Copper Homeostasis in the Model Organism C. elegans
Abstract
:1. Introduction
2. The Human Copper Proteome and Orthologs in C. elegans
| Enzyme Activity | HGNC Symbol a,b | UniProt-ID c | C. elegans Gene Ortholog b,d,f | Reference e |
|---|---|---|---|---|
| Amine oxidase | AOC1 | P19801 | no ortholog | [a], [b] |
| AOC2 | O75106 | |||
| AOC3 | Q16853 | |||
| Cytochrome c oxidase | MT-CO1 | P00395 | ctc-1/cox-1 | [a], [b] |
| MT-CO2 | P00403 | ctc-2/cox-2 | [a], [b] | |
| Dopamine β-hydroxylase | DBH | P09172 | tbh-1 | [a], [b] |
| MOXD1 | Q6UVY6 | [a], [b] | ||
| MOXD2P | A6NHM9 | [b] | ||
| Ecto-NOX disulfide-thiol exchanger | ENOX1 | Q8TC92 | no ortholog | [a], [b] |
| ENOX2 | Q16206 | |||
| Ferroxidase | CP | P00450 | F21D5.3 | [a], [b] |
| HEPH | Q9BQS7 | |||
| HEPHL1 | Q6MZM0 | |||
| Lysyl oxidase | LOX | P28300 | no ortholog | [a], [b] |
| LOXL1 | Q08397 | no ortholog | [a], [b] | |
| LOXL2 | Q9Y4K0 | C06B8.7 | [b] | |
| LOXL3 | P58215 | C06B8.7 | [b] | |
| LOXL4 | Q96JB6 | no ortholog | [a], [b] | |
| MAPK/ERK kinase | MAP2K1 | Q02750 | mek-2 * | [a], [b], [18] |
| Methanethiol oxidase | SELENBP1 | Q13228 | semo-1 * | [19] |
| Peptidyl-glycine α-amidating monooxygenase | PAM | P19021 | pamn-1, pgal-1, pghm-1 | [b] |
| Protein deglycase | PARK7 | Q99497 | djr-1.1, djr-1.2 | [a], [b] |
