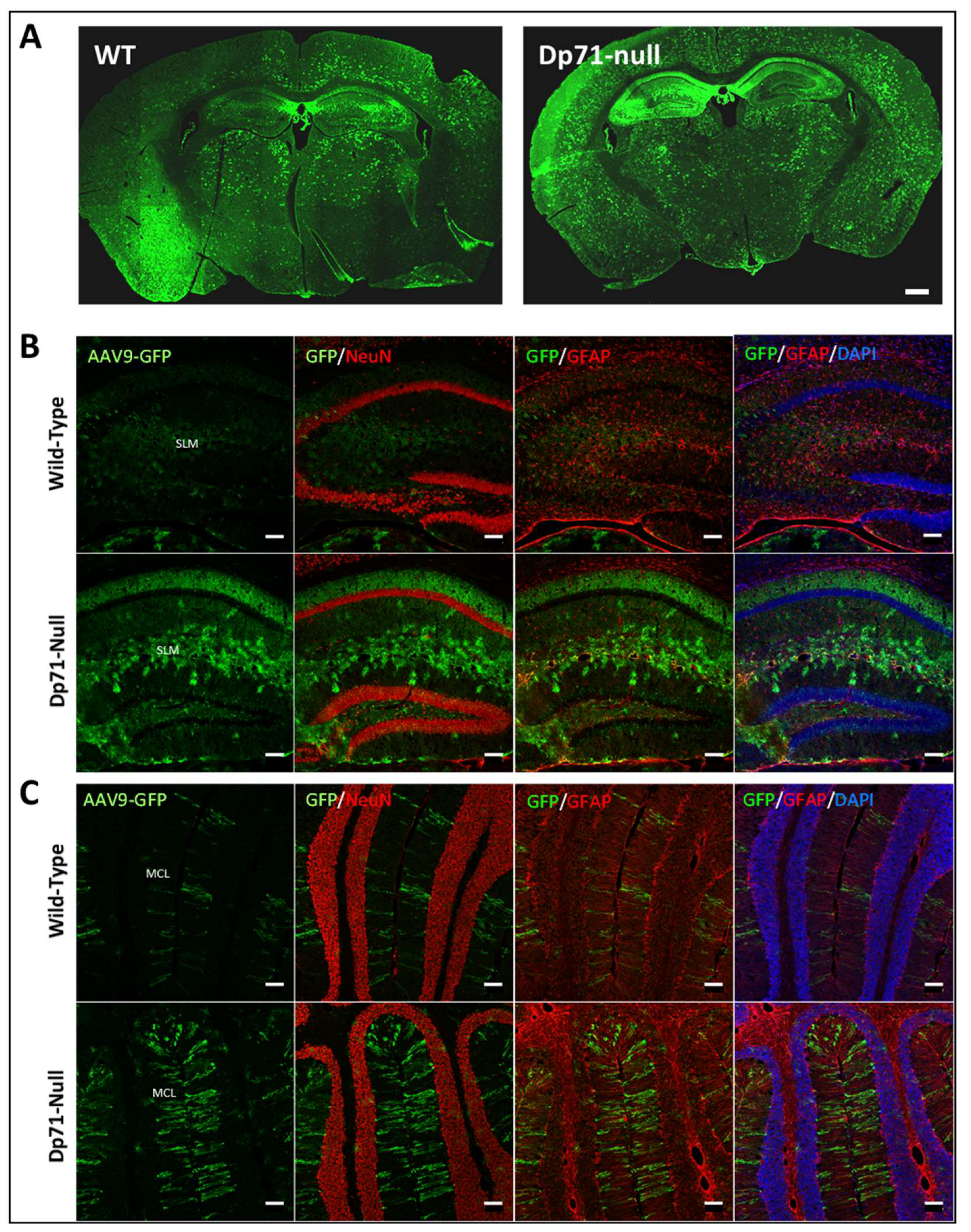
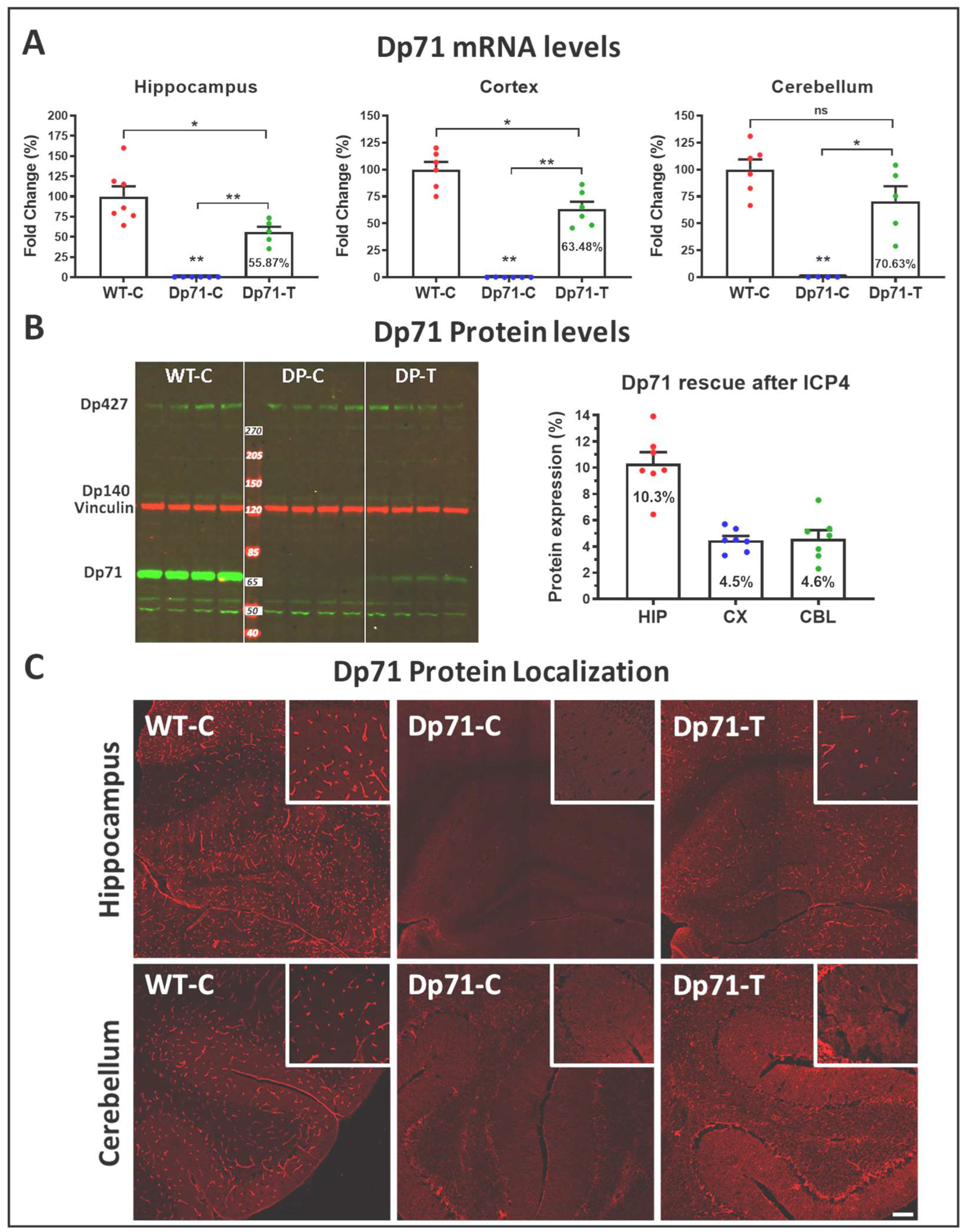
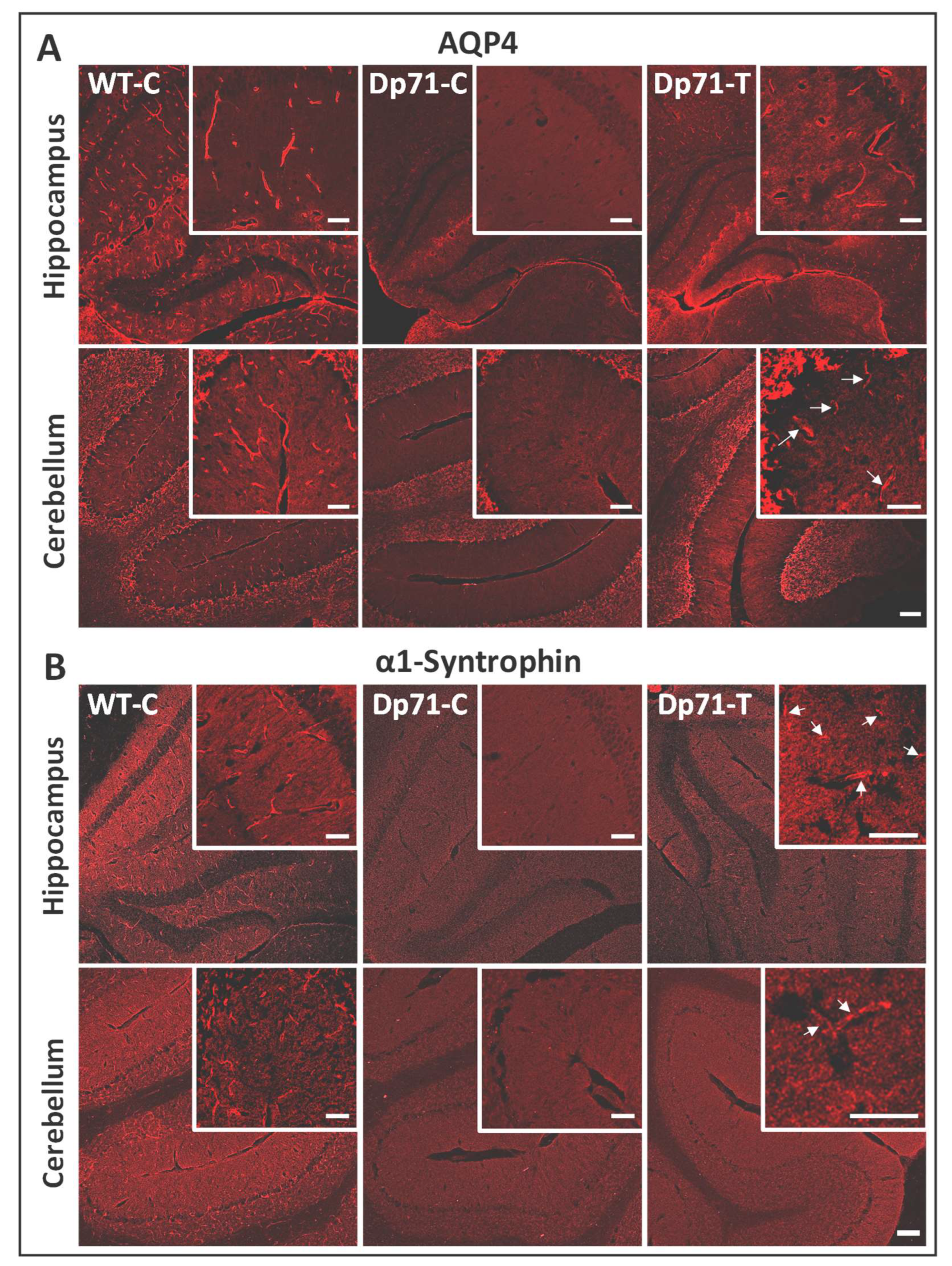
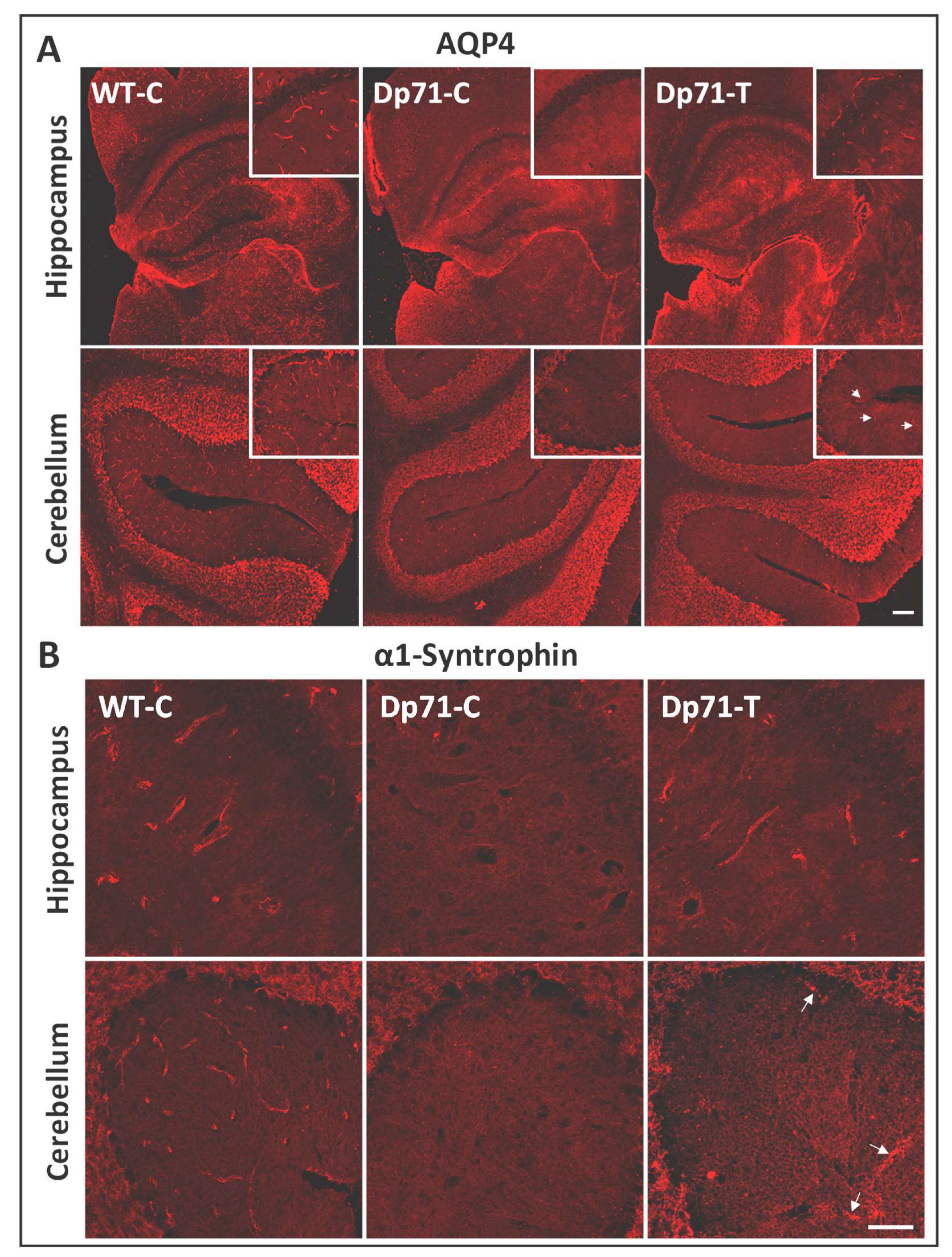
3.1. Rescue of Dp71 and Associated Proteins following Neonatal Intracardiac Injection
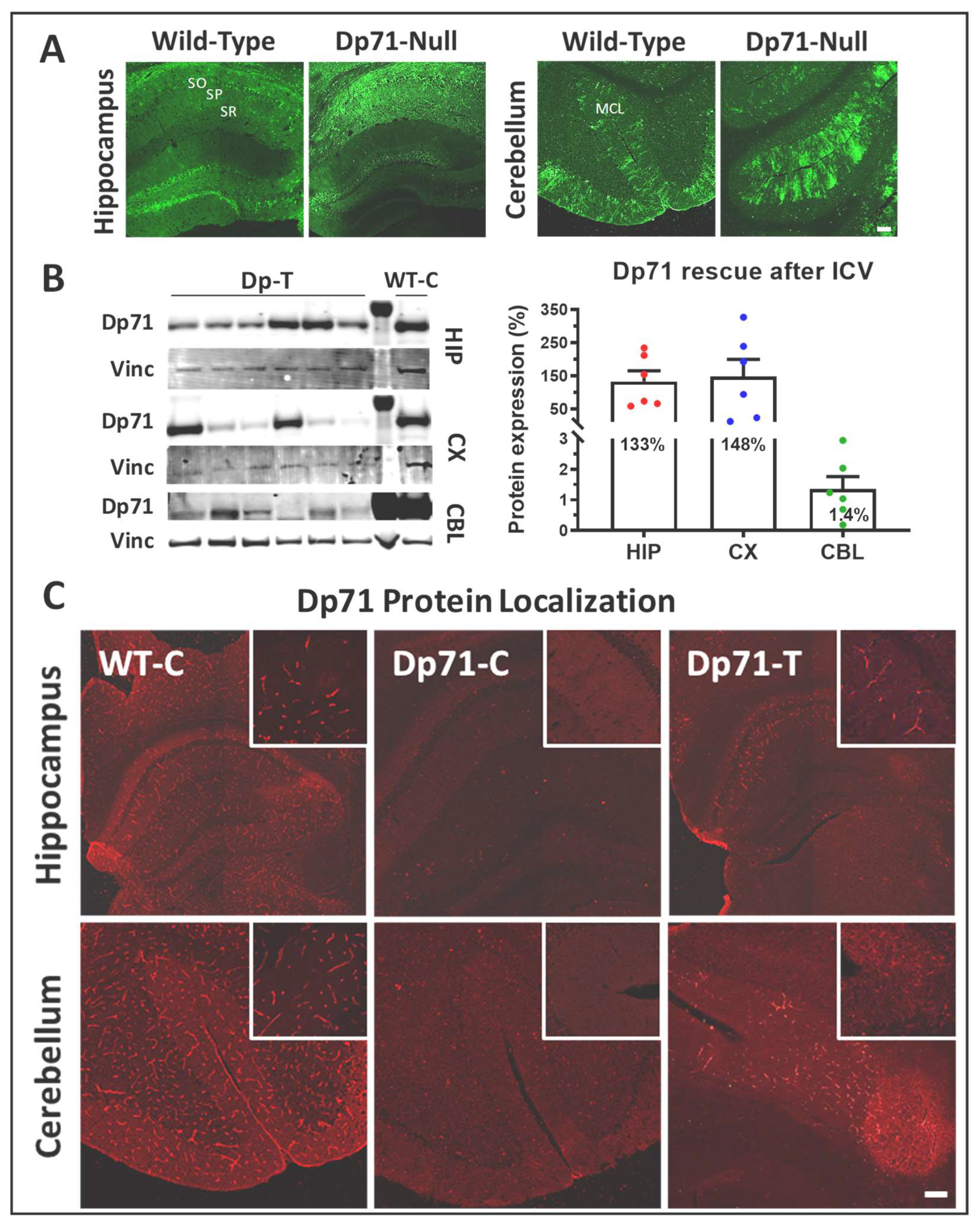
3.2. Rescue of Dp71 and Associated Proteins following Intracerebroventricular Injection in Adults
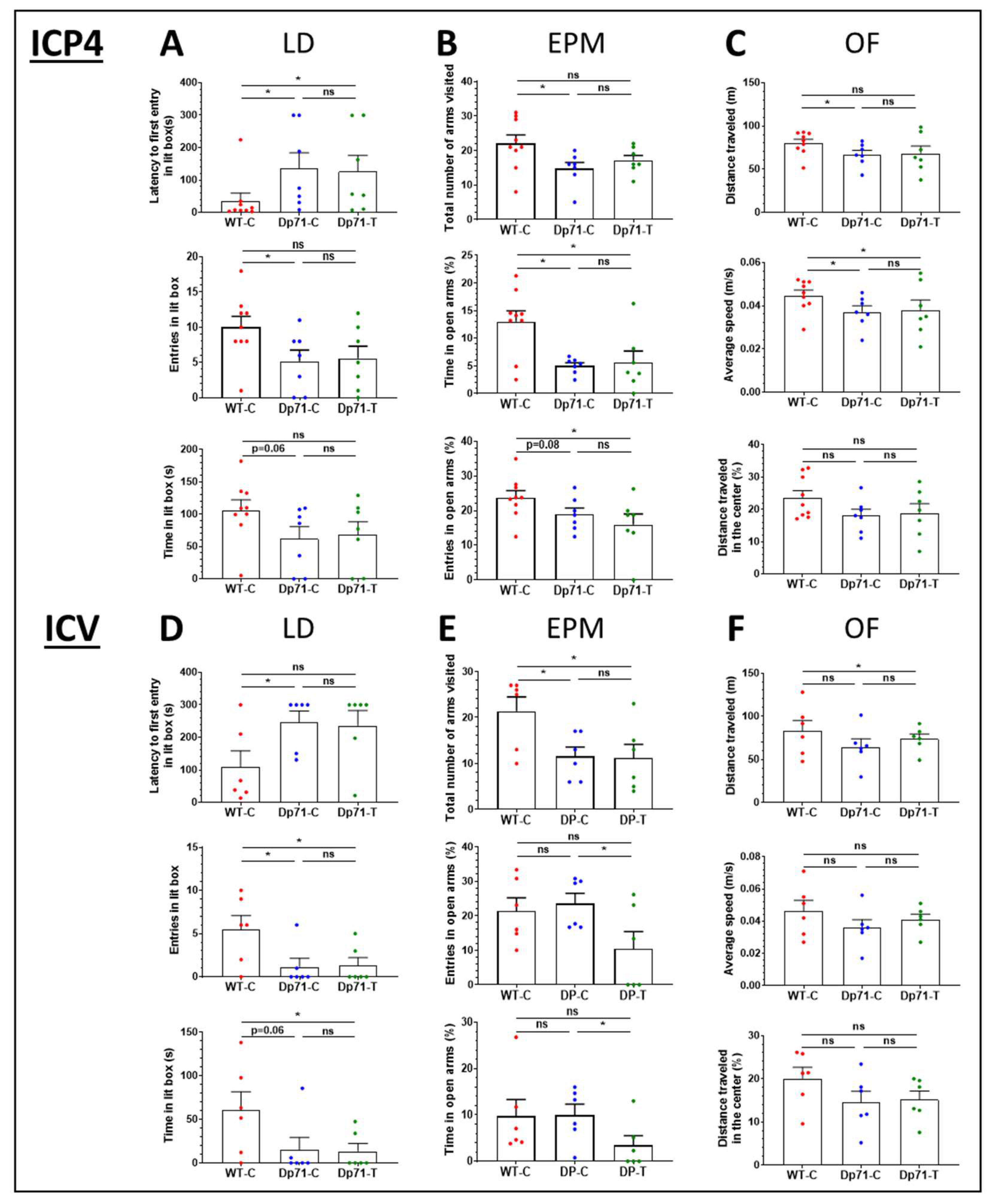
3.3. Impact of Dp71 Restoration on Behavioral Disturbances
3.3.1. Intracardiac Treatment at Postnatal Day 4 (P4)
3.3.2. Intracerebroventricular Treatment in Adult Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricotti, V.; Mandy, W.P.; Scoto, M.; Pane, M.; Deconinck, N.; Messina, S.; Mercuri, E.; Skuse, D.H.; Muntoni, F. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child Neurol. 2016, 58, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Waite, A.; Tinsley, C.L.; Locke, M.; Blake, D.J. The neurobiology of the dystrophin-associated glycoprotein complex. Ann. Med. 2009, 41, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Desguerre, I.; Christov, C.; Mayer, M.; Zeller, R.; Becane, H.M.; Bastuji-Garin, S.; Leturcq, F.; Chiron, C.; Chelly, J.; Gherardi, R.K. Clinical heterogeneity of duchenne muscular dystrophy (DMD): Definition of sub-phenotypes and predictive criteria by long-term follow-up. PLoS ONE 2009, 4, e4347. [Google Scholar] [CrossRef] [PubMed]
- Daoud, F.; Angeard, N.; Demerre, B.; Martie, I.; Benyaou, R.; Leturcq, F.; Cossee, M.; Deburgrave, N.; Saillour, Y.; Tuffery, S.; et al. Analysis of Dp71 contribution in the severity of mental retardation through comparison of Duchenne and Becker patients differing by mutation consequences on Dp71 expression. Hum. Mol. Genet. 2009, 18, 3779–3794. [Google Scholar] [CrossRef] [PubMed]
- Daoud, F.; Candelario-Martinez, A.; Billard, J.M.; Avital, A.; Khelfaoui, M.; Rozenvald, Y.; Guegan, M.; Mornet, D.; Jaillard, D.; Nudel, U.; et al. Role of mental retardation-associated dystrophin-gene product Dp71 in excitatory synapse organization, synaptic plasticity and behavioral functions. PLoS ONE 2009, 4, e6574. [Google Scholar] [CrossRef] [PubMed]
