Activin A, a Novel Chemokine, Induces Mouse NK Cell Migration via AKT and Calcium Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Reagents
2.3. Cell Lines
2.4. Isolation of NK Cells
2.5. Giemsa Staining
2.6. Flow Cytometry
2.7. CCK-8 Cell Viability Assay
2.8. NK Cell Cytotoxicity Assay
2.9. Transwell Chamber Cell Migration Assay
2.10. Microfluidic Cell Migration Assay
2.11. Transwell Chamber Cell Invasion Assay
2.12. Immunofluorescent Staining
2.13. Real-Time Cell Analysis
2.14. Western Blotting
2.15. Kinase Inhibition
2.16. Calcium Flux Assay
2.17. Calcium Chelation
2.18. RT-PCR
2.19. Enzyme-Linked Immunosorbent Assay
2.20. Establishment of Tumor-Bearing Mice with NS-1 Cells
2.21. Establishment of Tumor-Bearing Mice with 4T-1 Cells
2.22. Immunofluorescence Assay for NK Cells in Tumor Tissue
2.23. Statistical Analysis
3. Results
3.1. Activin A Does Not Affect the Viability and Cytotoxicity of Mouse NK Cells
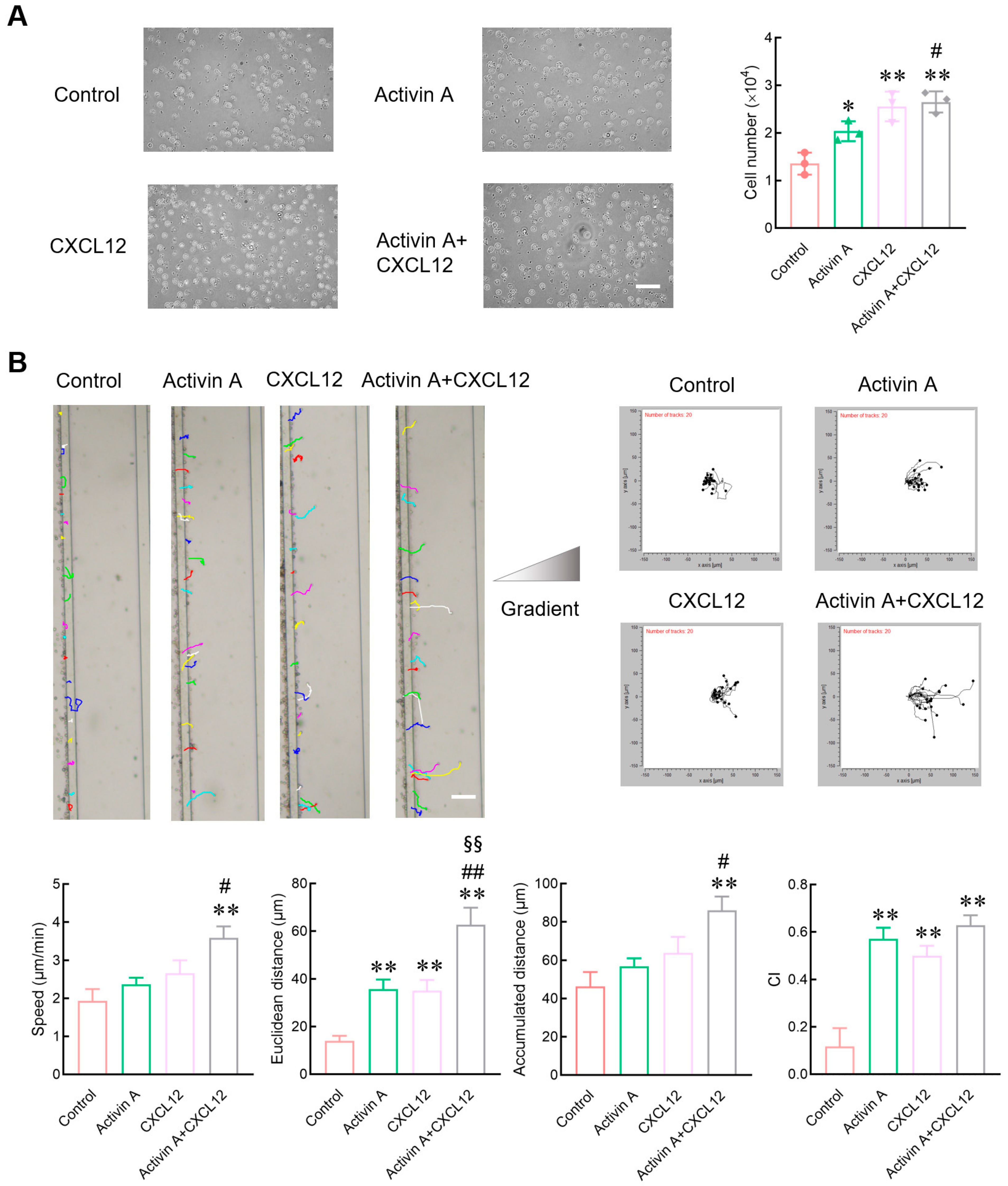
3.2. Activin A Induces Mouse NK Cell Migration
3.3. Activin A Increases the Invasion and Inhibits the Adhesion of Mouse NK Cells
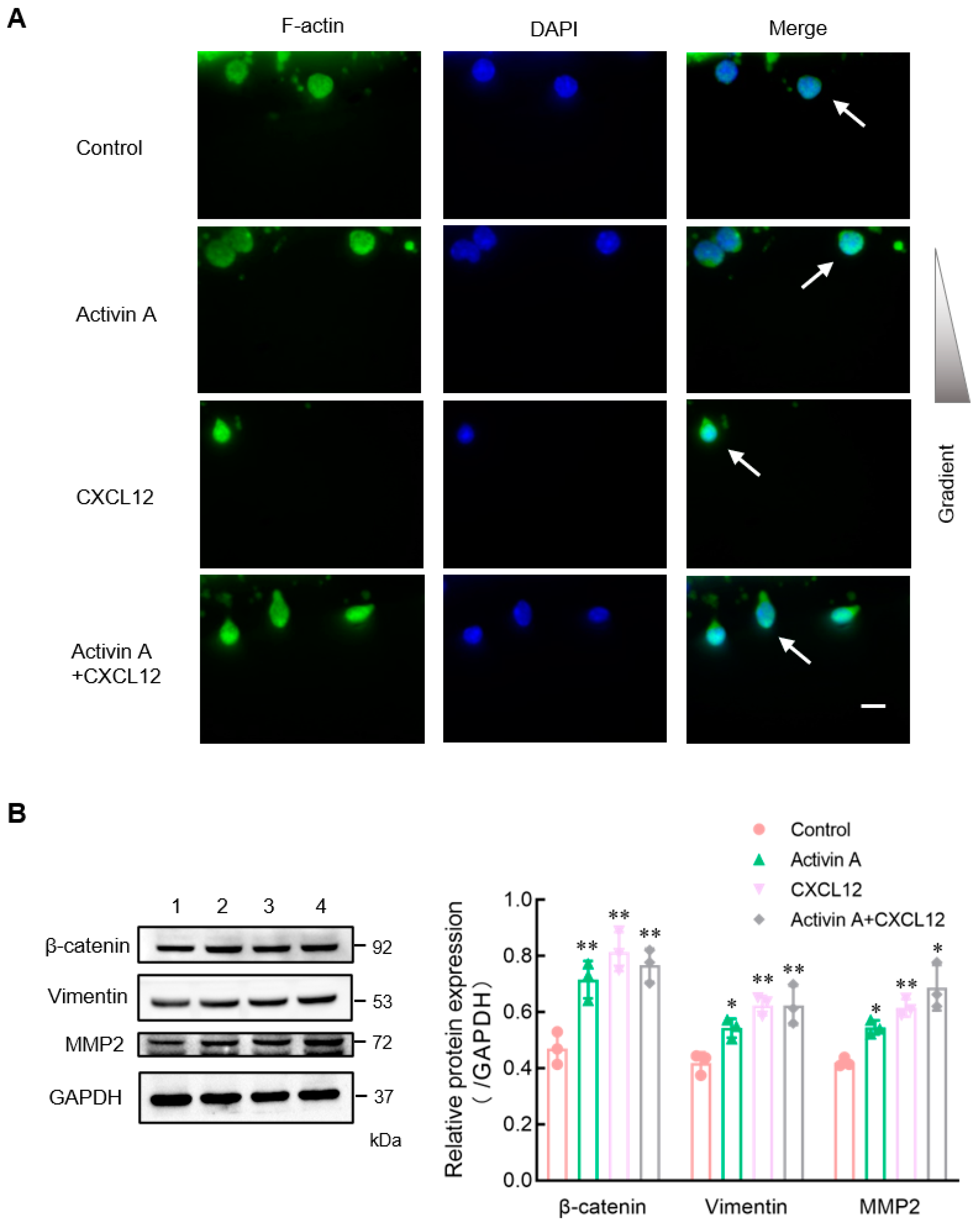
3.4. Activin A Promotes Polarization of Mouse NK Cells
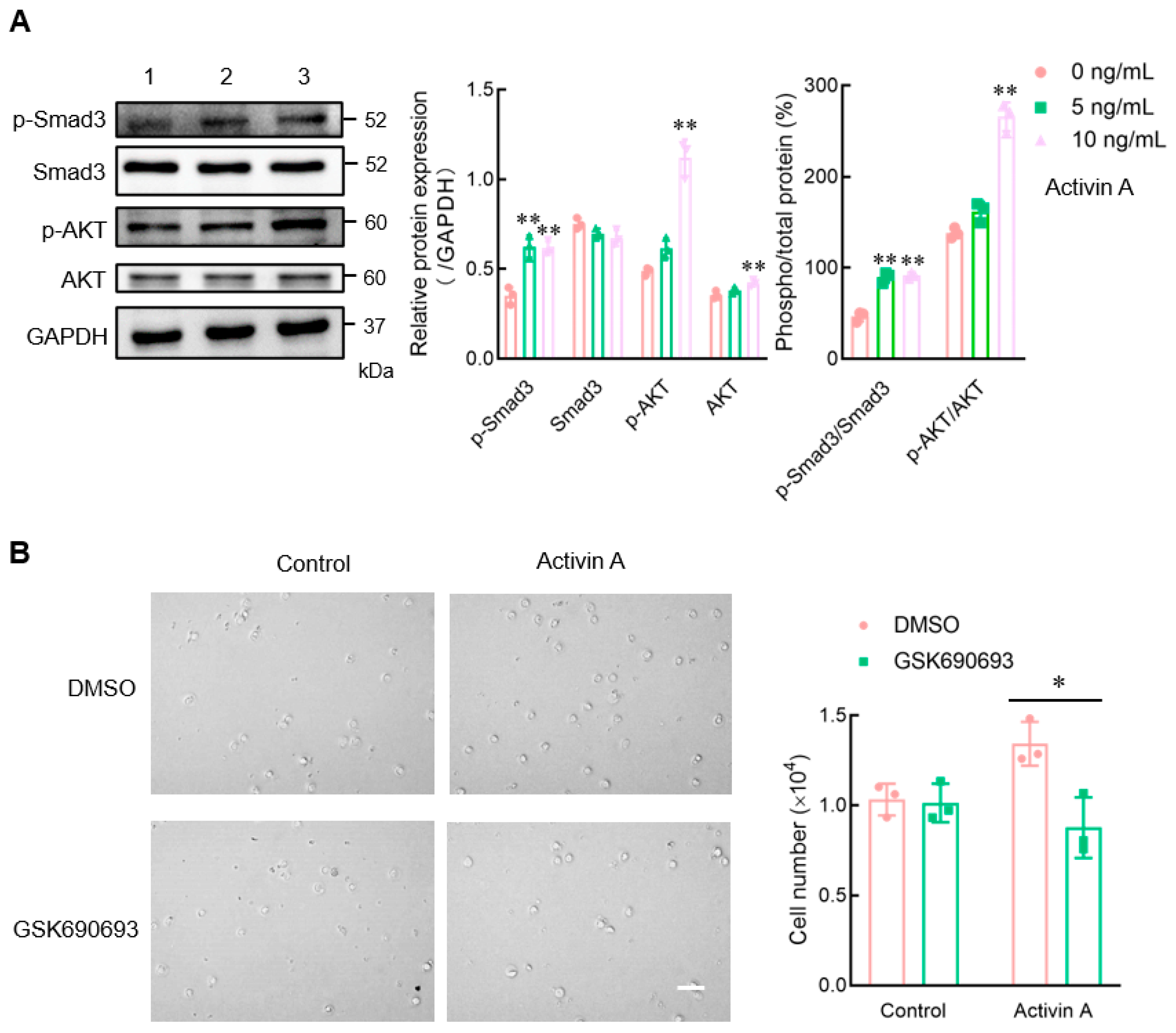
3.5. Activin A Increases the Levels of p-SMAD3 and p-AKT Proteins in Mouse NK Cells
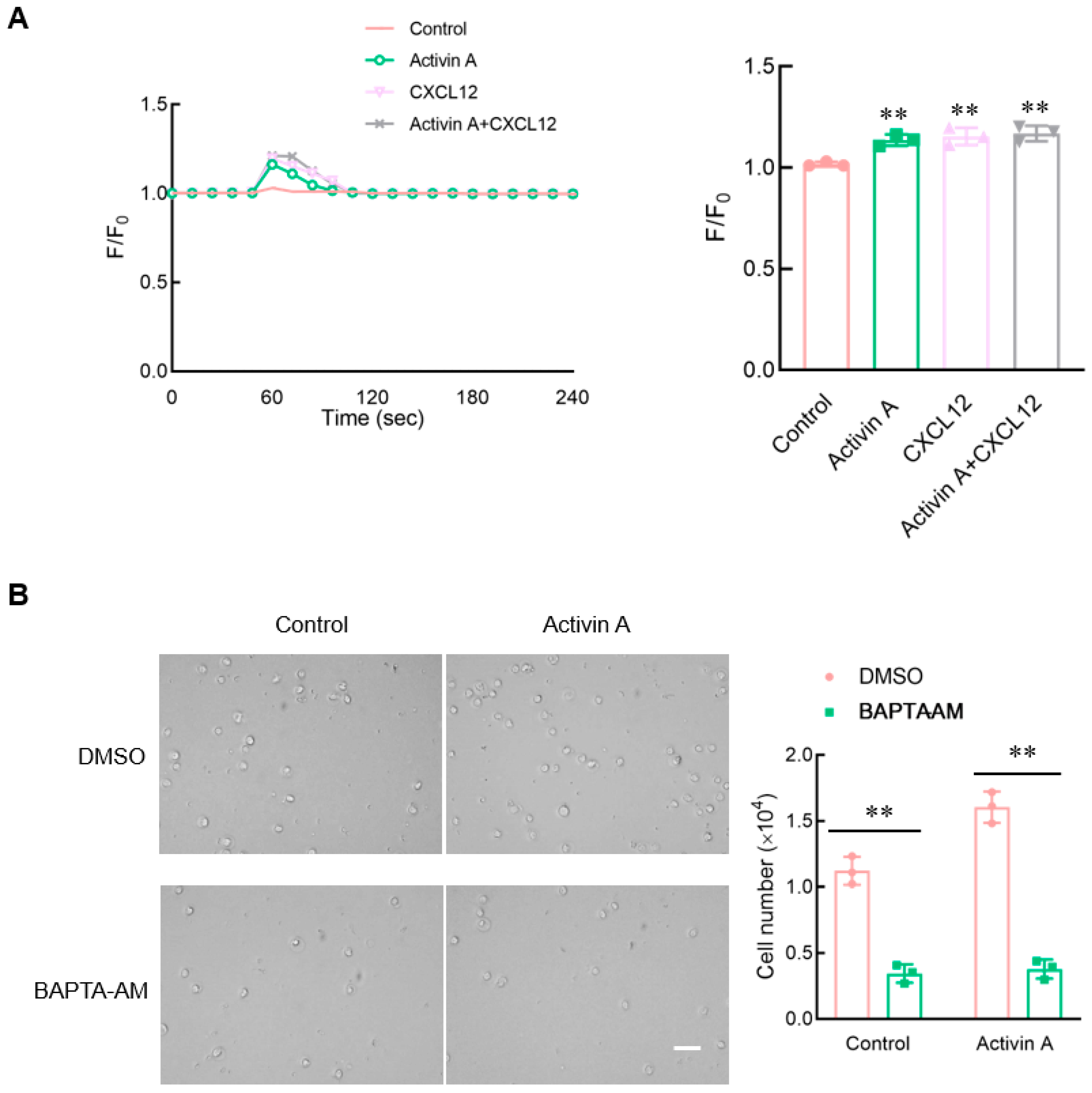
3.6. Activin A Increases Ca2+ Levels in Mouse NK Cells
3.7. Exogenous Activin A Increases NK Cell Infiltration into NS-1 Cell Solid Tumors
3.8. Blocking the Endogenous Activin A Reduces the Infiltration of NK Cells into 4T-1 Cell Solid Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azgomi, M.S.; Badami, G.D.; Pizzo, M.L.; Tamburini, B.; Dieli, C.; Manna, M.P.L.; Francesco Dieli, F.; Caccamo, N. Integrated