| Superoxide dismutase | SOD1 | P00441 | sod-1 *, sod-4 *, sod-5 * | [a], [b], [20] |
| SOD3 | P08294 | sod-4 *, sod-5 * | [a], [b], [20] | |
| Tyrosinase | TYR | P14679 | tyr-1 (and 5 others) | [a], [b] |
| TYRP1 | P17643 | tyr-5 (and 5 others) | [a], [b] |
3. Copper Homeostasis in C. elegans
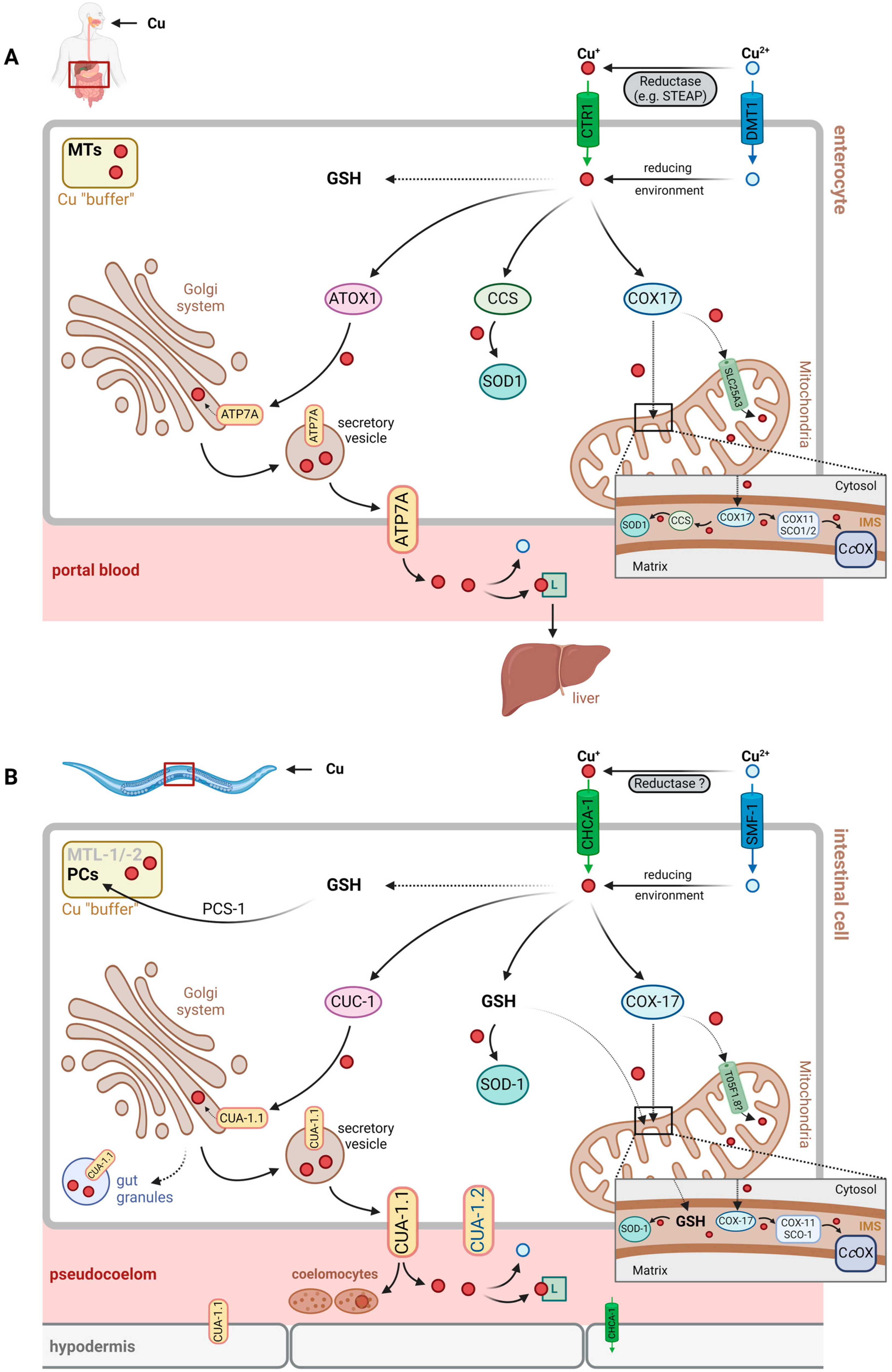
3.1. Copper Uptake and Distribution
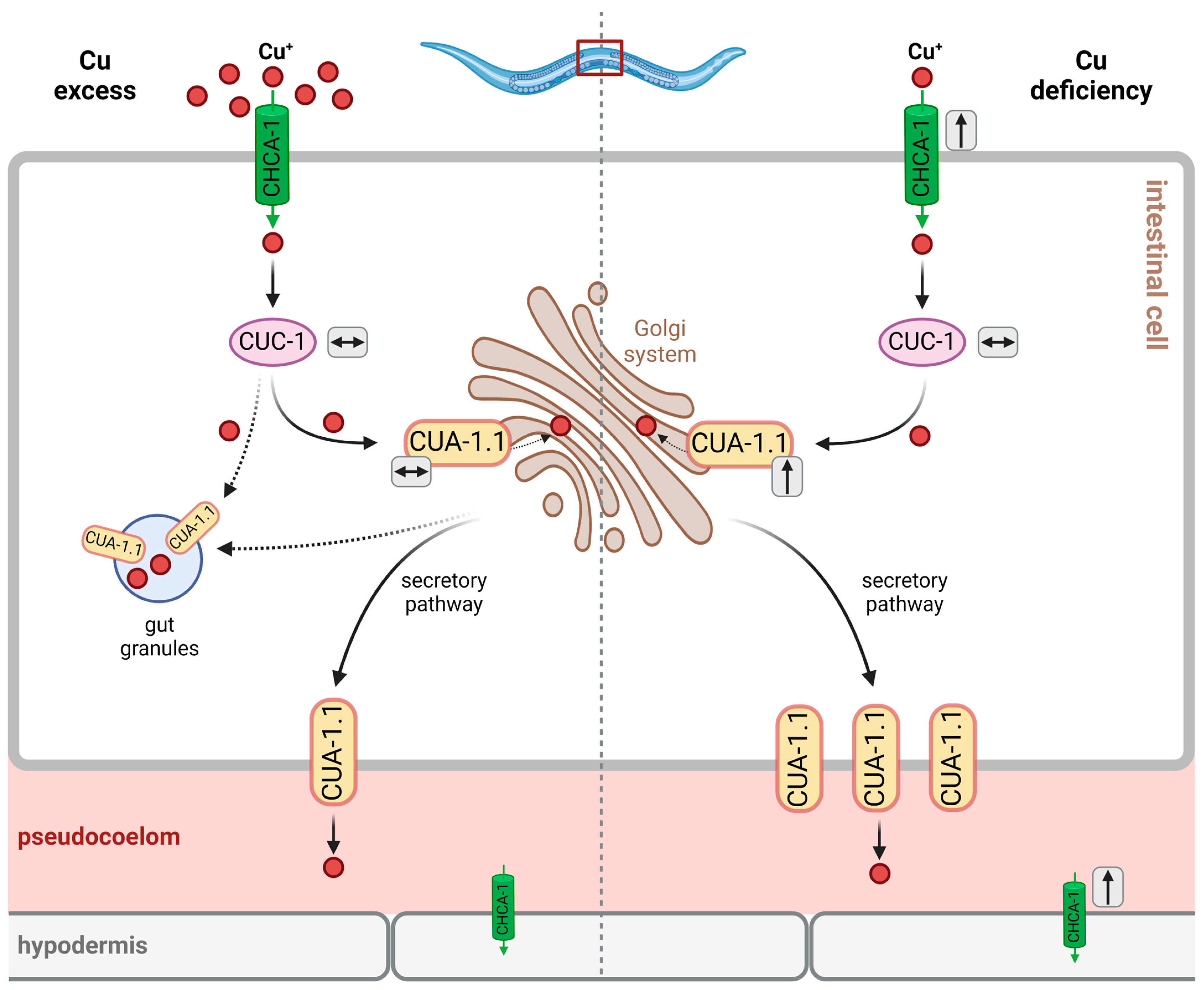
3.2. Cellular Cu Import, Distribution and Tolerance
3.2.1. Import
| HGNC Symbol a,b | UniProt-ID c | C. elegans Gene Ortholog b,d | Reference e |
|---|---|---|---|
| ATOX1 | O00244 | cuc-1 * | [37,38] |
| ATP7A | Q04656 | cua-1 * | [39] |
| ATP7B | P35670 | ||
| CCS | O14618 | no ortholog | [20] |
| COMMD1 | Q8N668 | no ortholog | [a], [b] |
| COX11 | Q9Y6N1 | cox-11 | [a], [b] |
| COX17 | Q14061 | cox-17 | [a], [b] |
| CUTC | Q9NTM9 | cutc-1 | [40] |
| SCO1 | O75880 | sco-1 | [a], [b] |
| SCO2 | O43819 | ||
| SLC25A3 | Q00325 | T05F1.8 (and 3 others) | [a], [b] |
| SLC31A1 (CTR1) | O15431 | chca-1 * | [36] |
| SLC31A2 (CTR2) | O15432 |
3.2.2. Intracellular Cu Distribution
- (A) The ATOX1→ATP7A/B Pathway in C. elegans: CUC-1→CUA-1
- (B) Glutathione-Dependent, but CCS-Independent, Cu Transfer to SODs in C. elegans
- (C) Mitochondrial Cu Chaperones
- (D) CUTC-1—A Chaperone?