- Haenggi, T.; Soontornmalai, A.; Schaub, M.C.; Fritschy, J.M. The role of utrophin and Dp71 for assembly of different dystrophin-associated protein complexes (DPCs) in the choroid plexus and microvasculature of the brain. Neuroscience 2004, 129, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Rossi, A.; Nudel, U.; Svelto, M.; Frigeri, A. Dystrophin-dependent and -independent AQP4 pools are expressed in the mouse brain. Glia 2008, 56, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Belmaati Cherkaoui, M.; Vacca, O.; Izabelle, C.; Boulay, A.C.; Boulogne, C.; Gillet, C.; Barnier, J.V.; Rendon, A.; Cohen-Salmon, M.; Vaillend, C. Dp71 contribution to the molecular scaffold anchoring aquaporine-4 channels in brain macroglial cells. Glia 2021, 69, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Vacca, O.; Charles-Messance, H.; El Mathari, B.; Sene, A.; Barbe, P.; Fouquet, S.; Aragon, J.; Darche, M.; Giocanti-Auregan, A.; Paques, M.; et al. AAV-mediated gene therapy in Dystrophin-Dp71 deficient mouse leads to blood-retinal barrier restoration and oedema reabsorption. Hum Mol Genet 2016, 25, 3070–3079. [Google Scholar] [CrossRef]
- Naidoo, M.; Anthony, K. Dystrophin Dp71 and the Neuropathophysiology of Duchenne Muscular Dystrophy. Mol. Neurobiol. 2020, 57, 1748–1767. [Google Scholar] [CrossRef]
- Wijekoon, N.; Gonawala, L.; Ratnayake, P.; Amaratunga, D.; Hathout, Y.; Mohan, C.; Steinbusch, H.W.M.; Dalal, A.; Hoffman, E.P.; de Silva, K.R.D. Duchenne Muscular Dystrophy from Brain to Muscle: The Role of Brain Dystrophin Isoforms in Motor Functions. J. Clin. Med. 2023, 12, 5637. [Google Scholar] [CrossRef] [PubMed]
- Connors, N.C.; Adams, M.E.; Froehner, S.C.; Kofuji, P. The potassium channel Kir4.1 associates with the dystrophin-glycoprotein complex via alpha-syntrophin in glia. J. Biol. Chem. 2004, 279, 28387–28392. [Google Scholar] [CrossRef] [PubMed]