Analysis of Single-Cell and Bulk RNA Sequencing Data Reveals Memory-like NK Cell Subset Associated with Mycobacterium tuberculosis Latency. Cells 2024, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Miyazato, K.; Takahashi, K.; Yoshimura, N.; Tahara, H.; Hayakawa, Y. Lung-resident natural killer cells control pulmonary tumor growth in mice. Cancer Sci. 2018, 109, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Billingsley, J.M.; Sharma, A.A.; Styles, T.M.; Govindaraj, S.; Shanmugasundaram, U.; Babu, H.; Riberio, S.P.; Ali, S.A.; Tharp, G.K.; et al. Lymph node CXCR5+ NK cells associate with control of chronic SHIV infection. JCI Insight 2022, 7, e155601. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Nandagopal, S.; Sow, I.; Lin, F.; Kung, S.K. Microfluidic-based, live-cell analysis allows assessment of NK-cell migration in response to crosstalk with dendritic cells. Eur. J. Immunol. 2014, 44, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Ma, S.; Tang, T.; Wu, X.; Mansour, A.G.; Lu, T.; Zhang, J.; Wang, L.S.; Caligiuri, M.A.; Yu, J. PDGF-D-PDGFRβ signaling enhances IL-15-mediated human natural killer cell survival. Proc. Natl. Acad. Sci. USA 2022, 119, e2114134119. [Google Scholar] [CrossRef]
- Barrow, A.D.; Edeling, M.A.; Trifonov, V.; Luo, J.; Goyal, P.; Bohl, B.; Bando, J.K.; Kim, A.H.; Walker, J.; Andahazy, M.; et al. Natural killer cells control tumor growth by sensing a growth factor. Cell 2018, 172, 534–548.e19. [Google Scholar] [CrossRef]
- Bloise, E.; Ciarmela, P.; Dela Cruz, C.; Luisi, S.; Petraglia, F.; Reis, F.M. Activin A in Mammalian Physiology. Physiol. Rev. 2019, 99, 739–780. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Z.; Shang, S.; Jiang, L.; Lv, X.; Qi, Y.; Cui, X.; Ge, J. Activin A regulates activities of peripheral blood natural killer cells of mouse in an autocrine and paracrine manner. Exp. Cell Res. 2019, 374, 114–121. [Google Scholar] [CrossRef]