3.2.3. Cellular Cu Buffers/Cu Tolerance
| HGNC Symbol a,b | UniProt-ID c | C. elegans Gene Ortholog b,d | Reference e |
|---|---|---|---|
| AFP | P02771 | no ortholog | [a], [b] |
| ALB | P02768 | no ortholog | [a], [b] |
| AHCY | P23526 | ahcy-1 | [a], [b] |
| APP | P05067 | apl-1 * | [14,74] |
| APLP2 | Q06481 | ||
| CUTA | O60888 | F35G12.7 | [a], [b] |
| F5 | P12259 | ddr-1 | [b] |
| GPC1 | P35052 | gpn-1 | [a], [b] |
| LTF | P02788 | no ortholog | [a], [b] |
| MEMO1 | Q9Y316 | memo-1 * | [75] |
| MT1 (9 isoforms) | various | mtl-1/mtl-2 * | [76,77] |
| MT2A | P02795 | ||
| MT3 | P25713 | ||
| MT4 | P47944 | ||
| MUC2 | Q02817 | T01D3.6 | [b] |
| MUC5AC | P98088 | Y69H2.10 (and 3 others) | [b] |
| MUC5B | Q9HC84 | Y69H2.10 (and 7 others) | [b] |
| PRNP | P04156 | no ortholog | [a], [b] |
| S100A12 | P80511 | no ortholog | [a], [b] |
| S100A13 | Q99584 | ||
| S100A5 | P33763 | ||
| S100B | P04271 | ||
| SNCA | P37840 | no ortholog | [a], [b] |
| SPARC | P09486 | ost-1 | [a], [b] |
- (A) Sequestration by Gut Granules
- (B) Thiol-Mediated Chelation of Cu: Metallothioneins, Phytochelatins, Glutathione
- (C) Coelomocytes Contribute to Cu Tolerance in C. elegans
3.3. From Orthologs to Conserved Mechanisms: Molecular Aspects of Intracellular Cu Transport
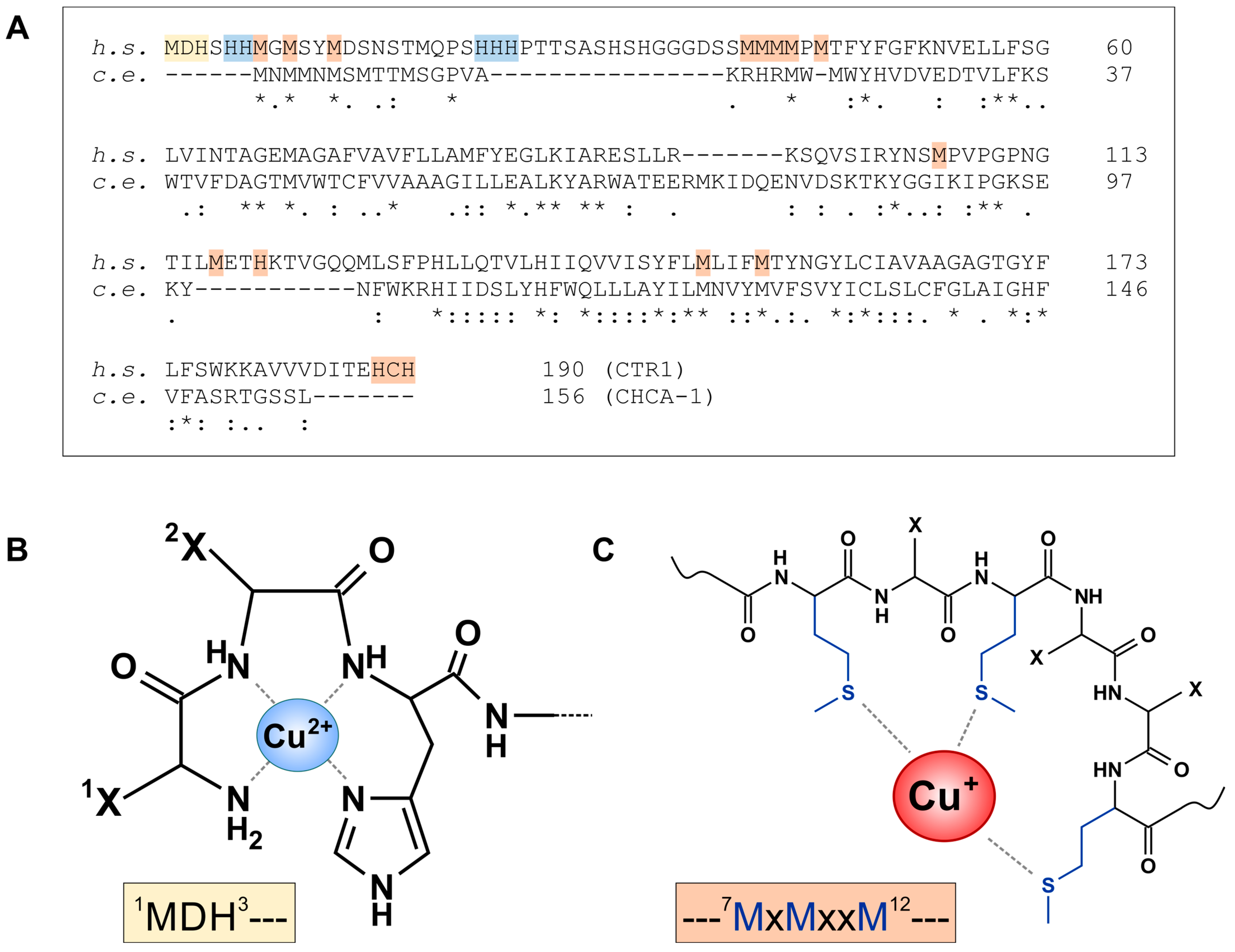
3.3.1. Conservation of Domains Involved in Cu Uptake by CTR1 and CHCA-1
3.3.2. Cu Binding by ATOX1/CUC-1 during Transfer to ATP7B/CUA-1
3.4. Regulatory Aspects of Cu Homeostasis in C. elegans: From Altered Gene Expression to Stress Response
4. Closing Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Klotz, L.O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef]
- Barthel, A.; Ostrakhovitch, E.A.; Walter, P.L.; Kampkötter, A.; Klotz, L.O. Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: Mechanisms and consequences. Arch. Biochem. Biophys. 2007, 463, 175–182. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Frezal, L.; Felix, M.A. C. elegans outside the Petri dish. eLife 2015, 4, e05849. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–399. [Google Scholar] [CrossRef]
- Kim, W.; Underwood, R.S.; Greenwald, I.; Shaye, D.D. OrthoList 2: A New Comparative Genomic Analysis of Human and Caenorhabditis elegans Genes. Genetics 2018, 210, 445–461. [Google Scholar] [CrossRef]
- Blockhuys, S.; Celauro, E.; Hildesjo, C.; Feizi, A.; Stal, O.; Fierro-Gonzalez, J.C.; Wittung-Stafshede, P. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics 2017, 9, 112–123. [Google Scholar] [CrossRef]
- Reznik, N.; Gallo, A.D.; Rush, K.W.; Javitt, G.; Fridmann-Sirkis, Y.; Ilani, T.; Nairner, N.A.; Fishilevich, S.; Gokhman, D.; Chacon, K.N.; et al. Intestinal mucin is a chaperone of multivalent copper. Cell 2022, 185, 4206–4215.e4211. [Google Scholar] [CrossRef]
- Philipp, T.M.; Gernoth, L.; Will, A.; Schwarz, M.; Ohse, V.A.; Kipp, A.P.; Steinbrenner, H.; Klotz, L.O. Selenium-binding protein 1 (SELENBP1) is a copper-dependent thiol oxidase. Redox Biol. 2023, 65, 102807. [Google Scholar] [CrossRef] [PubMed]
- Boulet, A.; Vest, K.E.; Maynard, M.K.; Gammon, M.G.; Russell, A.C.; Mathews, A.T.; Cole, S.E.; Zhu, X.; Phillips, C.B.; Kwong, J.Q.; et al. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Biol. Chem. 2018, 293, 1887–1896. [Google Scholar] [CrossRef]
- Calvo, J.; Jung, H.; Meloni, G. Copper metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.K.; Miles, L.A.; Crespi, G.A.; Morton, C.J.; Ng, H.L.; Barnham, K.J.; McKinstry, W.J.; Cappai, R.; Parker, M.W. Copper binding to the Alzheimer’s disease amyloid precursor protein. Eur. Biophys. J. 2008, 37, 269–279. [Google Scholar] [CrossRef]
- Vizan, P.; Di Croce, L.; Aranda, S. Functional and Pathological Roles of AHCY. Front. Cell Dev. Biol. 2021, 9, 654344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Walke, G.R.; Horvath, I.; Kumar, R.; Blockhuys, S.; Holgersson, S.; Walton, P.H.; Wittung-Stafshede, P. Memo1 binds reduced copper ions, interacts with copper chaperone Atox1, and protects against copper-mediated redox activity in vitro. Proc. Natl. Acad. Sci. USA 2022, 119, e2206905119. [Google Scholar] [CrossRef]
- Zhang, X.; Walke, G.; Wittung-Stafshede, P. Memo1 reduces copper-mediated reactive oxygen species in breast cancer cells. J. Inorg. Biochem. 2023, 247, 112335. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, M.; Guan, K.L. MEK-2, a Caenorhabditis elegans MAP kinase kinase, functions in Ras-mediated vulval induction and other developmental events. Genes Dev. 1995, 9, 742–755. [Google Scholar] [CrossRef]
- Philipp, T.M.; Gong, W.; Köhnlein, K.; Ohse, V.A.; Müller, F.I.; Priebs, J.; Steinbrenner, H.; Klotz, L.O. SEMO-1, a novel methanethiol oxidase in Caenorhabditis elegans, is a pro-aging factor conferring selective stress resistance. Biofactors 2022, 48, 699–706. [Google Scholar] [CrossRef]