- Galaz-Vega, R.; Hernandez-Kelly, L.C.; Mendez, J.A.; Cisneros, B.; Ortega, A. Glutamate regulates dystrophin-71 levels in glia cells. Neurochem. Res. 2005, 30, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fort, P.E.; Sene, A.; Pannicke, T.; Roux, M.J.; Forster, V.; Mornet, D.; Nudel, U.; Yaffe, D.; Reichenbach, A.; Sahel, J.A.; et al. Kir4.1 and AQP4 associate with Dp71- and utrophin-DAPs complexes in specific and defined microdomains of Muller retinal glial cell membrane. Glia 2008, 56, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Stam, K.; Yaoi, T.; Nakano, K.; Arai, T.; Okamura, T.; Itoh, K. Dystrophin Short Product, Dp71, Interacts with AQP4 and Kir4.1 Channels in the Mouse Cerebellar Glial Cells in Contrast to Dp427 at Inhibitory Postsynapses in the Purkinje Neurons. Mol. Neurobiol. 2023, 60, 3664–3677. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Nico, B.; Camassa, L.M.; Mola, M.G.; Loh, N.; Dermietzel, R.; Spray, D.C.; Svelto, M.; Frigeri, A. The role of aquaporin-4 in the blood-brain barrier development and integrity: Studies in animal and cell culture models. Neuroscience 2004, 129, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Vajda, Z.; Pedersen, M.; Fuchtbauer, E.M.; Wertz, K.; Stodkilde-Jorgensen, H.; Sulyok, E.; Doczi, T.; Neely, J.D.; Agre, P.; Frokiaer, J.; et al. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13131–13136. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Frydenlund, D.S.; Ottersen, O.P. Anchoring of aquaporin-4 in brain: Molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 2004, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Benabdesselam, R.; Sene, A.; Raison, D.; Benmessaoud-Mesbah, O.; Ayad, G.; Mornet, D.; Yaffe, D.; Rendon, A.; Hardin-Pouzet, H.; Dorbani-Mamine, L. A deficit of brain dystrophin 71 impairs hypothalamic osmostat. J. Neurosci. Res. 2010, 88, 324–334. [Google Scholar] [CrossRef]
- Lange, J.; Gillham, O.; Alkharji, R.; Eaton, S.; Ferrari, G.; Madej, M.; Flower, M.; Tedesco, F.S.; Muntoni, F.; Ferretti, P. Dystrophin deficiency affects human astrocyte properties and response to damage. Glia 2022, 70, 466–490. [Google Scholar] [CrossRef]