- Zhang, F.; Qi, Y.; Li, J.; Liu, B.; Liu, Z.; Cui, X. Activin A induces apoptosis of human lung adenocarcinoma A549 cells through endoplasmic reticulum stress pathway. Oncol. Rep. 2024, 51, 29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, B.; Qi, Y.; Zhu, L.; Cui, X.; Liu, Z. Antagonistic effects of activin A and TNF-α on the activation of L929 fibroblast cells via Smad3-independent signaling. Sci. Rep. 2020, 10, 20623. [Google Scholar] [CrossRef] [PubMed]
- Seeger, P.; Bosisio, D.; Parolini, S.; Badolato, R.; Gismondi, A.; Santoni, A.; Sozzani, S. Activin A as a mediator of NK-dendritic cell functional interactions. J. Immunol. 2014, 192, 1241–1248. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, L.; Wu, C.; Li, J.; Wang, H.; Wang, S.; Chen, X.; Cui, X.; Liu, Z. Activin A impairs ActRIIA(+) neutrophil recruitment into infected skin of mice. iScience 2021, 24, 102080. [Google Scholar] [CrossRef]
- Ma, C.; Qi, Y.; Liu, H.; Wu, C.; Cui, X.; Liu, Z. Inhibitory effect of activin A on IL-9 production by mouse NK cells through Smad3 signaling. Biol. Chem. 2020, 401, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, Y.; Qi, J.; Wu, J.; Lin, F.; Cui, X.; Ge, J.; Liu, Z. Activin A is a novel chemoattractant for migration of microglial BV2 cells. J. Neuroimmunol. 2022, 371, 577929. [Google Scholar] [CrossRef] [PubMed]