- Jensen, L.T.; Culotta, V.C. Activation of CuZn superoxide dismutases from Caenorhabditis elegans does not require the copper chaperone CCS. J. Biol. Chem. 2005, 280, 41373–41379. [Google Scholar]
- Hu, Y.; Flockhart, I.; Vinayagam, A.; Bergwitz, C.; Berger, B.; Perrimon, N.; Mohr, S.E. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinform. 2011, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology, C.; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef] [PubMed]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S.; Ami, G.O.H.; Web Presence Working, G. AmiGO: Online access to ontology and annotation data. Bioinformatics 2009, 25, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Höss, S.; Schlottmann, K.; Traunspurger, W. Toxicity of ingested cadmium to the nematode Caenorhabditis elegans. Environ. Sci. Technol. 2011, 45, 10219–10225. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Zhang, J.; Yin, D.Q. Toxic and recovery effects of copper on Caenorhabditis elegans by various food-borne and water-borne pathways. Chemosphere 2012, 87, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Shafer, C.M.; Tseng, A.; Allard, P.; McEvoy, M.M. Strength of Cu-efflux response in Escherichia coli coordinates metal resistance in Caenorhabditis elegans and contributes to the severity of environmental toxicity. J. Biol. Chem. 2021, 297, 101060. [Google Scholar] [CrossRef] [PubMed]
- Kirsipuu, T.; Zadorožnaja, A.; Smirnova, J.; Friedemann, M.; Plitz, T.; Tõugu, V.; Palumaa, P. Copper(II)-binding equilibria in human blood. Sci. Rep. 2020, 10, 5686. [Google Scholar] [CrossRef] [PubMed]
- Fares, H.; Greenwald, I. Genetic analysis of endocytosis in Caenorhabditis elegans: Coelomocyte uptake defective mutants. Genetics 2001, 159, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Yanowitz, J.; Fire, A. Cyclin D involvement demarcates a late transition in C. elegans embryogenesis. Dev. Biol. 2005, 279, 244–251. [Google Scholar] [CrossRef]
- Boyd, W.A.; Cole, R.D.; Anderson, G.L.; Williams, P.L. The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ. Toxicol. Chem. 2003, 22, 3049–3055. [Google Scholar] [CrossRef]
- Tvermoes, B.E.; Boyd, W.A.; Freedman, J.H. Molecular characterization of numr-1 and numr-2: Genes that increase both resistance to metal-induced stress and lifespan in Caenorhabditis elegans. J. Cell Sci. 2010, 123, 2124–2134. [Google Scholar] [CrossRef]
- Jackson, B.P.; Williams, P.L.; Lanzirotti, A.; Bertsch, P.M. Evidence for biogenic pyromorphite formation by the nematode Caenorhabditis elegans. Environ. Sci. Technol. 2005, 39, 5620–5625. [Google Scholar] [CrossRef]
- Kar, S.; Sen, S.; Maji, S.; Saraf, D.; Ruturaj; Paul, R.; Dutt, S.; Mondal, B.; Rodriguez-Boulan, E.; Schreiner, R.; et al. Copper(II) import and reduction are dependent on His-Met clusters in the extracellular amino terminus of human copper transporter-1. J. Biol. Chem. 2022, 298, 101631. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef]
- Yuan, S.; Sharma, A.K.; Richart, A.; Lee, J.; Kim, B.E. CHCA-1 is a copper-regulated CTR1 homolog required for normal development, copper accumulation, and copper-sensing behavior in Caenorhabditis elegans. J. Biol. Chem. 2018, 293, 10911–10925. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Nakamura, N.; Sambongi, Y.; Wada, Y.; Oka, T.; Futai, M. Identification of the copper chaperone, CUC-1, in Caenorhabditis elegans: Tissue specific co-expression with the copper transporting ATPase, CUA-1. FEBS Lett. 1998, 440, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Blockhuys, S.; Devkota, R.; Pilon, M.; Wittung-Stafshede, P. The Caenorhabditis elegans homolog of human copper chaperone Atox1, CUC-1, aids in distal tip cell migration. BioMetals 2020, 33, 147–157. [Google Scholar] [CrossRef]
- Chun, H.; Sharma, A.K.; Lee, J.; Chan, J.; Jia, S.; Kim, B.E. The Intestinal Copper Exporter CUA-1 Is Required for Systemic Copper Homeostasis in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Calafato, S.; Swain, S.; Hughes, S.; Kille, P.; Stürzenbaum, S.R. Knock down of Caenorhabditis elegans cutc-1 exacerbates the sensitivity toward high levels of copper. Toxicol. Sci. 2008, 106, 384–391. [Google Scholar] [CrossRef]
- Yanatori, I.; Kishi, F. DMT1 and iron transport. Free Radic. Biol. Med. 2019, 133, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.; Muñoz, P.; Mura, C.V.; Núñez, M.T. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am. J. Physiol.-Cell Physiol. 2003, 284, C1525–C1530. [Google Scholar] [CrossRef] [PubMed]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate Profile and Metal-ion Selectivity of Human Divalent Metal-ion Transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, Z.; Wang, T.; Chen, C.; James Kang, Y. Copper uptake by DMT1: A compensatory mechanism for CTR1 deficiency in human umbilical vein endothelial cells. Metallomics 2015, 7, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Au, C.; Benedetto, A.; Anderson, J.; Labrousse, A.; Erikson, K.; Ewbank, J.J.; Aschner, M. SMF-1, SMF-2 and SMF-3 DMT1 Orthologues Regulate and Are Regulated Differentially by Manganese Levels in C. elegans. PLoS ONE 2009, 4, e7792. [Google Scholar] [CrossRef] [PubMed]
