L-carnitine and Ginkgo biloba Supplementation In Vivo Ameliorates HCD-Induced Steatohepatitis and Dyslipidemia by Regulating Hepatic Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
2.3. Serum Biochemical Analysis
2.4. Antioxidant Markers
2.5. Histological and Immunohistochemical Techniques
2.6. Histological Lesion Scoring and Image Analysis
2.7. Ultrastructure Study
2.8. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Biochemical Parameters
3.2.1. Glucose Homeostasis
3.2.2. Lipid Profile
3.2.3. Liver Enzymes
3.3. Antioxidant Markers
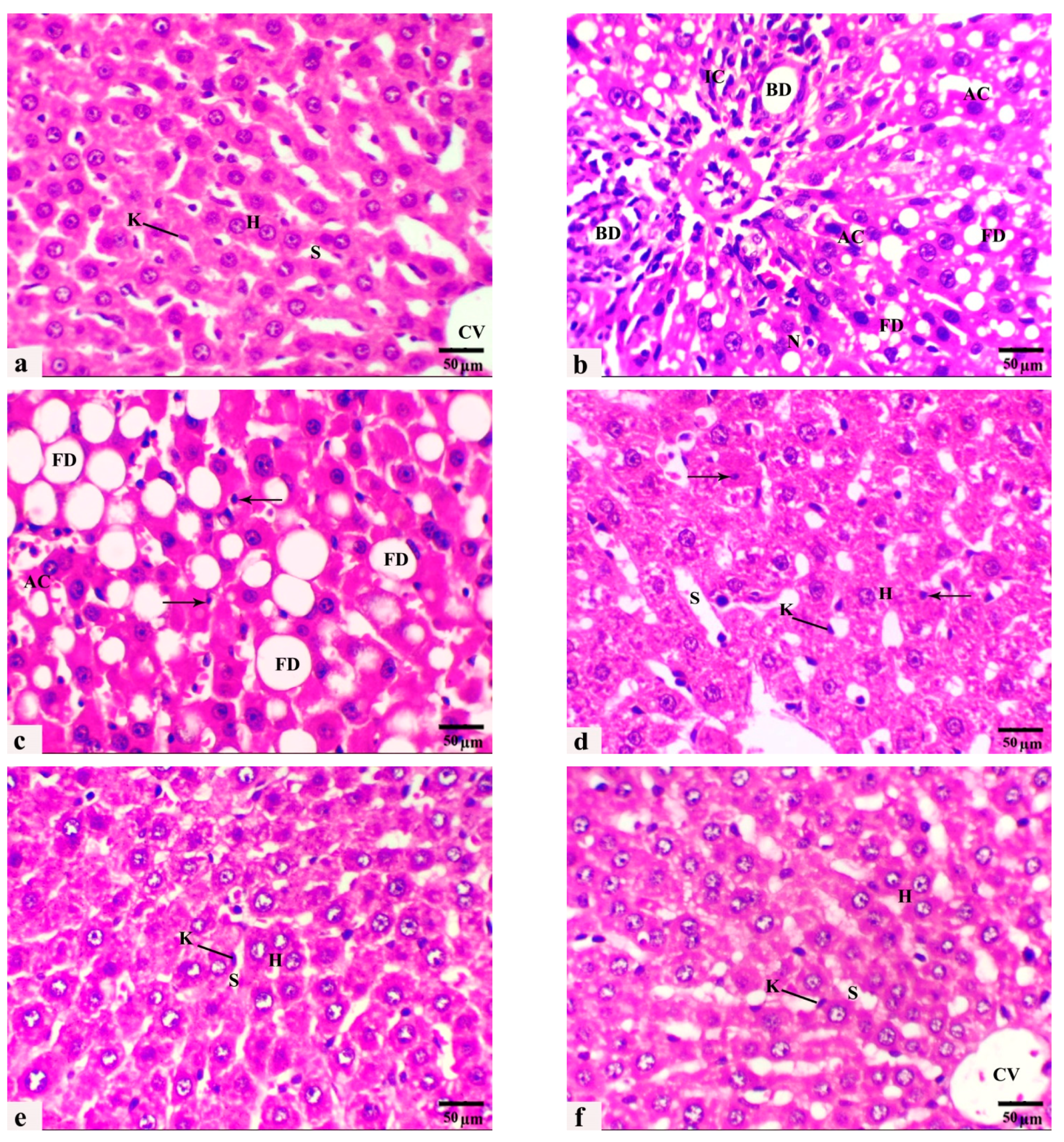
3.4. The Histological Observations
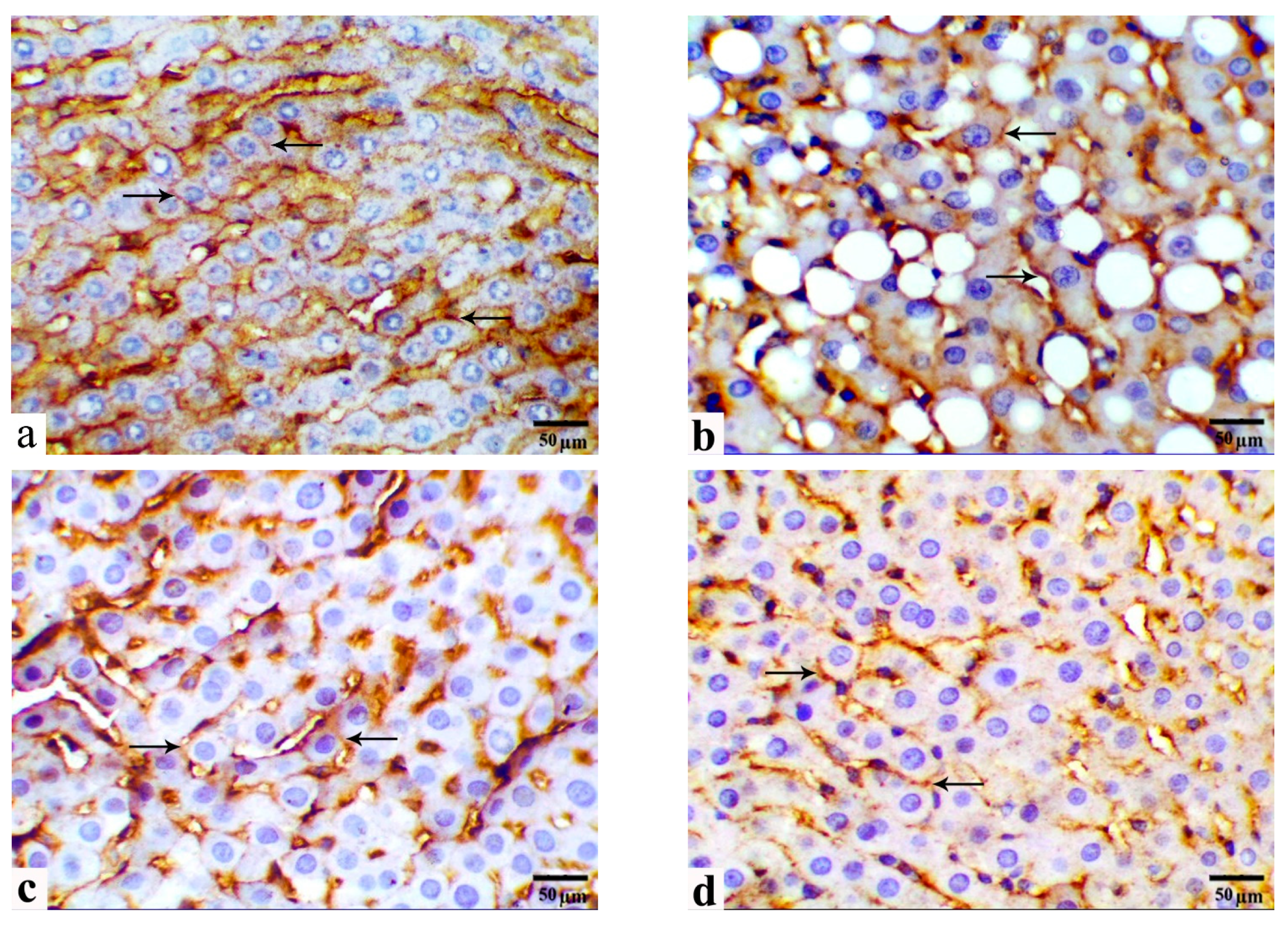
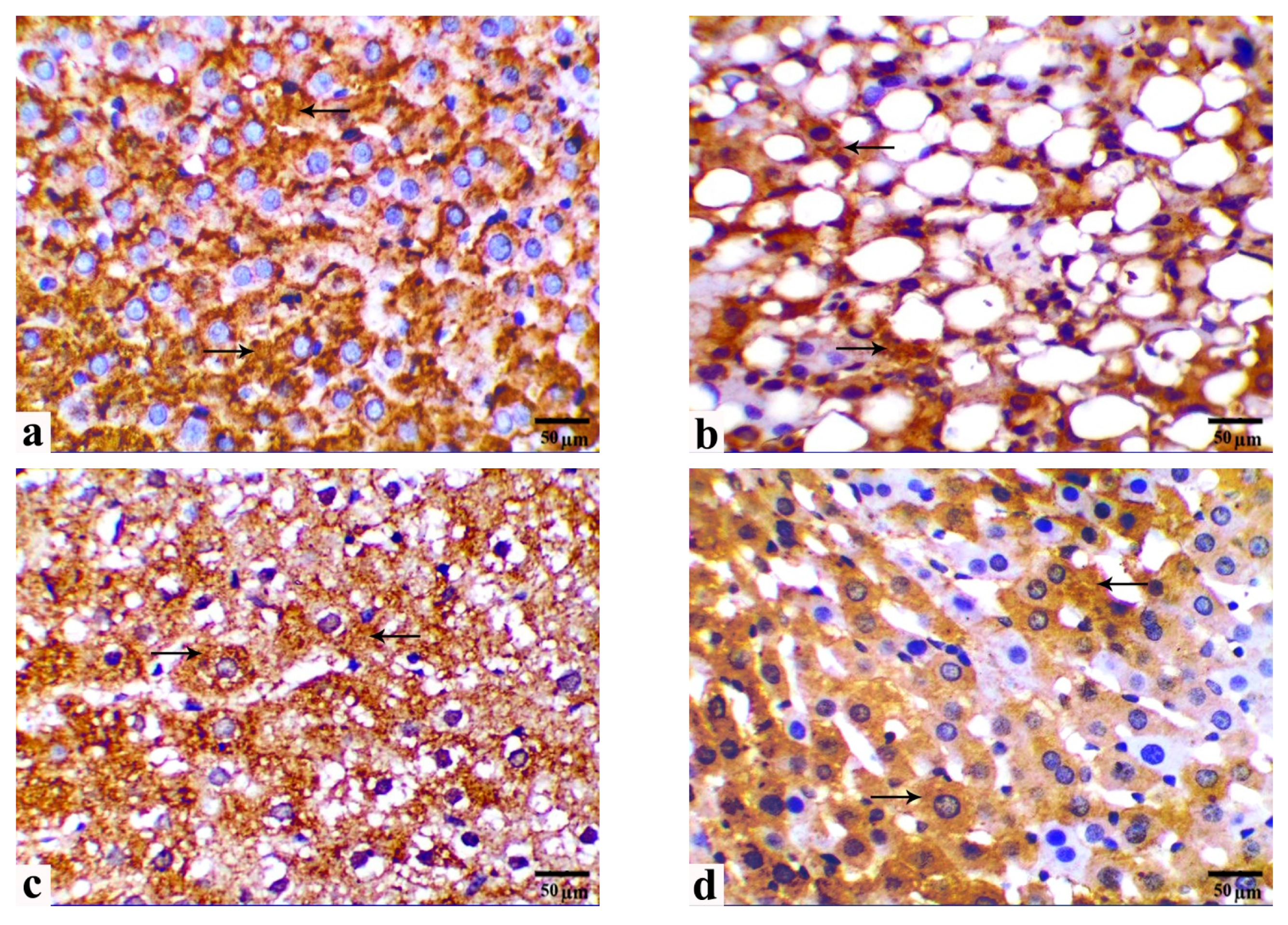
3.5. Immunohistochemical Observations
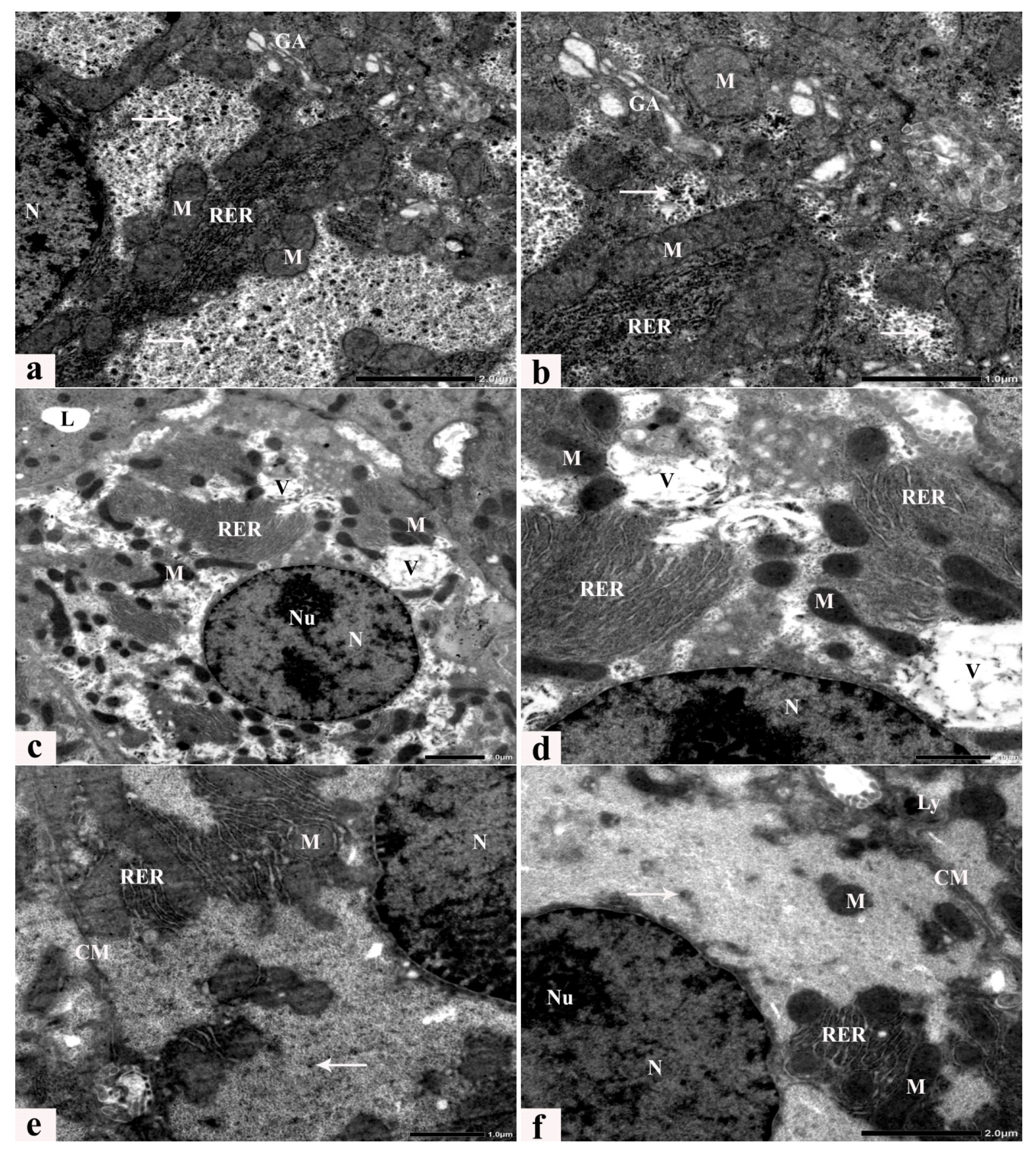
3.6. Ultrastructure Alterations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bence, K.K.; Birnbaum, M.J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 2021, 50, 101143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, K.; Yang, C.; Lin, X.; Zhang, X.; Wang, Y.; Liu, G.; Xian, X. Dietary Cholesterol Is Highly Associated with Severity of Hyperlipidemia and Atherosclerotic Lesions in Heterozygous LDLR-Deficient Hamsters. Int. J. Mol. Sci. 2019, 20, 3515. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Goodspeed, L.; Wang, S.; Kim, J.; Zeng, L.; Ioannou, G.N.; Haigh, W.G.; Yeh, M.M.; Kowdley, K.V.; O’Brien, K.D.; et al. Dietary Cholesterol Exacerbates Hepatic Steatosis and Inflammation in Obese LDL Receptor-Deficient Mice. J. Lipid Res. 2011, 52, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Walenbergh, S.M.; Shiri-Sverdlov, R. Cholesterol is a Significant Risk Factor for Non-Alcoholic Steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.E.; AboShabaan, H.S.; Fayyad, R.M.; Ereba, R.E.; Omar, N.A.; Elsharkawy, S.M.; Elberri, A.I. Immunostimulatory and Anti-Inflammatory Impact of Fragaria Ananassa Methanol Extract in a Rat Model of Cadmium Chloride-Induced Pulmonary Toxicity. Front. Immunol. 2023, 14, 1297315. [Google Scholar] [PubMed]
- Ali, A.F. Histological and Immunohistochemical Study on the Effect of Valporic Acid on the Bone of Adult Male Guinea Pigs and the Possible Protective Role of L-Carnitine. Egypt. J. Histol. 2019, 43, 718–731. [Google Scholar] [CrossRef]