- Pinjusic, K.; Dubey, O.A.; Egorova, O.; Nassiri, S.; Meylan, E.; Faget, J.; Constam, D.B. Activin-A impairs CD8 T cell-mediated immunity and immune checkpoint therapy response in melanoma. J. Immunother. Cancer 2022, 10, e004533. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cui, X.; Ge, J.; Li, J.; Niu, L.; Liu, H.; Qi, Y.; Liu, Z.; Wang, Y. Activin A inhibits activities of lipopolysaccharide-activated macrophages via TLR4, not of TLR2. Biochem. Biophys. Res. Commun. 2013, 435, 222–228. [Google Scholar] [CrossRef]
- Chen, S.; Tang, W.; Yu, G.; Tang, Z.; Liu, E. CXCL12/CXCR4 Axis is Involved in the Recruitment of NK Cells by HMGB1 Contributing to Persistent Airway Inflammation and AHR During the Late Stage of RSV Infection. J. Microbiol. 2023, 61, 461–469. [Google Scholar] [CrossRef]
- Xie, D.; Liu, Z.; Wu, J.; Feng, W.; Yang, K.; Deng, J.; Tian, G.; Santos, S.; Cui, X.; Lin, F. The effects of activin A on the migration of human breast cancer cells and neutrophils and their migratory interaction. Exp. Cell Res. 2017, 357, 107–115. [Google Scholar] [CrossRef]
- Jiang, L.; Qi, Y.; Kong, X.; Wang, R.; Qi, J.; Lin, F.; Cui, X.; Liu, Z. Activin A as a Novel Chemokine Induces Migration of L929 Fibroblasts by ERK Signaling in Microfluidic Devices. Front. Cell Dev. Biol. 2021, 9, 660316. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, X.; Gao, C.; Hua, C.; Hong, C.; Zhu, L. A Novel Microfluidic Device for the Neutrophil Functional Phenotype Analysis: Effects of Glucose and Its Derivatives AGEs. Micromachines 2021, 12, 944. [Google Scholar] [CrossRef]
- Noda, M.; Omatsu, Y.; Sugiyama, T.; Oishi, S.; Fujii, N.; Nagasawa, T. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood 2011, 117, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.K.; Aspalter, I.M.; Sixt, M. Focal Adhesion-Independent Cell Migration. Annu. Rev. Cell Dev. Biol. 2016, 32, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, M.K.; Srinivas, C.S.; Deokate, N.; Rakshit, S. The strong propensity of Cadherin-23 for aggregation inhibits cell migration. Mol. Oncol. 