CD4+ T-Cell Senescence in Neurodegenerative Disease: Pathogenesis and Potential Therapeutic Targets
Abstract
:1. Introduction
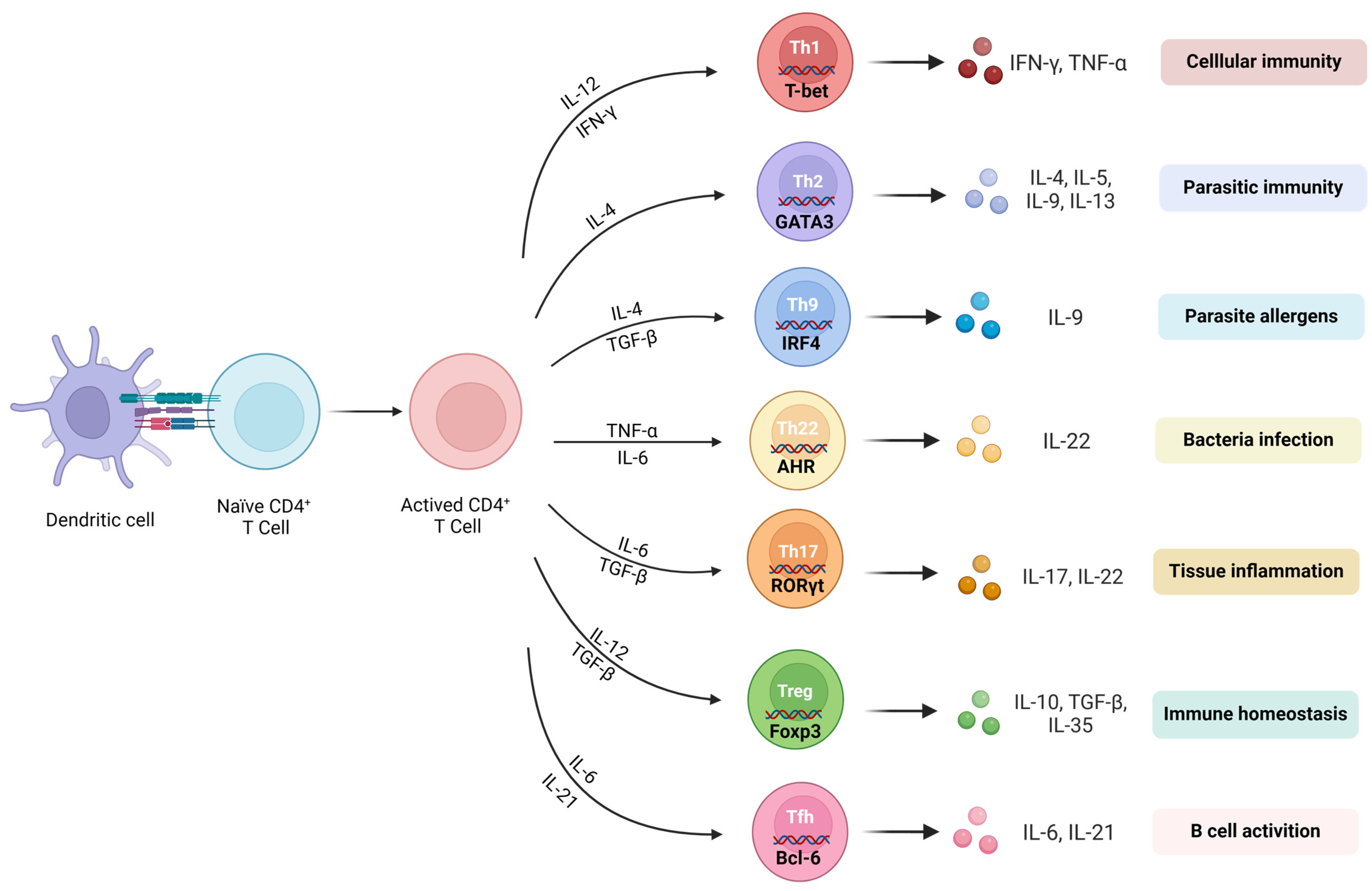
2. Subsets of CD4+ T-Cells and Their Roles in Neurodegenerative Diseases
2.1. Naïve CD4+ T-Cells
2.2. Effector CD4+ T-Cells
2.3. Memory CD4+ T-Cells
3. Features of CD4+ T-Cell Senescence
3.1. Thymic Involution
3.2. Imbalance of Naïve CD4+ T and Memory CD4+ T
3.3. Decrease in CD4+ T-Cell Plasticity
3.4. The Metabolic Changes in CD4+ T-Cells
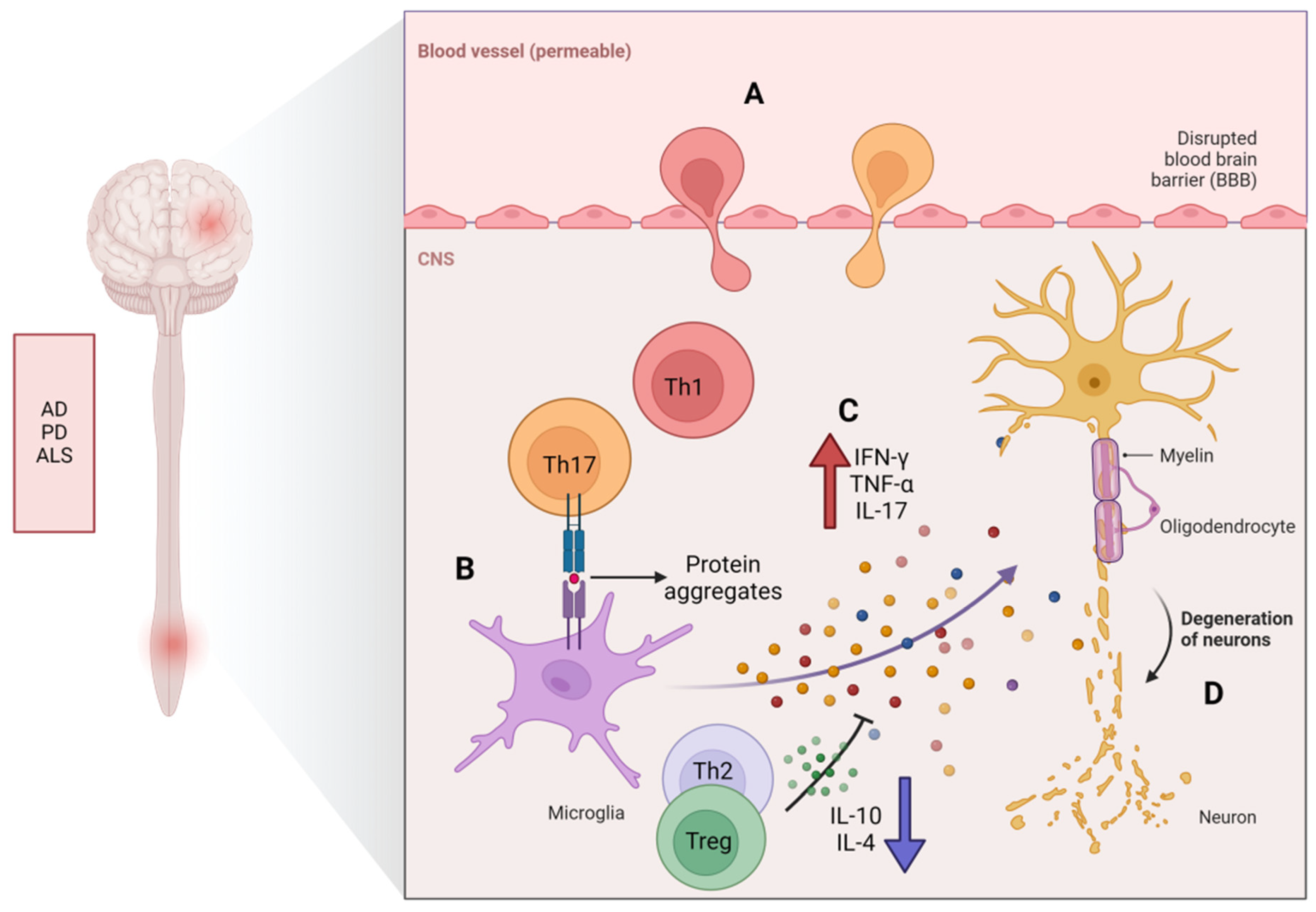
4. The Potential Role of CD4+ T-Cell Senescence in Neurodegenerative Diseases
4.1. Multiple Sclerosis
4.2. Alzheimer’s Disease
4.3. Parkinson’s Disease
4.4. Amyotrophic Lateral Sclerosis
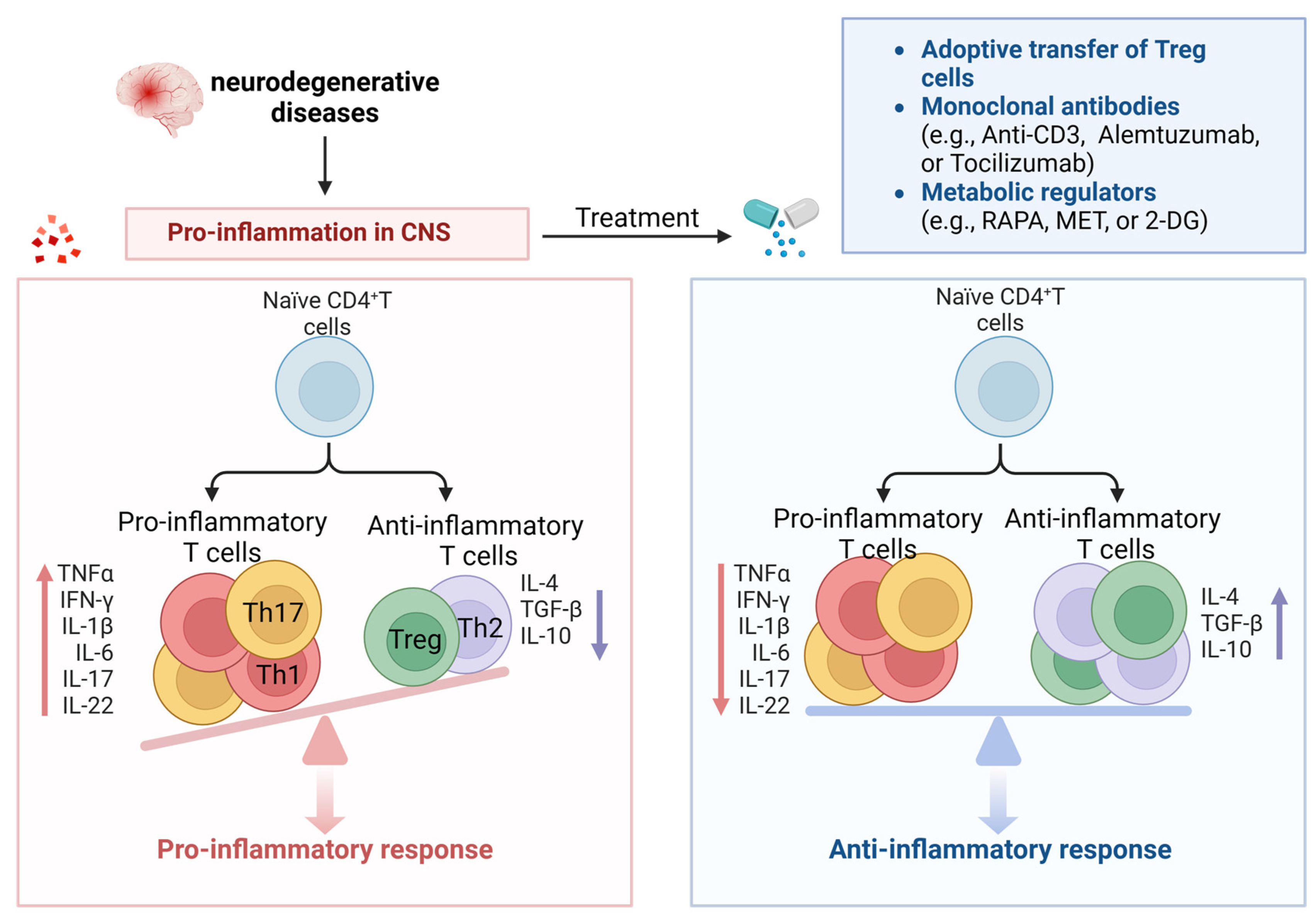
5. The Dawn of Targeting Senescent CD4+ T-Cells for the Treatment of Neurodegenerative Diseases
5.1. Adoptive Transfer of Treg
5.2. Monoclonal Antibodies
5.3. Hormone and Cytokines
5.4. Metabolic Regulators
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rex, N.; Melk, A.; Schmitt, R. Cellular senescence and kidney aging. Clin. Sci. 2023, 137, 1805–1821. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Kunkl, M.; Frascolla, S.; Amormino, C.; Volpe, E.; Tuosto, L. T Helper Cells: The Modulators of Inflammation in Multiple Sclerosis. Cells 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Wang, C.; Han, T.; Liu, H.; Sun, L.; Hong, J.; Hashimoto, M.; Wei, J. The reciprocal interactions between microglia and T cells in Parkinson’s disease: A double-edged sword. J. Neuroinflamm. 2023, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Sheikhi, A.; Jafarzadeh, Z.; Nemati, M. Differential roles of regulatory T cells in Alzheimer’s disease. Cell Immunol. 2023, 393–394, 104778. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, I.; Heath, P.; Shaw, P.J.; Kirby, J. The involvement of regulatory T cells in amyotrophic lateral sclerosis and their therapeutic potential. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef]