- Vaillend, C.; Ungerer, A. Behavioral characterization of mdx3cv mice deficient in C-terminal dystrophins. Neuromuscul. Disord. 1999, 9, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Helleringer, R.; Le Verger, D.; Li, X.; Izabelle, C.; Chaussenot, R.; Belmaati-Cherkaoui, M.; Dammak, R.; Decottignies, P.; Daniel, H.; Galante, M.; et al. Cerebellar synapse properties and cerebellum-dependent motor and non-motor performance in Dp71-null mice. Dis. Model. Mech. 2018, 11, dmm033258. [Google Scholar] [CrossRef] [PubMed]
- Chaussenot, R.; Amar, M.; Fossier, P.; Vaillend, C. Dp71-Dystrophin Deficiency Alters Prefrontal Cortex Excitation-Inhibition Balance and Executive Functions. Mol. Neurobiol. 2019, 56, 2670–2684. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarria, L.; et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243. [Google Scholar] [CrossRef] [PubMed]
- Filonova, G.; Aartsma-Rus, A. Next steps for the optimization of exon therapy for Duchenne muscular dystrophy. Expert Opin. Biol. Ther. 2023, 23, 133–143. [Google Scholar] [CrossRef]
- Saoudi, A.; Barberat, S.; le Coz, O.; Vacca, O.; Doisy Caquant, M.; Tensorer, T.; Sliwinski, E.; Garcia, L.; Muntoni, F.; Vaillend, C.; et al. Partial restoration of brain dystrophin by tricyclo-DNA antisense oligonucleotides alleviates emotional deficits in mdx52 mice. Mol. Ther. Nucleic Acids. 2023, 32, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Zarrouki, F.; Relizani, K.; Bizot, F.; Tensorer, T.; Garcia, L.; Vaillend, C.; Goyenvalle, A. Partial Restoration of Brain Dystrophin and Behavioral Deficits by Exon Skipping in the Muscular Dystrophy X-Linked (mdx) Mouse. Ann. Neurol. 2022, 92, 213–229. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Zushida, K.; Yoshida, M.; Maekawa, M.; Kamichi, S.; Yoshida, M.; Sahara, Y.; Yuasa, S.; Takeda, S.; Wada, K. A deficit of brain dystrophin impairs specific amygdala GABAergic transmission and enhances defensive behaviour in mice. Brain 2009, 132, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Vacca, O.; Darche, M.; Schaffer, D.V.; Flannery, J.G.; Sahel, J.A.; Rendon, A.; Dalkara, D. AAV-mediated gene delivery in Dp71-null mouse model with compromised barriers. Glia 2014, 62, 468–476. [Google Scholar] [CrossRef]