2019, 13, 1092–1109. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Xu, D.; Cui, J.; Wang, Y.; Su, Y.; Liu, Y.; Shen, Y.; Jing, X.; Bai, W. The immunolocalization of cluster of differentiation 31, phalloidin and alpha smooth muscle actin on vascular network of normal and ischemic rat brain. Sci. Rep. 2022, 12, 22288. [Google Scholar] [CrossRef] [PubMed]
- Mazloom-Farsibaf, H.; Farzam, F.; Fazel, M.; Wester, M.J.; Meddens, M.B.M.; Lidke, K.A. Comparing lifeact and phalloidin for super-resolution imaging of actin in fixed cells. PLoS ONE 2021, 16, e0246138. [Google Scholar] [CrossRef]
- Edsparr, K.; Johansson, B.R.; Goldfarb, R.H.; Basse, P.H.; Nannmark, U.; Speetjens, F.M.; Kuppen, P.J.; Lennernäs, B.; Albertsson, P. Human NK cell lines migrate differentially in vitro related to matrix interaction and MMP expression. Immunol. Cell Biol. 2009, 87, 489–495. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Wennerberg, E.; Kremer, V.; Childs, R.; Lundqvist, A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol. Immunother. 2015, 64, 225–235. [Google Scholar] [CrossRef]
- Bernardini, G.; Gismondi, A.; Santoni, A. Chemokines and NK cells: Regulators of development, trafficking and functions. Immunol. Lett. 2012, 145, 39–46. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Morianos, I.; Papadopoulou, G.; Semitekolou, M.; Xanthou, G. Activin-A in the regulation of immunity in health and disease. J. Autoimmun. 2019, 104, 102314. [Google Scholar] [CrossRef] [PubMed]