- Mashock, M.J.; Zanon, T.; Kappell, A.D.; Petrella, L.N.; Andersen, E.C.; Hristova, K.R. Copper Oxide Nanoparticles Impact Several Toxicological Endpoints and Cause Neurodegeneration in Caenorhabditis elegans. PLoS ONE 2016, 11, e0167613. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Lutsenko, S. The Role of Copper Chaperone Atox1 in Coupling Redox Homeostasis to Intracellular Copper Distribution. Antioxidants 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- La Fontaine, S.; Mercer, J.F. Trafficking of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis. Arch. Biochem. Biophys. 2007, 463, 149–167. [Google Scholar] [CrossRef]
- Ruturaj; Mishra, M.; Saha, S.; Maji, S.; Rodriguez-Boulan, E.; Schreiner, R.; Gupta, A. Regulation of the apico-basolateral trafficking polarity of the homologous copper-ATPases ATP7A and ATP7B. J. Cell Sci. 2024, 137, jcs261258. [Google Scholar] [CrossRef]
- Sambongi, Y.; Wakabayashi, T.; Yoshimizu, T.; Omote, H.; Oka, T.; Futai, M. Caenorhabditis elegans cDNA for a Menkes/Wilson Disease Gene Homologue and Its Function in a Yeast CCC2 Gene Deletion Mutant. J. Biochem. 1997, 121, 1169–1175. [Google Scholar] [CrossRef]
- Catalano, F.; O’Brien, T.J.; Mekhova, A.A.; Sepe, L.V.; Elia, M.; De Cegli, R.; Gallotta, I.; Santonicola, P.; Zampi, G.; Ilyechova, E.Y.; et al. A new Caenorhabditis elegans model to study copper toxicity in Wilson disease. Traffic 2024, 25, e12920. [Google Scholar] [CrossRef] [PubMed]
- Sturtz, L.A.; Diekert, K.; Jensen, L.T.; Lill, R.; Culotta, V.C. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001, 276, 38084–38089. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ali, M.; Fischer, M.; Riemer, J. Human copper chaperone for superoxide dismutase 1 mediates its own oxidation-dependent import into mitochondria. Nat. Commun. 2013, 4, 2430. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Tainer, J.A.; Getzoff, E.D.; Beem, K.M.; Richardson, J.S.; Richardson, D.C. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J. Mol. Biol. 1982, 160, 181–217. [Google Scholar] [CrossRef]
- Doonan, R.; McElwee, J.J.; Matthijssens, F.; Walker, G.A.; Houthoofd, K.; Back, P.; Matscheski, A.; Vanfleteren, J.R.; Gems, D. Against the oxidative damage theory of aging: Superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008, 22, 3236–3241. [Google Scholar] [CrossRef]
- Miranda-Vizuete, A.; Veal, E.A. Caenorhabditis elegans as a model for understanding ROS function in physiology and disease. Redox Biol. 2017, 11, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wu, T.H.; Song, Y.X.; Ge, M.H.; Su, C.M.; Niu, W.P.; Li, L.L.; Xu, Z.J.; Ge, C.L.; Al-Mhanawi, M.T.; et al. Reciprocal inhibition between sensory ASH and ASI neurons modulates nociception and avoidance in Caenorhabditis elegans. Nat. Commun. 2015, 6, 5655. [Google Scholar] [CrossRef]
- Wu, J.J.; Yin, S.W.; Liu, H.; Li, R.; Huang, J.H.; Wang, P.Z.; Xu, Y.; Zhao, J.L.; Wu, P.P.; Wu, Z.X. Positive interaction between ASH and ASK sensory neurons accelerates nociception and inhibits behavioral adaptation. iScience 2022, 25, 105287. [Google Scholar] [CrossRef]
- Boyd, S.D.; Ullrich, M.S.; Skopp, A.; Winkler, D.D. Copper Sources for Sod1 Activation. Antioxidants 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Maghool, S.; Fontaine, S.; Roberts, B.R.; Kwan, A.H.; Maher, M.J. Human glutaredoxin-1 can transfer copper to isolated metal binding domains of the P(1B)-type ATPase, ATP7B. Sci. Rep. 2020, 10, 4157. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.D.; Bridge, W.J. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox Biol. 2019, 24, 101171. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.T.; Branco, V.; Vale, F.F.; Coppo, L. Glutaredoxin: Discovery, redox defense and much more. Redox Biol. 2021, 43, 101975. [Google Scholar] [CrossRef] [PubMed]
- Field, L.S.; Furukawa, Y.; O’Halloran, T.V.; Culotta, V.C. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J. Biol. Chem. 2003, 278, 28052–28059. [Google Scholar] [CrossRef] [PubMed]
- Zischka, H.; Einer, C. Mitochondrial copper homeostasis and its derailment in Wilson disease. Int. J. Biochem. Cell Biol. 2018, 102, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Boulet, A.; Buckley, K.M.; Phillips, C.B.; Gammon, M.G.; Oldfather, L.E.; Moore, S.A.; Leary, S.C.; Cobine, P.A. Mitochondrial copper and phosphate transporter specificity was defined early in the evolution of eukaryotes. eLife 2021, 10, e64690. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Weishaupt, A.K.; Lamann, K.; Tallarek, E.; Pezacki, A.T.; Matier, C.D.; Schwerdtle, T.; Aschner, M.; Chang, C.J.; Stürzenbaum, S.R.; Bornhorst, J. Dysfunction in atox-1 and ceruloplasmin alters labile Cu levels and consequently Cu homeostasis in C. elegans. Front. Mol. Biosci. 2024, 11, 1354627. [Google Scholar] [CrossRef]