- Barboni, M.T.S.; Vaillend, C.; Joachimsthaler, A.; Liber, A.M.P.; Khabou, H.; Roux, M.J.; Vacca, O.; Vignaud, L.; Dalkara, D.; Guillonneau, X.; et al. Rescue of Defective Electroretinographic Responses in Dp71-Null Mice With AAV-Mediated Reexpression of Dp71. Investig. Ophthalmol. Vis. Sci. 2020, 61, 11. [Google Scholar] [CrossRef]
- Cearley, C.N.; Wolfe, J.H. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 2006, 13, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, K.; Muramatsu, S.I. Adeno-associated virus vector-based gene therapies for pediatric diseases. Pediatr. Neonatol. 2023, 64 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Sarig, R.; Mezger-Lallemand, V.; Gitelman, I.; Davis, C.; Fuchs, O.; Yaffe, D.; Nudel, U. Targeted inactivation of Dp71, the major non-muscle product of the DMD gene: Differential activity of the Dp71 promoter during development. Hum. Mol. Genet. 1999, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Daly, T.; Gao, C.; Flotte, T.R.; Song, S.; Byrne, B.J.; Sands, M.S.; Parker Ponder, K. CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum. Gene. Ther. 2001, 12, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Yamamura, K.; Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 108, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.; Asokan, A.; Haberman, R.A.; Samulski, R.J. Production of recombinant adeno-associated viral vectors. Curr. Protoc. Hum. Genet. 2007, 16, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Aurnhammer, C.; Haase, M.; Muether, N.; Hausl, M.; Rauschhuber, C.; Huber, I.; Nitschko, H.; Busch, U.; Sing, A.; Ehrhardt, A.; et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods 2012, 23, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1984; pp. 142–143. [Google Scholar]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009, 27, 59–65. [Google Scholar] [CrossRef]