- Robson, N.C.; Wei, H.; McAlpine, T.; Kirkpatrick, N.; Cebon, J.; Maraskovsky, E. Activin-A attenuates several human natural killer cell functions. Blood 2009, 113, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Suri, S.; Sargent, I.L.; Redman, C.W.; Muttukrishna, S. Maternal circulating levels of activin A, inhibin A, sFlt-1 and endoglin at parturition in normal pregnancy and pre-eclampsia. PLoS ONE 2009, 4, e4453. [Google Scholar] [CrossRef] [PubMed]
- Shahul, S.; Ramadan, H.; Nizamuddin, J.; Mueller, A.; Patel, V.; Dreixler, J.; Tung, A.; Lang, R.M.; Weinert, L.; Nasim, R.; et al. Activin A and Late Postpartum Cardiac Dysfunction Among Women With Hypertensive Disorders of Pregnancy. Hypertension 2018, 72, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Kremer, V.; Ligtenberg, M.A.; Zendehdel, R.; Seitz, C.; Duivenvoorden, A.; Wennerberg, E.; Colón, E.; Scherman-Plogell, A.H.; Lundqvist, A. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J. Immunother. Cancer 2017, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- de Boer, L.L.; Vanes, L.; Melgrati, S.; Biggs O’May, J.; Hayward, D.; Driscoll, P.C.; Day, J.; Griffiths, A.; Magueta, R.; Morrell, A.; et al. T cell migration requires ion and water influx to regulate actin polymerization. Nat. Commun. 2023, 14, 7844. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef]
- Ramírez-Santiago, G.; Robles-Valero, J.; Morlino, G.; Cruz-Adalia, A.; Pérez-Martínez, M.; Zaldivar, A.; Torres-Torresano, M.; Chichón, F.J.; Sorrentino, A.; Pereiro, E.; et al. Clathrin regulates lymphocyte migration by driving actin accumulation at the cellular leading edge. Eur. J. Immunol. 2016, 46, 2376–2387. [Google Scholar] [CrossRef]
- Freeley, M.; O’Dowd, F.; Paul, T.; Kashanin, D.; Davies, A.; Kelleher, D.; Long, A. L-plastin regulates polarization and migration in chemokine-stimulated human T lymphocytes. J. Immunol. 2012, 188, 6357–6370. [Google Scholar] [CrossRef] [PubMed]