- Sallam, A.O. The Ameliorative Effects of L-Carnitine against Cisplatin-Induced Gonadal Toxicity in Rats. Pak. Vet. J. 2021, 41, 147–151. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Ibrahim, F.M.; Orabi, S.H.; Hassan, A.; El Sabagh, H.S. L-Carnitine-Induced Amelioration of HFD-Induced Hepatic Dysfunction is Accompanied by a Reduction in Hepatic TNF-α and TGF-Β1. Biochem. Cell Biol. 2018, 96, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, M.; Elsayd, F.; Soliman, A.; Fadl, S.E.; El-Shafey, A.; Abdelhiee, E.Y. L-Carnitine and Vitamin E Ameliorate Cardiotoxicity Induced by Tilmicosin in Rats. Environ. Sci. Pollut. Res. 2020, 27, 23026–23034. [Google Scholar]
- Yapar, K.; Kart, A.; Karapehlivan, M.; Atakisi, O.; Tunca, R.; Erginsoy, S.; Citil, M. Hepatoprotective Effect of L-Carnitine against Acute Acetaminophen Toxicity in Mice. Exp. Toxicol. Pathol. 2007, 59, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Fisher-Wellman, K.H.; Tucker, P.S. Effect of Oral Acetyl L-Carnitine Arginate on Resting and Postprandial Blood Biomarkers in Pre-Diabetics. Nutr. Metab. 2009, 6, 25. [Google Scholar] [CrossRef]
- Zambrano, S.; Blanca, A.J.; Ruiz-Armenta, M.V.; Miguel-Carrasco, J.L.; Arévalo, M.; Vázquez, M.J.; Mate, A.; Vázquez, C.M. L-Carnitine Protects against Arterial Hypertension-Related Cardiac Fibrosis through Modulation of PPAR-γ Expression. Biochem. Pharmacol. 2013, 85, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G. Carnitine in Type 2 Diabetes. Ann. N. Y. Acad. Sci. 2004, 1033, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Takashima, H.; Maruyama, T.; Abe, M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients 2021, 13, 1219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Liu, Y.-Y.; Liu, G.-H.; Lu, H.-B.; Mao, C.-Y. L-Carnitine and Heart Disease. Life Sci. 2018, 194, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Mao, Y.Z.; He, S.M.; Yang, Y.; Chen, X. Quantitative Efficacy of L-Carnitine Supplementation on Glycemic Control in Type 2 Diabetes Mellitus Patients. Expert Rev. Clin. Pharmacol. 2021, 14, 919–926. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Montoro, P. Chemical Analysis and Quality Control of Ginkgo biloba Leaves, Extracts, and Phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, Z.; Shen, M.; Lin, W.; Wang, Y.; Wang, S.; Li, C.; Wang, S.; Chen, M.; Shan, W.; et al. Insight into Ginkgo biloba L. Extract on the Improved Spatial Learning and Memory by Chemogenomics Knowledgebase, Molecular Docking, Molecular Dynamics Simulation, and Bioassay Validations. ACS Omega 2020, 5, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- DeFeudis, F.V.; Papadopoulos, V.; Drieu, K. Ginkgo biloba Extracts and Cancer: A Research Area in Its Infancy. Fundam. Clin. Pharmacol. 2003, 17, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.Y.; Lee, J.; Kim, Y.J.; Do, A.; Choi, J.Y.; Cho, S.J.; Jung, U.J.; Lee, M.K.; Park, Y.B.; Choi, M.S. Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity. Nutrients 2017, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Sikder, K.; Kesh, S.B.; Das, N.; Manna, K.; Dey, S. The High Antioxidative Power of Quercetin (Aglycone Flavonoid) and Its Glycone (Rutin) Avert High Cholesterol Diet Induced Hepatotoxicity and Inflammation in Swiss Albino Mice. Food Funct. 2014, 5, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, G.; A, J.Y.; Wu, D.; Zhu, L.; Ma, B.; Du, Y. Application of GC/MS-Based Metabonomic Profiling in Studying the Lipid-Regulating Effects of Ginkgo biloba Extract on Diet-Induced Hyperlipidemia in Rats. Acta Pharmacol. Sin. 2009, 30, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- He, S.X.; Luo, J.Y.; Wang, Y.P.; Wang, Y.L.; Fu, H.; Xu, J.L.; Zhao, G.; Liu, E.Q. Effects of Extract from Ginkgo biloba on Carbon Tetrachloride-Induced Liver Injury in Rats. World J. Gastroenterol. 2006, 12, 3924. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Yang, F.; Zhao, X.; Xue, S.; Gong, F. Ginkgo biloba Extract 50 (GBE50) Ameliorates Insulin Resistance, Hepatic Steatosis and Liver Injury in High Fat Diet-Fed Mice. J. Inflamm. Res. 2021, 14, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Tousson, E.; Keshta, A.T.H.; Hussein, Y.; Fekry, R.M.; Abo-Ghaneima, W.K. Renal Protective Effect of Ginkgo biloba and L-Carnitine Extracts against Pentylenetetrazol Induced Toxicity, Oxidative Stress, Injury and Proliferation Alternation in Epileptic Rats. Annu. Res. Rev. Biol. 2019, 32, 1–13. [Google Scholar] [CrossRef]
- Essawy, A.E.; El-Sayed, S.A.; Tousson, E.; Abd El-gawad, H.S.; Alhasani, R.H.; Abd Elkader, H.-T.A.E. Anti-Kindling Effect of Ginkgo biloba Leaf Extract and L-Carnitine in the Pentylenetetrazol Model of Epilepsy. Environ. Sci. Pollut. Res. 2022, 29, 48573–48587. [Google Scholar] [CrossRef] [PubMed]