- Lowenstein, P.R. Crossing the rubicon. Nat. Biotechnol. 2009, 27, 42–44. [Google Scholar] [CrossRef]
- Saunders, N.R.; Joakim Ek, C.; Dziegielewska, K.M. The neonatal blood-brain barrier is functionally effective, and immaturity does not explain differential targeting of AAV9. Nat. Biotechnol. 2009, 27, 804. [Google Scholar] [CrossRef] [PubMed]
- Reemst, K.; Noctor, S.C.; Lucassen, P.J.; Hol, E.M. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci. 2016, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.Y.; de Vin, F.; Duque, S.I.; Fripont, S.; Castaldo, S.A.; Bouhuijzen-Wenger, J.; Holt, M.G. Widespread transduction of astrocytes and neurons in the mouse central nervous system after systemic delivery of a self-complementary AAV-PHP.B vector. Gene Ther. 2018, 25, 83–92. [Google Scholar] [CrossRef]
- McCarty, D.M. Self-complementary AAV vectors; advances and applications. Mol. Ther. 2008, 16, 1648–1656. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, J.Y.; Madrakhimov, S.; Park, H.Y.; Park, K.; Park, T.K. Adeno-Associated Viral Vector 2 and 9 Transduction Is Enhanced in Streptozotocin-Induced Diabetic Mouse Retina. Mol. Ther. Methods Clin. Dev. 2019, 13, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Saoudi, A.; Zarrouki, F.; Sebrie, C.; Izabelle, C.; Goyenvalle, A.; Vaillend, C. Emotional behavior and brain anatomy of the mdx52 mouse model of Duchenne muscular dystrophy. Dis. Model. Mech. 2021, 14, dmm049028. [Google Scholar] [CrossRef] [PubMed]
- Maresh, K.; Papageorgiou, A.; Ridout, D.; Harrison, N.A.; Mandy, W.; Skuse, D.; Muntoni, F. Startle responses in Duchenne muscular dystrophy: A novel biomarker of brain dystrophin deficiency. Brain 2023, 146, 252–265. [Google Scholar] [CrossRef]
- Alemdaroglu-Gurbuz, I.; Ipek, C.; Bulut, N.; Karaduman, A.; Yilmaz, O. The Impact of “Fear of Falling” on Physical Performance, Balance, and Ambulation in Duchenne Muscular Dystrophy. Neuropediatrics 2022, 53, 330–337. [Google Scholar] [CrossRef]