- Fionda, C.; Stabile, H.; Molfetta, R.; Kosta, A.; Peruzzi, G.; Ruggeri, S.; Zingoni, A.; Capuano, C.; Soriani, A.; Paolini, R.; et al. Cereblon regulates NK cell cytotoxicity and migration via Rac1 activation. Eur. J. Immunol. 2021, 51, 2607–2617. [Google Scholar] [CrossRef]
- Nieto, M.; Navarro, F.; Perez-Villar, J.J.; del Pozo, M.A.; González-Amaro, R.; Mellado, M.; Frade, J.M.; Martínez, A.C.; López-Botet, M.; Sánchez-Madrid, F. Roles of chemokines and receptor polarization in NK-target cell interactions. J. Immunol. 1998, 161, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, S.; Yang, P.; Han, C.; Wang, J.; Liu, J.; Han, Y.; Yu, Y.; Cao, X. Fibronectin maintains survival of mouse natural killer (NK) cells via CD11b/Src/beta-catenin pathway. Blood 2009, 114, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, H.B.; Zhang, Q.; Liu, W.Y.; Jiang, Y.H.; Peng, G.; Wu, F.Z.; Liu, X.; Yang, P.Y.; Liu, F. Enhancement of E-cadherin expression and processing and driving of cancer cell metastasis by ARID1A deficiency. Oncogene 2021, 40, 5468–5481. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T. Systemic Activation of Activin A Signaling Causes Chronic Kidney Disease-Mineral Bone Disorder. Int. J. Mol. Sci. 2018, 19, 2490. [Google Scholar] [CrossRef]
- Dean, M.; Davis, D.A.; Burdette, J.E. Activin A stimulates migration of the fallopian tube epithelium, an origin of high-grade serous ovarian cancer, through non-canonical signaling. Cancer Lett. 2017, 391, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.S.; Kahana, J.A.; Kumar, R. AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines. Blood 2009, 113, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, S.; Zhang, G.; Wang, W.; Shi, Y. Effect of AKT inhibition on epithelial-mesenchymal transition and ZEB1-potentiated radiotherapy in nasopharyngeal carcinoma. Oncol. Lett. 2013, 6, 1234–1240. [Google Scholar] [CrossRef]