- Fleige, H.; Bosnjak, B.; Permanyer, M.; Ristenpart, J.; Bubke, A.; Willenzon, S.; Sutter, G.; Luther, S.A.; Förster, R. Manifold Roles of CCR7 and Its Ligands in the Induction and Maintenance of Bronchus-Associated Lymphoid Tissue. Cell Rep. 2018, 23, 783–795. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Herz, J.; Wall, M.; Dykstra, T.; de Lima, K.A.; Norris, G.T.; Dabhi, N.; Kennedy, T.; Baker, W.; Kipnis, J. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and β-amyloid pathology. Sci. Adv. 2021, 7, eabe4601. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jankovic, D.; Oler, A.J.; Wei, G.; Sharma, S.; Hu, G.; Guo, L.; Yagi, R.; Yamane, H.; Punkosdy, G.; et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 2012, 37, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ke, K.F.; Liu, Z.; Qiu, Y.H.; Peng, Y.P. Th17 cell-mediated neuroinflammation is involved in neurodegeneration of aβ1-42-induced Alzheimer’s disease model rats. PLoS ONE 2013, 8, e75786. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Yeapuri, P.; Lu, Y.; Foster, E.; Chikhale, R.; Herskovitz, J.; Namminga, K.L.; Olson, K.E.; Abdelmoaty, M.M.; Gao, J.; et al. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J. Neuroinflamm. 2021, 18, 272. [Google Scholar] [CrossRef] [PubMed]
- Browne, T.C.; McQuillan, K.; McManus, R.M.; O’Reilly, J.A.; Mills, K.H.; Lynch, M.A. IFN-γ Production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J. Immunol. 2013, 190, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A.; McKenzie, A.N.J. T(H)2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhu, J. Transcriptional regulators dictate innate lymphoid cell fates. Protein Cell 2017, 8, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Günther, R.; Akgün, K.; Hermann, A.; Ziemssen, T. Peripheral proinflammatory Th1/Th17 immune cell shift is linked to disease severity in amyotrophic lateral sclerosis. Sci. Rep. 2020, 10, 5941. [Google Scholar] [CrossRef]
- Dardalhon, V.; Awasthi, A.; Kwon, H.; Galileos, G.; Gao, W.; Sobel, R.A.; Mitsdoerffer, M.; Strom, T.B.; Elyaman, W.; Ho, I.C.; et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 2008, 9, 1347–1355. [Google Scholar] [CrossRef]