- Tousson, E.; Bayomy, M.; ElSendiony, B. Effects of L-Carnitine and Ginkgo biloba on Cerebral Cortex in Experimentally-Induced Epileptic Seizures Disease in Rat. J. Biosci. Appl. 2015, 1, 2356–9182. [Google Scholar]
- Seenak, P.; Kumphune, S.; Malakul, W.; Chotima, R.; Nernpermpisooth, N. Pineapple Consumption Reduced Cardiac Oxidative Stress and Inflammation in High Cholesterol Diet-Fed Rats. Nutr. Metab. 2021, 18, 1–10. [Google Scholar]
- Metwally, F.M.; Rashad, H.; Mahmoud, A.A. Morus alba L. Diminishes Visceral Adiposity, Insulin Resistance, Behavioral Alterations via Regulation of Gene Expression of Leptin, Resistin and Adiponectin in Rats Fed a High-Cholesterol Diet. Physiol. Behav. 2019, 201, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Rao, N.; Research, K.Y.-B. The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen. Biochem. Biophys. 1972, 46, 849–852. [Google Scholar]
- Aebi, H. [13] Catalase in Vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Suvarna, K.; Layton, C.; Bancroft, J. Bancroft’s Theory and Practice of Histological Techniques EBook, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kiernan, J. Histological and Histochemical Methods, 5th ed.; Scion Publishing Ltd.: Banbury, UK, 2015. [Google Scholar]
- Jensen, C.B.; Pyke, C.; Rasch, M.G.; Dahl, A.B.; Knudsen, L.B.; Secher, A. Characterization of the Glucagon like Peptide-1 Receptor in Male Mouse Brain Using a Novel Antibody and In Situ Hybridization. Endocrinology 2018, 159, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lee, J.; Nambiar, S.M.; Hu, M.; Rui, W.; Bao, Q.; Chan, J.Y.; Dai, G. Nrf2 Is Involved in Maintaining Hepatocyte Identity during Liver Regeneration. PLoS ONE 2014, 9, e107423. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; DenK, H.; Desmet, V.; Korb, G.; MacSween, R.N.M.; et al. Histological Grading and Staging of Chronic Hepatitis. J. Hepatol. 1995, 22, 696–704. [Google Scholar] [CrossRef]
- Rasband, W.S.; ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. 2011. Available online: https://www.semanticscholar.org/paper/ImageJ%2C-U.S.-National-Institutes-of-Health%2C-USA-Rasband/034dbc2e4c735500c519183180f8cf6033fcb28d (accessed on 18 March 2024).