- Hammad, A.S.; Machaca, K. Store Operated Calcium Entry in Cell Migration and Cancer Metastasis. Cells 2021, 10, 1246. [Google Scholar] [CrossRef]
- Liu, L.; Wu, N.; Wang, Y.; Zhang, X.; Xia, B.; Tang, J.; Cai, J.; Zhao, Z.; Liao, Q.; Wang, J. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. J. Exp. Clin. Cancer Res. 2019, 38, 106. [Google Scholar] [CrossRef] [PubMed]
- Cózar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zessner-Spitzenberg, J.; Thomas, A.L.; Krett, N.L.; Jung, B. TGFβ and activin A in the tumor microenvironment in colorectal cancer. Gene Rep. 2019, 17, 100501. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer | Sequence (5′-3′) | Fragment Size (bp) | Tm (°C) |
|---|---|---|---|---|
| GAPDH | F | GATTGTTGCCATCAACGACC | 372 | 56 |
| R | GTGCAGGATGCATTGCTGAC | |||
| Activin-βA | F | CGGGTATGTGGAGATAGAGGA | 148 | 56 |
| R | CAGGTCACTGCCTTCCTTGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Z.; Qi, Y.; Wu, J.; Liu, B.; Cui, X. Activin A, a Novel Chemokine, Induces Mouse NK Cell Migration via AKT and Calcium Signaling. Cells 2024, 13, 728. https://doi.org/10.3390/cells13090728
Wang Y, Liu Z, Qi Y, Wu J, Liu B, Cui X. Activin A, a Novel Chemokine, Induces Mouse NK Cell Migration via AKT and Calcium Signaling. Cells. 2024; 13(9):728. https://doi.org/10.3390/cells13090728
Chicago/Turabian StyleWang, Yunfeng, Zhonghui Liu, Yan Qi, Jiandong Wu, Boyang Liu, and Xueling Cui. 2024. "Activin A, a Novel Chemokine, Induces Mouse NK Cell Migration via AKT and Calcium Signaling" Cells 13, no. 9: 728. https://doi.org/10.3390/cells13090728