- Liu, Y.; Teige, I.; Birnir, B.; Issazadeh-Navikas, S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat. Med. 2006, 12, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Finiasz, M.R.; Franco, M.C.; de la Barrera, S.; Rutitzky, L.; Pizzariello, G.; del Carmen Sasiain, M.; Renauld, J.C.; Van Snick, J.; Fink, S. IL-9 promotes anti-Mycobacterium leprae cytotoxicity: Involvement of IFNgamma. Clin. Exp. Immunol. 2007, 147, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Elyaman, W.; Bradshaw, E.M.; Uyttenhove, C.; Dardalhon, V.; Awasthi, A.; Imitola, J.; Bettelli, E.; Oukka, M.; van Snick, J.; Renauld, J.C.; et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA 2009, 106, 12885–12890. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, J.B.; Van Roost, E.; Stevens, M.; Groner, B.; Renauld, J.C. Distinct roles for STAT1, STAT3, and STAT5 in differentiation gene induction and apoptosis inhibition by interleukin-9. J. Biol. Chem. 1999, 274, 25855–25861. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, J.B.; Uyttenhove, C.; Van Roost, E.; DeLestré, B.; Donckers, D.; Van Snick, J.; Renauld, J.C. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol. Cell Biol. 1996, 16, 4710–4716. [Google Scholar] [CrossRef]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef]
- Kanamori, M.; Nakatsukasa, H.; Okada, M.; Lu, Q.; Yoshimura, A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016, 37, 803–811. [Google Scholar] [CrossRef]
- Zhou, X.; Bailey-Bucktrout, S.L.; Jeker, L.T.; Penaranda, C.; Martínez-Llordella, M.; Ashby, M.; Nakayama, M.; Rosenthal, W.; Bluestone, J.A. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009, 10, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, Y.P.; Niec, R.E.; Josefowicz, S.; Li, L.; Darce, J.; Mathis, D.; Benoist, C.; Rudensky, A.Y. Stability of the regulatory T cell lineage in vivo. Science 2010, 329, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Rentzos, M.; Evangelopoulos, E.; Sereti, E.; Zouvelou, V.; Marmara, S.; Alexakis, T.; Evdokimidis, I. Alterations of T cell subsets in ALS: A systemic immune activation? Acta Neurol. Scand. 