- Gupta, S.D.; Lee, B.T.; Camakaris, J.; Wu, H.C. Identification of cutC and cutF (nlpE) genes involved in copper tolerance in Escherichia coli. J. Bacteriol. 1995, 177, 4207–4215. [Google Scholar] [CrossRef]
- Li, J.; Ji, C.; Chen, J.; Yang, Z.; Wang, Y.; Fei, X.; Zheng, M.; Gu, X.; Wen, G.; Xie, Y.; et al. Identification and characterization of a novel Cut family cDNA that encodes human copper transporter protein CutC. Biochem. Biophys. Res. Commun. 2005, 337, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, J.; Zhang, P.; Ding, J. Crystal structure of human copper homeostasis protein CutC reveals a potential copper-binding site. J. Struct. Biol. 2010, 169, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kunjunni, R.; Sathianathan, S.; Behari, M.; Chattopadhyay, P.; Subbiah, V. Silencing of Human CutC Gene (hCutC) Induces Apoptosis in HepG2 Cells. Biol. Trace Elem. Res. 2016, 172, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.; Young, T.R.; Barnham, K.J.; Wedd, A.G.; Hinds, M.G.; Xiao, Z.; Cappai, R. Quantification of copper binding to amyloid precursor protein domain 2 and its Caenorhabditis elegans ortholog. Implications for biological function. Metallomics 2014, 6, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ewald, C.Y.; Hourihan, J.M.; Bland, M.S.; Obieglo, C.; Katic, I.; Moronetti Mazzeo, L.E.; Alcedo, J.; Blackwell, T.K.; Hynes, N.E. NADPH oxidase-mediated redox signaling promotes oxidative stress resistance and longevity through memo-1 in C. elegans. eLife 2017, 6, e19493. [Google Scholar] [CrossRef] [PubMed]
- Höckner, M.; Dallinger, R.; Stürzenbaum, S.R. Nematode and snail metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Essig, Y.J.; Leszczyszyn, O.I.; Almutairi, N.; Harrison-Smith, A.; Blease, A.; Zeitoun-Ghandour, S.; Webb, S.M.; Blindauer, C.A.; Stürzenbaum, S.R. Juggling cadmium detoxification and zinc homeostasis: A division of labour between the two C. elegans metallothioneins. Chemosphere 2024, 350, 141021. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.C.; Collier, S.; Guthrie, J.; Robertson, J.D.; Kornfeld, K. Lysosome-Related Organelles in Intestinal Cells Are a Zinc Storage Site in C. elegans. Cell Metab. 2012, 15, 88–99. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. The Bioinorganic Chemistry of Mammalian Metallothioneins. Chem. Rev. 2021, 121, 14594–14648. [Google Scholar] [CrossRef]
- Hedera, P. Clinical management of Wilson disease. Ann. Transl. Med. 2019, 7, S66. [Google Scholar] [CrossRef]
- Günther, V.; Lindert, U.; Schaffner, W. The taste of heavy metals: Gene regulation by MTF-1. Biochim. Et Biophys. Acta 2012, 1823, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.C.; Keusekotten, K.; Baumeister, R.; Stürzenbaum, S.R. C. elegans Metallothioneins: New Insights into the Phenotypic Effects of Cadmium Toxicosis. J. Mol. Biol. 2004, 341, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.H.; Slice, L.W.; Dixon, D.; Fire, A.; Rubin, C.S. The novel metallothionein genes of Caenorhabditis elegans. Structural organization and inducible, cell-specific expression. J. Biol. Chem. 1993, 268, 2554–2564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, C.; Zhang, H.; Lu, Q.; Zhou, J.; Liu, R.; Wang, S.; Pu, Y.; Yin, L. Trans-generational effects of copper on nerve damage in Caenorhabditis elegans. Chemosphere 2021, 284, 131324. [Google Scholar] [CrossRef] [PubMed]
- Bofill, R.; Orihuela, R.; Romagosa, M.; Domenech, J.; Atrian, S.; Capdevila, M. Caenorhabditis elegans metallothionein isoform specificity—Metal binding abilities and the role of histidine in CeMT1 and CeMT2. FEBS J. 2009, 276, 7040–7056. [Google Scholar] [CrossRef] [PubMed]
- Ibiam, U.; Grant, A. The function of metallothionein in Caenorhabditis elegans: Is detoxification of copper or of cadmium more important? Middle-East J. Sci. Res. 2015, 23, 186–191. [Google Scholar] [CrossRef]
- Cioci, L.K.; Qiu, L.; Freedman, J.H. Transgenic strains of the nematode Caenorhabditis elegans as biomonitors of metal contamination. Environ. Toxicol. Chem. 2000, 19, 2122–2129. [Google Scholar] [CrossRef]
- Ma, H.; Glenn, T.C.; Jagoe, C.H.; Jones, K.L.; Williams, P.L. A transgenic strain of the nematode Caenorhabditis elegans as a biomonitor for heavy metal contamination. Environ. Toxicol. Chem. 2009, 28, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The role of glutathione in copper metabolism and toxicity. J. Biol. Chem. 1989, 264, 5598–5605. [Google Scholar] [CrossRef]
- Ciriolo, M.R.; Desideri, A.; Paci, M.; Rotilio, G. Reconstitution of Cu,Zn-superoxide dismutase by the Cu(I).glutathione complex. J. Biol. Chem. 1990, 265, 11030–11034. [Google Scholar] [CrossRef]