- Hayat, M. Principles and Techniques of Electron Microscopy, 4th ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Després, J.; Lemieux, I. Abdominal Obesity and Metabolic Syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Ponmurugan, P.; Deepa, M.A.; Senthilkumar, B. Anti-obesity Action of Gingerol: Effect on Lipid Profile, Insulin, Leptin, Amylase and Lipase in Male Obese Rats Induced by a High-fat Diet. J. Sci. Food Agric. 2014, 94, 2972–2977. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Kim, D.; Sung, K.S.; Jung, S.; Kim, H.J.; Jo, C. The Effect of Dietary α-Lipoic Acid, Betaine, L-Carnitine, and Swimming on the Obesity of Mice Induced by a High-Fat Diet. Food Funct. 2014, 5, 1966–1974. [Google Scholar] [CrossRef]
- Hussein, A.A.; Assad, H.C.; Rabeea, I.S. Antihyperlipidemic, Antioxidant and Anti-Inflammatory Effects of Ginkgo biloba in High Cholesterol Fed Rabbits. J. Pharm. Sci. Res. 2017, 9, 2163–2167. [Google Scholar]
- Salman, M.; Kamel, M.A.; Hassab El-Nabi, S.E.; Ismail, A.H.A.; Ullah, S.; Al-Ghamdi, A.; Hathout, H.M.R.; El-Garawani, I.M. The Regulation of HBP1, SIRT1, and SREBP-1c Genes and the Related MicroRNAs in Nonalcoholic Fatty Liver Rats: The Association with the Folic Acid Anti-Steatosis. PLoS ONE 2022, 17, e0265455. [Google Scholar] [CrossRef] [PubMed]
- Wudel, L.J.; Wright, J.K.; Debelak, J.P.; Allos, T.M.; Shyr, Y.; Chapman, W.C. Prevention of Gallstone Formation in Morbidly Obese Patients Undergoing Rapid Weight Loss: Results of a Randomized Controlled Pilot Study. J. Surg. Res. 2002, 102, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Treatment of Nonalcoholic Fatty Liver Disease. Ann. Hepatol. 2002, 1, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ihedioha, J.I.; Noel-Uneke, O.A.; Ihedioha, T.E. Reference Values for the Serum Lipid Profile of Albino Rats (Rattus norvegicus) of Varied Ages and Sexes. Comp. Clin. Path. 2013, 22, 93–99. [Google Scholar] [CrossRef]
- Fidèle, N.; Joseph, B.; Emmanuel, T.; Théophile, D. Hypolipidemic, Antioxidant and Anti-Atherosclerogenic Effect of Aqueous Extract Leaves of Cassia. occidentalis Linn (Caesalpiniaceae) in Diet-Induced Hypercholesterolemic Rats. BMC Complement. Altern. Med. 2017, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Orabi, S.H.; Al-Sabbagh, E.S.; Khalifa, H.K.; Mohamed, M.A.E.G.; Elhamouly, M.; Gad-Allah, S.M.; Abdel-Daim, M.M.; Abd Eldaim, M.A. Commiphora Myrrha Resin Alcoholic Extract Ameliorates High Fat Diet Induced Obesity via Regulation of UCP1 and Adiponectin Proteins Expression in Rats. Nutrients 2020, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, K.Y.; Kim, M.S.; Lee, J.H.; Lee, K.P.; Park, T. A Mixture of the Aqueous Extract of Garcinia Cambogia, Soy Peptide and L-Carnitine Reduces the Accumulation of Visceral Fat Mass in Rats Rendered Obese by a High Fat Diet. Genes Nutr. 2008, 2, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Mayes, P.A.; Botham, K.M.; Bender, D. Lipid transport and storage, oxidation of fatty acids, gluconeogenesis and control of blood glucose. In Harper’s Illustrated Biochemistry, 26th ed.; Murray, R.K., Granner, D.K., Mayes, P.A., Rodwell, V.W., Eds.; Lange Medical Books/McGraw Hill: New York, NY, USA, 2003; pp. 153–205. [Google Scholar]
- González-Ortiz, M.; Hernández-González, S.O.; Hernández-Salazar, E.; Martínez-Abundis, E. Effect of Oral L-Carnitine Administration on Insulin Sensitivity and Lipid Profile in Type 2 Diabetes Mellitus Patients. Ann. Nutr. Metab. 2008, 52, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-C.; Chang, C.-S.; Chou, C.-H.; Wu, Y.-H.S.; Yang, K.-T.; Tseng, J.-K.; Chang, Y.-Y.; Chen, Y.-C. L-Carnitine Ameliorates Dyslipidemic and Hepatic Disorders Induced by a High-Fat Diet via Regulating Lipid Metabolism, Self-Antioxidant Capacity, and Inflammatory Response. J. Funct. Foods 2015, 15, 497–508. [Google Scholar] [CrossRef]
- Fan, Y.; Jin, X.; Man, C.; Gong, D. Does Adjuvant Treatment with Ginkgo biloba to Statins Have Additional Benefits in Patients with Dyslipidemia? Front. Pharmacol. 2018, 9, 659. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative Stress, Insulin Resistance, Dyslipidemia and Type 2 Diabetes Mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-I.; Jeong, D.H.; Do, S.H.; Kim, Y.K.; Park, H.Y.; Kwon, O.D.; Kim, T.H.; Jeong, K.S. Mild Hepatic Fibrosis in Cholesterol and Sodium Cholate Diet-Fed Rats. J. Vet. Med. Sci. 2005, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Eisvand, F.; Razavi, B.M.; Hosseinzadeh, H. The Effects of Ginkgo biloba on Metabolic Syndrome: A Review. Phyther. Res. 2020, 34, 1798–1811. [Google Scholar] [CrossRef]