2012, 125, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Dansokho, C.; Ait Ahmed, D.; Aid, S.; Toly-Ndour, C.; Chaigneau, T.; Calle, V.; Cagnard, N.; Holzenberger, M.; Piaggio, E.; Aucouturier, P.; et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 2016, 139 Pt 4, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, D.; Ma, W.; Wang, Y.; Yan, H. Bcl-6 controlled TFH polarization and memory: The known unknowns. Curr. Opin. Immunol. 2014, 28, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Förster, R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Nohra, R.; Beyeen, A.D.; Guo, J.P.; Khademi, M.; Sundqvist, E.; Hedreul, M.T.; Sellebjerg, F.; Smestad, C.; Oturai, A.B.; Harbo, H.F.; et al. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Genes Immun. 2010, 11, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Ito, M.; Srirat, T.; Kondo, T.; Yoshimura, A. Memory T cell, exhaustion, and tumor immunity. Immunol. Med. 2020, 43, 1–9. [Google Scholar] [CrossRef]
- Soon, M.S.; Engel, J.A.; Lee, H.J.; Haque, A. Development of circulating CD4(+) T-cell memory. Immunol. Cell Biol. 2019, 97, 617–624. [Google Scholar] [CrossRef]
- Murphy, A.C.; Lalor, S.J.; Lynch, M.A.; Mills, K.H. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2010, 24, 641–651. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.M.; Higgins, S.C.; Mills, K.H.; Lynch, M.A. Respiratory infection promotes T cell infiltration and amyloid-β deposition in APP/PS1 mice. Neurobiol. Aging 2014, 35, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.E.; Goldberg, G.L.; Chidgey, A.; Van den Brink, M.R.; Boyd, R.; Sempowski, G.D. Thymic involution and immune reconstitution. Trends Immunol. 2009, 30, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.S.; Velardi, E.; Dudakov, J.A.; van den Brink, M.R. Thymus: The next (re)generation. Immunol. Rev. 2016, 271, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Minato, N.; Hattori, M.; Hamazaki, Y. Physiology and pathology of T-cell aging. Int. Immunol. 2020, 32, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Wang, W.; Su, D.M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Monsonego, A.; Zota, V.; Karni, A.; Krieger, J.I.; Bar-Or, A.; Bitan, G.; Budson, A.E.; Sperling, R.; Selkoe, D.J.; Weiner, H.L. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J. Clin. Investig. 2003, 112, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dema, M.; Eixarch, H.; Villar, L.M.; Montalban, X.; Espejo, C. Immunosenescence in multiple sclerosis: The identification of new therapeutic targets. Autoimmun. Rev. 2021, 20, 102893. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, C.B.; Jakimovski, D.; Kavak, K.S.; Ramanathan, M.; Benedict, R.H.B.; Zivadinov, R.; Weinstock-Guttman, B. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat. Rev. Neurol. 2019, 15, 329–342. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Lei, L.; Sun, L.; Jiao, A.; Zhu, K.; Xie, T.; Liu, H.; Zhang, X.; Su, Y.; et al. Age-Related Gene Alteration in Naïve and Memory T cells Using Precise Age-Tracking Model. Front. Cell Dev. Biol. 2020, 8, 624380. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Shen, Y. Insights into T-cell dysfunction in Alzheimer’s disease. Aging Cell 2021, 20, e13511. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Peng, K.; Li, R.; Zhang, Z.; Pan, L.; Zhang, T.; Lin, A.; Hong, R.; Nie, Z.; Guan, Q.; et al. Changes of T lymphocyte subpopulations and their roles in predicting the risk of Parkinson’s disease. J. Neurol. 2022, 269, 5368–5381. [Google Scholar] [CrossRef]
- Weng, N.P.; Akbar, A.N.; Goronzy, J. CD28(-) T cells: Their role in the age-associated decline of immune function. Trends Immunol. 2009, 30, 306–312. [Google Scholar] [CrossRef]
- Broux, B.; Pannemans, K.; Zhang, X.; Markovic-Plese, S.; Broekmans, T.; Eijnde, B.O.; Van Wijmeersch, B.; Somers, V.; Geusens, P.; van der Pol, S.; et al. CX(3)CR1 drives cytotoxic CD4(+)CD28(-) T cells into the brain of multiple sclerosis patients. J. Autoimmun. 2012, 38, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.P.; Effros, R.B. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar] [CrossRef]