- Ascone, I.; Longo, A.; Dexpert, H.; Ciriolo, M.R.; Rotilio, G.; Desideri, A. An X-ray absorption study of the reconstitution process of bovine Cu,Zn superoxide dismutase by Cu(I)-glutathione complex. FEBS Lett. 1993, 322, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Mercer, S.W.; La Fontaine, S.; Warr, C.G.; Burke, R. Reduced glutathione biosynthesis in Drosophila melanogaster causes neuronal defects linked to copper deficiency. J. Neurochem. 2016, 137, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.; Tsitsipatis, D.; Hausig, F.; Kreuzer, K.; Erler, K.; Stein, V.; Ristow, M.; Steinbrenner, H.; Klotz, L.O. Non-linear impact of glutathione depletion on C. elegans life span and stress resistance. Redox Biol. 2017, 11, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Schroeder, J.I.; Degenkolb, T. Caenorhabditis elegans expresses a functional phytochelatin synthase. Eur. J. Biochem. 2001, 268, 3640–3643. [Google Scholar] [CrossRef] [PubMed]
- Vatamaniuk, O.K.; Bucher, E.A.; Ward, J.T.; Rea, P.A. A new pathway for heavy metal detoxification in animals. Phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 2001, 276, 20817–20820. [Google Scholar] [CrossRef] [PubMed]
- Vatamaniuk, O.K.; Bucher, E.A.; Ward, J.T.; Rea, P.A. Worms take the ‘phyto’ out of ‘phytochelatins’. Trends Biotechnol. 2002, 20, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.S.; Benci, J.L.; Selote, D.S.; Sharma, A.K.; Chen, A.G.; Dang, H.; Fares, H.; Vatamaniuk, O.K. Detoxification of multiple heavy metals by a half-molecule ABC transporter, HMT-1, and coelomocytes of Caenorhabditis elegans. PLoS ONE 2010, 5, e9564. [Google Scholar] [CrossRef]
- Hughes, S.L.; Bundy, J.G.; Want, E.J.; Kille, P.; Stürzenbaum, S.R. The metabolomic responses of Caenorhabditis elegans to cadmium are largely independent of metallothionein status, but dominated by changes in cystathionine and phytochelatins. J. Proteome Res. 2009, 8, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sharma, A.K.; Vatamaniuk, O.K. N-Terminal Extension and C-Terminal Domains Are Required for ABCB6/HMT-1 Protein Interactions, Function in Cadmium Detoxification, and Localization to the Endosomal-Recycling System in Caenorhabditis elegans. Front. Physiol. 2018, 9, 885. [Google Scholar] [CrossRef]
- Lutsenko, S. Copper trafficking to the secretory pathway. Metallomics 2016, 8, 840–852. [Google Scholar] [CrossRef]
- Magistrato, A.; Pavlin, M.; Qasem, Z.; Ruthstein, S. Copper trafficking in eukaryotic systems: Current knowledge from experimental and computational efforts. Curr. Opin Struct. Biol. 2019, 58, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Pushie, M.J.; Shaw, K.; Franz, K.J.; Shearer, J.; Haas, K.L. Model Peptide Studies Reveal a Mixed Histidine-Methionine Cu(I) Binding Site at the N-Terminus of Human Copper Transporter 1. Inorg. Chem. 2015, 54, 8544–8551. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, H.; Wang, X.; Liu, Q.; Ni, J.; Sun, H. Kinetics and thermodynamics of metal binding to the N-terminus of a human copper transporter, hCTR1. Chem. Commun. 2013, 49, 9134–9136. [Google Scholar] [CrossRef] [PubMed]
- Rubino, J.T.; Riggs-Gelasco, P.; Franz, K.J. Methionine motifs of copper transport proteins provide general and flexible thioether-only binding sites for Cu(I) and Ag(I). J. Biol. Inorg. Chem. 2010, 15, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Tsigelny, I.F.; Sharikov, Y.; Greenberg, J.P.; Miller, M.A.; Kouznetsova, V.L.; Larson, C.A.; Howell, S.B. An all-atom model of the structure of human copper transporter 1. Cell Biochem. Biophys. 2012, 63, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Maryon, E.B.; Molloy, S.A.; Ivy, K.; Yu, H.; Kaplan, J.H. Rate and regulation of copper transport by human copper transporter 1 (hCTR1). J. Biol. Chem. 2013, 288, 18035–18046. [Google Scholar] [CrossRef] [PubMed]
- Kahra, D.; Kovermann, M.; Wittung-Stafshede, P. The C-Terminus of Human Copper Importer Ctr1 Acts as a Binding Site and Transfers Copper to Atox1. Biophys. J. 2016, 110, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Clasen, S.; Hasan, N.M.; Barry, A.N.; Lutsenko, S. Functional partnership of the copper export machinery and glutathione balance in human cells. J. Biol. Chem. 2012, 287, 26678–26687. [Google Scholar] [CrossRef]
- Hussain, F.; Rodriguez-Granillo, A.; Wittung-Stafshede, P. Lysine-60 in copper chaperone Atox1 plays an essential role in adduct formation with a target Wilson disease domain. J. Am. Chem. Soc. 2009, 131, 16371–16373. [Google Scholar] [CrossRef]
- Xi, Z.; Shi, C.; Tian, C.; Liu, Y. Conserved residue modulates copper-binding properties through structural dynamics in human copper chaperone Atox1. Metallomics 2013, 5, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, I.; Banci, L.; Bertini, I.; Cantini, F.; Katsari, E.; Rosato, A. Solution structure of the apo and copper(I)-loaded human metallochaperone HAH1. Biochemistry 2004, 43, 13046–13053. [Google Scholar] [CrossRef] [PubMed]