- Jing, F.-Y.; Zhou, Y.-Z.; Wang, H.-Y.; Yin, X.-L.; Zhang, Y.-Q. Enhancing Antioxidant and Anti-Hyperglycaemic Functions of Gingko Biloba L. Seeds Using Thermal Detoxification. J. Funct. Foods 2021, 87, 104819. [Google Scholar] [CrossRef]
- Sharma, S.; Nasir, A.; Prabhu, K.; Murthy, P.; Dev, G. Hypoglycaemic and Hypolipidemic Effect of Ethanolic Extract of Seeds of Eugenia jambolana in Alloxan-Induced Diabetic Rabbits. J. Ethnopharmacol. 2003, 85, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, S.; Nazari, L.; Hoseini, S.; Moghaddam, P.B.; Gachkar, L. Effects of L-Carnitine on Polycystic Ovary Syndrome. JBRA Assist. Reprod. 2019, 23, 392–395. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Hasanavand, A.; Falahi, E.; Kashkooli, S.; Asbaghi, O.; Choghakhori, R. Effect of L-Carnitine Supplementation on Lipid Profiles of Patients with Liver Disease: A Systematic Review and Meta-Analysis. Prev. Nutr. Food Sci. 2020, 25, 124. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.; Nagy, M.A. Effect of Carnitine and Herbal Mixture Extract on Obesity Induced by High Fat Diet in Rats. Diabetol. Metab. Syndr. 2009, 1, 1–14. [Google Scholar]
- Sharma, B.; Yadav, D.K. L-Carnitine and Chronic Kidney Disease: A Comprehensive Review on Nutrition and Health Perspectives. J. Pers. Med. 2023, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, G.; Tao, S.; Xia, P.; Chaudhry, N.; Kaura, S.; Stone, S.S.; Liu, M. Ginkgo biloba Extract Reduces Cardiac and Brain Inflammation in Rats Fed a HFD and Exposed to Chronic Mental Stress through NF-κ B Inhibition. Mediat. Inflamm. 2022, 2022, 2408598. [Google Scholar] [CrossRef] [PubMed]
- Milagro, F.; Campión, J.; Martínez, J. Weight Gain Induced by High-fat Feeding Involves Increased Liver Oxidative Stress. Obesity 2006, 14, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity: Implications for Metabolic Syndrome, Diabetes, Hypertension, Dyslipidemia, Atherosclerosis, and Cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Biju, V.; Yoshida, Y. In Vivo ROS Production and Use of Oxidative Stress-Derived Biomarkers to Detect the Onset of Diseases Such as Alzheimer’s Disease, Parkinson’s Disease, and Diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Aboubakr, M.; Elshafae, S.M.; Abdelhiee, E.Y.; Fadl, S.E.; Soliman, A.; Abdelkader, A.; Abdel-Daim, M.M.; Bayoumi, K.A.; Baty, R.S.; Elgendy, E.; et al. Antioxidant and Anti-Inflammatory Potential of Thymoquinone and Lycopene Mitigate the Chlorpyrifos-Induced Toxic Neuropathy. Pharmaceuticals 2021, 14, 940. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Yuksel, A.; Seven, M. The Effects of Carbamazepine and Valproic Acid on the Erythrocyte Glutathione, Glutathione Peroxidase, Superoxide Dismutase and Serum Lipid Peroxidation in Epileptic. Pharmacol. Res. 2000, 41, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.E.; Okdah, Y.A.; Rady, M.I.; Hassaan, H.Z. Gum Acacia Attenuates Cisplatin Toxic Effect Spermatogenesis Dysfunction and Infertility in Rats. Int. J. Biol. Macromol. 2023, 240, 124292. [Google Scholar] [CrossRef] [PubMed]
- Raafat, B.; El-Barbary, A.; Touson, E.; Aziz, S. Di-Mercapto Succinic Acid (DMSA) and Vitamin C Chelating Potency in Lead Intoxication, Regarding Oxidative Stress and Apoptotic Related Proteins in Rabbits. J. Genet. Eng. Biotechnol. 2011, 9, 121–131. [Google Scholar] [CrossRef]
- Ellatif, M.A.; El Karib, A.O.; Dallak, M.; Eid, R.A.; Al-Ani, R.; Haidara, M.A. Vitamin E Protects Against Hepatocyte Ultrastructural Damage Induced by High Fat Diet in a Rat Model of Pre-Diabetes. Int. J. Morphol. 2018, 36, 1350–1355. [Google Scholar] [CrossRef]
- Tandra, S.; Yeh, M.M.; Brunt, E.M.; Vuppalanchi, R.; Cummings, O.W.; Ünalp-Arida, A.; Wilson, L.A.; Chalasani, N. Presence and Significance of Microvesicular Steatosis in Nonalcoholic Fatty Liver Disease. J. Hepatol. 2011, 55, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Duavy, S.M.P.; Salazar, G.J.T.; de Oliveira Leite, G.; Ecker, A.; Barbosa, N.V. Effect of Dietary Supplementation with Olive and Sunflower Oils on Lipid Profile and Liver Histology in Rats Fed High Cholesterol Diet. Asian Pac. J. Trop. Med. 2017, 10, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Mattenni, C.; Younossi, Z.; Gramlich, T.; Boparai, N.; Liu, Y.; Mccullough, A. Nonalcoholic Fatty Liver Disease: A Spectrum of Clinical and Pathological Severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Neuschwander-Tetri, B.A.; Oliver, D.; Wehmeier, K.R.; Bacon, B.R. Nonalcoholic Steatohepatitis: Histologic Features and Clinical Correlations with 30 Blinded Biopsy Specimens. Hum. Pathol. 2004, 35, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Emam, R.A.; Ahmed, E.A.; Ali, M.F. The Protective Role of L-Carnitine against 1st- and 2nd-Generation Antihistamine-Induced Liver Injury in Mice. Comp. Clin. Path. 2020, 29, 213–221. [Google Scholar] [CrossRef]