- Austin, R.C.; Morris, G.E.; Howard, P.L.; Klamut, H.J.; Ray, P.N. Expression and synthesis of alternatively spliced variants of Dp71 in adult human brain. Neuromuscul. Disord. 2000, 10, 187–193. [Google Scholar] [CrossRef]
- Tillfors, M.; Furmark, T.; Marteinsdottir, I.; Fredrikson, M. Cerebral blood flow during anticipation of public speaking in social phobia: A PET study. Biol. Psychiatry 2002, 52, 1113–1119. [Google Scholar] [CrossRef]
- Evans, K.C.; Wright, C.I.; Wedig, M.M.; Gold, A.L.; Pollack, M.H.; Rauch, S.L. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress. Anxiety 2008, 25, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Kumano, H.; Nishikawa, M.; Sakano, Y.; Kaiya, H.; Imabayashi, E.; Ohnishi, T.; Matsuda, H.; Yasuda, A.; Sato, A.; et al. Cerebral glucose metabolism associated with a fear network in panic disorder. Neuroreport 2005, 16, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, J.; Zhang, J.; Zhan, W.; Li, L.; Wu, M.; Huang, H.; Zhu, H.; Kemp, G.J.; Gong, Q. Altered resting-state functional activity in posttraumatic stress disorder: A quantitative meta-analysis. Sci. Rep. 2016, 6, 27131. [Google Scholar] [CrossRef] [PubMed]
- Chao, O.Y.; Pathak, S.S.; Zhang, H.; Augustine, G.J.; Christie, J.M.; Kikuchi, C.; Taniguchi, H.; Yang, Y.M. Social memory deficit caused by dysregulation of the cerebellar vermis. Nat. Commun. 2023, 14, 6007. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, B.; Haenzi, B.; Hilton, S.; Carnicer-Lombarte, A.; Hobo, B.; Verhaagen, J.; Fawcett, J.W. Optimization of adeno-associated viral vector-mediated transduction of the corticospinal tract: Comparison of four promoters. Gene Ther. 2021, 28, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; Dayton, R.D.; Tatom, J.B.; Henderson, K.M.; Henning, P.P. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: Effects of serotype, promoter and purification method. Mol. Ther. 2008, 16, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Busuttil, R.W.; Lipshutz, G.S. RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J. Gene Med. 2010, 12, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Pietersz, K.L.; Plessis, F.D.; Pouw, S.M.; Liefhebber, J.M.; van Deventer, S.J.; Martens, G.J.M.; Konstantinova, P.S.; Blits, B. PhP.B Enhanced Adeno-Associated Virus Mediated-Expression Following Systemic Delivery or Direct Brain Administration. Front. Bioeng. Biotechnol. 2021, 9, 679483. [Google Scholar] [CrossRef] [PubMed]
- Surdyka, M.; Jesion, E.; Niewiadomska-Cimicka, A.; Trottier, Y.; Kalinowska-Poska, Z.; Figiel, M. Selective transduction of cerebellar Purkinje and granule neurons using delivery of AAV-PHP.eB and AAVrh10 vectors at axonal terminal locations. Front. Mol. Neurosci. 2022, 15, 947490. [Google Scholar] [CrossRef]
- de Brouwer, A.P.; Nabuurs, S.B.; Verhaart, I.E.; Oudakker, A.R.; Hordijk, R.; Yntema, H.G.; Hordijk-Hos, J.M.; Voesenek, K.; de Vries, B.B.; van Essen, T.; et al. A 3-base pair deletion, c.9711_9713del, in DMD results in intellectual disability without muscular dystrophy. Eur. J. Hum. Genet. 2014, 22, 480–485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vacca, O.; Zarrouki, F.; Izabelle, C.; Belmaati Cherkaoui, M.; Rendon, A.; Dalkara, D.; Vaillend, C. AAV-Mediated Restoration of Dystrophin-Dp71 in the Brain of Dp71-Null Mice: Molecular, Cellular and Behavioral Outcomes. Cells 2024, 13, 718. https://doi.org/10.3390/cells13080718
Vacca O, Zarrouki F, Izabelle C, Belmaati Cherkaoui M, Rendon A, Dalkara D, Vaillend C. AAV-Mediated Restoration of Dystrophin-Dp71 in the Brain of Dp71-Null Mice: Molecular, Cellular and Behavioral Outcomes. Cells. 2024; 13(8):718. https://doi.org/10.3390/cells13080718
Chicago/Turabian StyleVacca, Ophélie, Faouzi Zarrouki, Charlotte Izabelle, Mehdi Belmaati Cherkaoui, Alvaro Rendon, Deniz Dalkara, and Cyrille Vaillend. 2024. "AAV-Mediated Restoration of Dystrophin-Dp71 in the Brain of Dp71-Null Mice: Molecular, Cellular and Behavioral Outcomes" Cells 13, no. 8: 718. https://doi.org/10.3390/cells13080718