- Ariöz, C.; Li, Y.; Wittung-Stafshede, P. The six metal binding domains in human copper transporter, ATP7B: Molecular biophysics and disease-causing mutations. Biometals 2017, 30, 823–840. [Google Scholar] [CrossRef]
- Achila, D.; Banci, L.; Bertini, I.; Bunce, J.; Ciofi-Baffoni, S.; Huffman, D.L. Structure of human Wilson protein domains 5 and 6 and their interplay with domain 4 and the copper chaperone HAH1 in copper uptake. Proc. Natl. Acad. Sci. USA 2006, 103, 5729–5734. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, N.; Korzhnev, D.M.; Velyvis, A.; Sarkar, B.; Forman-Kay, J.D. NMR characterization of copper-binding domains 4-6 of ATP7B. Biochemistry 2010, 49, 8468–8477. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granillo, A.; Crespo, A.; Estrin, D.A.; Wittung-Stafshede, P. Copper-transfer mechanism from the human chaperone Atox1 to a metal-binding domain of Wilson disease protein. J. Phys. Chem. B 2010, 114, 3698–3706. [Google Scholar] [CrossRef]
- Yang, G.M.; Xu, L.; Wang, R.M.; Tao, X.; Zheng, Z.W.; Chang, S.; Ma, D.; Zhao, C.; Dong, Y.; Wu, S.; et al. Structures of the human Wilson disease copper transporter ATP7B. Cell Rep. 2023, 42, 112417. [Google Scholar] [CrossRef] [PubMed]
- Brose, J.; La Fontaine, S.; Wedd, A.G.; Xiao, Z. Redox sulfur chemistry of the copper chaperone Atox1 is regulated by the enzyme glutaredoxin 1, the reduction potential of the glutathione couple GSSG/2GSH and the availability of Cu(I). Metallomics 2014, 6, 793–808. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Lu, L.; Zhao, C.; Zhang, H.; Liu, R.; Pu, Y.; Yin, L. Integrating Transcriptomics and Free Fatty Acid Profiling Analysis Reveal Cu Induces Shortened Lifespan and Increased Fat Accumulation and Oxidative Damage in C. elegans. Oxid. Med. Cell Longev. 2022, 2022, 5297342. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Lordnejad, M.R.; Schliess, F.; Sies, H.; Klotz, L.O. Copper ions strongly activate the phosphoinositide-3-kinase/Akt pathway independent of the generation of reactive oxygen species. Arch. Biochem. Biophys. 2002, 397, 232–239. [Google Scholar] [CrossRef]
- Walter, P.L.; Kampkötter, A.; Eckers, A.; Barthel, A.; Schmoll, D.; Sies, H.; Klotz, L.O. Modulation of FoxO signaling in human hepatoma cells by exposure to copper or zinc ions. Arch. Biochem. Biophys. 2006, 454, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hamann, I.; Petroll, K.; Grimm, L.; Hartwig, A.; Klotz, L.O. Insulin-like modulation of Akt/FoxO signaling by copper ions is independent of insulin receptor. Arch. Biochem. Biophys. 2014, 558, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Shomer, N.; Kadhim, A.Z.; Grants, J.M.; Cheng, X.; Alhusari, D.; Bhanshali, F.; Poon, A.F.; Lee, M.Y.Y.; Muhuri, A.; Park, J.I.; et al. Mediator subunit MDT-15/MED15 and Nuclear Receptor HIZR-1/HNF4 cooperate to regulate toxic metal stress responses in Caenorhabditis elegans. PLoS Genet. 2019, 15, e1008508. [Google Scholar] [CrossRef] [PubMed]
- Köhnlein, K.; Urban, N.; Guerrero-Gómez, D.; Steinbrenner, H.; Urbánek, P.; Priebs, J.; Koch, P.; Kaether, C.; Miranda-Vizuete, A.; Klotz, L.O. A Caenorhabditis elegans ortholog of human selenium-binding protein 1 is a pro-aging factor protecting against selenite toxicity. Redox Biol. 2020, 28, 101323. [Google Scholar] [CrossRef]
- Pol, A.; Renkema, G.H.; Tangerman, A.; Winkel, E.G.; Engelke, U.F.; de Brouwer, A.P.M.; Lloyd, K.C.; Araiza, R.S.; van den Heuvel, L.; Omran, H.; et al. Mutations in SELENBP1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat. Genet. 2018, 50, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Philipp, T.M.; Will, A.; Richter, H.; Winterhalter, P.R.; Pohnert, G.; Steinbrenner, H.; Klotz, L.O. A coupled enzyme assay for detection of selenium-binding protein 1 (SELENBP1) methanethiol oxidase (MTO) activity in mature enterocytes. Redox Biol. 2021, 43, 101972. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Köhnlein, K.; Urban, N.; Steinbrenner, H.; Guerrero-Gomez, D.; Miranda-Vizuete, A.; Kaether, C.; Klotz, L.O. Selenite-induced expression of a Caenorhabditis elegans pro-aging factor and ortholog of human selenium-binding protein 1. Curr. Nutraceuticals 2020, 1, 73–79. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L., Jr.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohse, V.A.; Klotz, L.-O.; Priebs, J. Copper Homeostasis in the Model Organism C. elegans. Cells 2024, 13, 727. https://doi.org/10.3390/cells13090727
Ohse VA, Klotz L-O, Priebs J. Copper Homeostasis in the Model Organism C. elegans. Cells. 2024; 13(9):727. https://doi.org/10.3390/cells13090727
Chicago/Turabian StyleOhse, Verena Alexia, Lars-Oliver Klotz, and Josephine Priebs. 2024. "Copper Homeostasis in the Model Organism C. elegans" Cells 13, no. 9: 727. https://doi.org/10.3390/cells13090727