- Hassan, N.; Rashad, M.; Elleithy, E.; Sabry, Z.; Ali, G.; Elmosalamy, S. L-Carnitine Alleviates Hepatic and Renal Mitochondrial-Dependent Apoptotic Progression Induced by Letrozole in Female Rats through Modulation of Nrf-2, Cyt c and CASP-3 Signaling. Drug Chem. Toxicol. 2023, 46, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Rashad, W.A.; Saadawy, S.F.; Refaay, N.E. Mitigating Effect of L-Carnitine against Atrazine-Induced Hepatotoxicity: Histopathological and Biochemical Analyses in Albino Rats. Environ. Sci. Pollut. Res. 2022, 30, 22034–22045. [Google Scholar] [CrossRef] [PubMed]
- Tousson, E.; Alghabban, A.J.M.; Harga, H.A. Thyroidectomy Induced Hepatic Toxicity and Possible Amelioration by Ginkgo biloba Leaf Extract. Biomed. Prev. Nutr. 2014, 4, 391–397. [Google Scholar] [CrossRef]
- Alisi, A.; Bedogni, G.; De Vito, R.; Comparcola, D.; Manco, M.; Nobili, V. Relationship between Portal Chronic Inflammation and Disease Severity in Paediatric Non-Alcoholic Fatty Liver Disease. Dig. Liver Dis. 2011, 43, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D. Special Considerations in Interpreting Liver Function Tests. Am. Fam. Physician 1999, 59, 2223–2230. [Google Scholar] [PubMed]
- Muniz, L.B.; Alves-Santos, A.M.; Camargo, F.; Martins, D.B.; Celes, M.R.N.; Naves, M.M.V. High-Lard and High-Cholesterol Diet, but Not High-Lard Diet, Leads to Metabolic Disorders in a Modified Dyslipidemia Model. Arq. Bras. Cardiol. 2019, 113, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Y.; Wang, R.; Wang, Y.; Peng, R.; Wu, Y. Ginkgo biloba Extract Mitigates Liver Fibrosis and Apoptosis by Regulating P38 MAPK, NF-κB/IκBα, and Bcl-2/Bax Signaling. Drug Des. Devel. Ther. 2015, 9, 6303–6317. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Park, C.H.; Jun, D.W. Impact of L-Carnitine Supplementation on Liver Enzyme Normalization in Patients with Chronic Liver Disease: A Meta-Analysis of Randomized Trials. J. Pers. Med. 2022, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Manco, M.; Devito, R.; Di Ciommo, V.; Comparcola, D.; Sartorelli, M.R.; Piemonte, F.; Marcellini, M.; Angulo, P. Lifestyle Intervention and Antioxidant Therapy in Children with Nonalcoholic Fatty Liver Disease: A Randomized, Controlled Trial. Hepatology 2008, 48, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.F.; Oneta, C.M.; Gonvers, J.J.; Bihl, F.; Cerny, A.; Cereda, J.M.; Zala, J.F.; Helbling, B.; Steuerwald, M.; Zimmermann, A.; et al. Randomized placebo-controlled trial of ursodeoxycholic acid with Vitamin E in nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2006, 4, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Tveden-Nyborg, P.; Birck, M.M.; Ipsen, D.H.; Thiessen, T.; de Bie Feldmann, L.; Lindblad, M.M.; Jensen, H.E.; Lykkesfeldt, J. Diet-Induced Dyslipidemia Leads to Nonalcoholic Fatty Liver Disease and Oxidative Stress in Guinea Pigs. Transl. Res. 2016, 168, 146–160. [Google Scholar] [CrossRef]
- Rady, M.; Nofal, A.; Okdah, Y.; Hassaan, H. Hepatoprotective Effect of Gum Arabic versus Cisplatin Hepatotoxicity in Adult Male Rats. Egypt. J. Chem. 2023, 66, 377–387. [Google Scholar] [CrossRef]
- Gao, Z.; Song, G.-Y.; Ren, L.-P.; Ma, H.-J.; Ma, B.-Q.; Chen, S.-C. β-Catenin Mediates the Effect of GLP-1 Receptor Agonist on Ameliorating Hepatic Steatosis Induced by High Fructose Diet. Eur. J. Histochem. 2020, 64. [Google Scholar] [CrossRef]
- Torre, C.; Perret, C.; Colnot, S. Transcription Dynamics in a Physiological Process: β-Catenin Signaling Directs Liver Metabolic Zonation. Int. J. Biochem. Cell Biol. 2011, 43, 271–278. [Google Scholar] [CrossRef]
- Monga, S.P. β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Lan, B.Y.A.; Bedolli, M.; Feng, S.; Hebrok, M. Liver-Specific Loss of β-Catenin Blocks Glutamine Synthesis Pathway Activity and Cytochrome P450 Expression in Mice. Hepatology 2006, 43, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Apte, U.; Zeng, G.; Thompson, M.D.; Muller, P.; Micsenyi, A.; Cieply, B.; Kaestner, K.H.; Monga, S.P.S. β-Catenin Is Critical for Early Postnatal Liver Growth. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1578–G1585. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Favero, G.; Lavazza, A.; Golic, I.; Aleksic, M.; Korac, A.; Rodella, L.F.; Rezzani, R. Hepatic Macrosteatosis Is Partially Converted to Microsteatosis by Melatonin Supplementation in Ob/Ob Mice Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0148115. [Google Scholar] [CrossRef]
- Lehwald, N.; Tao, G.; Jang, K.Y.; Papandreou, I.; Liu, B.; Lia, B.; Pysz, M.A.; Willmann, J.K.; Knoefel, W.T.; Denko, N.C.; et al. β-Catenin Regulates Hepatic Mitochondrial Function and Energy Balance in Mice. Gastroenterology 2012, 143, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Fathi, E.; Farahzadi, R.; Charoudeh, H.N. L-Carnitine Contributes to Enhancement of Neurogenesis from Mesenchymal Stem Cells through Wnt/β-Catenin and PKA Pathway. Exp. Biol. Med. 2017, 242, 482–486. [Google Scholar]
- Duca, F.A.; Côté, C.D.; Rasmussen, B.A.; Zadeh-Tahmasebi, M.; Rutter, G.A.; Filippi, B.M.; Lam, T.K.T. Metformin Activates a Duodenal Ampk–Dependent Pathway to Lower Hepatic Glucose Production in Rats. Nat. Med. 2015, 21, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Vrang, N.; Larsen, P.J. Preproglucagon Derived Peptides GLP-1, GLP-2 and Oxyntomodulin in the CNS: Role of Peripherally Secreted and Centrally Produced Peptides. Prog. Neurobiol. 2010, 92, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, W.; Tian, Y.; Tian, F.; Wang, Y.; Sun, X.; Gong, Y. Eugenol Alleviated Nonalcoholic Fatty Liver Disease in Rat via a Gut-Brain-Liver Axis Involving Glucagon-like Peptide-1. Arch. Biochem. Biophys. 2022, 725, 109269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, Y.W.; Zhao, Z.H.; Yang, R.X.; Xin, F.Z.; Liu, X.L.; Pan, Q.; Zhou, H.; Fan, J.G. Sodium Butyrate Reduces High-Fat Diet-Induced Non-Alcoholic Steatohepatitis through Upregulation of Hepatic GLP-1R Expression. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- El-Derany, M.O.; El-Dermerdash, E. Pyrvinium Pamoate Attenuates Non-Alcoholic Steatohepatitis: Insight on Hedgehog/Gli and Wnt/β-Catenin Signaling Crosstalk. Biochem. Pharmacol. 2020, 177, 113942. [Google Scholar] [CrossRef] [PubMed]







| Groups | Body Weight | Glucose (mg/L) | Insulin (uIU/mL) | HOMA-IR | ALT(U/L) | AST (U/L) |
|---|---|---|---|---|---|---|
| Control | 157.67 ± 5.28 | 86.17 ± 8.1 | 16.67 ± 1.15 | 3.73 ± 0.39 | 48.17 ± 4.67 | 94.5 ± 9.42 |
| HCD | 259.83 ± 5.27 acde | 138.22 ± 4.22 acde | 21.6 ± 3.51 e | 5.1 ± 0.55 a | 91.67 ± 4.18 acde | 166 ± 11.08 acde |
| HCD + LC | 218.83 ± 3.43 abe | 119.12 ± 4.02 abe | 18.83 ± 2.52 | 4.88 ± 0.46 a | 62.5 ± 5.86 ab | 118 ± 8.74 ab |
| HCD + GB | 228 ± 7.48 abe | 119.28 ± 4.83 abe | 16.63 ± 1.31 | 4.78 ± 0.48 a | 62 ± 5.18 ab | 118.33 ± 5.47 abc |
| HCD + LC + GB | 191.83 ± 5.19 abcd | 94.76 ± 3.79 bcd | 15.23 ± 1.68 b | 4.087 ± 0.44 | 55 ± 4 b | 113.33 ± 3.72 ab |
| Groups | Control (Standard Diet) | HCD | HCD + LC | HCD + GB | HCD + LC + GB |
|---|---|---|---|---|---|
| Lesions | |||||
| Hepatic steatosis | 0.00 ± 0.00 b | 2.70 ± 0.00 a | 1.00 ± 0.00 ab | 1.20 ± 0.00 ab | 0.50 ± 0.00 b |
| Ballooning degeneration | 0.00 ± 0.00 b | 2.00 ± 0.00 a | 1.00 ± 0.00 ab | 0.60 ± 0.00 ab | 0.20 ± 0.00 b |
| Lobular inflammation | 0.00 ± 0.00 b | 2.00 ± 0.00 a | 0.60 ± 0.00 ab | 0.40 ± 0.00 b | 0.10 ± 0.00 b |
| Hepatic fibrosis | 0.00 ± 0.00 b | 3.00 ± 0.00 a | 1.00 ± 0.00 a | 0.50 ± 0.00 b | 0.30 ± 0.00 b |
| Steatosis grade; 5% (0), 5–33% (1), 34–66% (2), 66% (3). | |||||
| Hepatocellular ballooning: none (0), little ballooned hepatocytes (1), several ballooned hepatocytes (2). | |||||
| Lobular inflammation: no foci (0), 2 foci (1), 2–4 foci (2), 4 foci (3) per 20× field. | |||||
| Hepatic fibrosis: No fibrosis (0), fibrous with or without short septa in some portal areas (1), fibrous with or without short septa in most of the portal tracts (2), fibrous with portal-portal bridges in most portal areas (3), fibrous with portal-portal bridges and portal enter bridges in portal areas (4), noticeable portal-portal bridges or/and bridges with occasional nodules (5), and cirrhosis (6). | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nofal, A.E.; AboShabaan, H.S.; Fadda, W.A.; Ereba, R.E.; Elsharkawy, S.M.; Hathout, H.M. L-carnitine and Ginkgo biloba Supplementation In Vivo Ameliorates HCD-Induced Steatohepatitis and Dyslipidemia by Regulating Hepatic Metabolism. Cells 2024, 13, 732. https://doi.org/10.3390/cells13090732
Nofal AE, AboShabaan HS, Fadda WA, Ereba RE, Elsharkawy SM, Hathout HM. L-carnitine and Ginkgo biloba Supplementation In Vivo Ameliorates HCD-Induced Steatohepatitis and Dyslipidemia by Regulating Hepatic Metabolism. Cells. 2024; 13(9):732. https://doi.org/10.3390/cells13090732
Chicago/Turabian StyleNofal, Amany E., Hind S. AboShabaan, Walaa A. Fadda, Rafik E. Ereba, Sherin M. Elsharkawy, and Heba M. Hathout. 2024. "L-carnitine and Ginkgo biloba Supplementation In Vivo Ameliorates HCD-Induced Steatohepatitis and Dyslipidemia by Regulating Hepatic Metabolism" Cells 13, no. 9: 732. https://doi.org/10.3390/cells13090732