- Elyahu, Y.; Hekselman, I.; Eizenberg-Magar, I.; Berner, O.; Strominger, I.; Schiller, M.; Mittal, K.; Nemirovsky, A.; Eremenko, E.; Vital, A.; et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci. Adv. 2019, 5, eaaw8330. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Xie, Y.; Chen, X.; Tian, L.; Liang, Y.; Li, H.; Zhang, J.; Liu, Y.; Yu, X. Interleukin-6 Knockout Inhibits Senescence of Bone Mesenchymal Stem Cells in High-Fat Diet-Induced Bone Loss. Front. Endocrinol. 2020, 11, 622950. [Google Scholar] [CrossRef]
- Uciechowski, P.; Kahmann, L.; Plümäkers, B.; Malavolta, M.; Mocchegiani, E.; Dedoussis, G.; Herbein, G.; Jajte, J.; Fulop, T.; Rink, L. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp. Gerontol. 2008, 43, 493–498. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Saresella, M.; Calabrese, E.; Marventano, I.; Piancone, F.; Gatti, A.; Alberoni, M.; Nemni, R.; Clerici, M. Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav. Immun. 2011, 25, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Oberstein, T.J.; Taha, L.; Spitzer, P.; Hellstern, J.; Herrmann, M.; Kornhuber, J.; Maler, J.M. Imbalance of Circulating T(h)17 and Regulatory T Cells in Alzheimer’s Disease: A Case Control Study. Front. Immunol. 2018, 9, 1213. [Google Scholar] [CrossRef] [PubMed]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e46. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.L.; Kuehn, H.S.; Zhao, F.; Niemela, J.E.; Deenick, E.K.; Palendira, U.; Avery, D.T.; Moens, L.; Cannons, J.L.; Biancalana, M.; et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 2014, 15, 88–97. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, T.; Gao, J.; Liu, X.; Hou, H.; Cao, R.; Li, B.; Quan, M.; Guo, L. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J. Neuroimmunol. 2016, 292, 58–67. [Google Scholar] [CrossRef]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Bektas, A.; Schurman, S.H.; Gonzalez-Freire, M.; Dunn, C.A.; Singh, A.K.; Macian, F.; Cuervo, A.M.; Sen, R.; Ferrucci, L. Age-associated changes in human CD4(+) T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging 2019, 11, 9234–9263. [Google Scholar] [CrossRef]
- Desdín-Micó, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabandé-Rodríguez, E.; Blanco, E.M.; Alfranca, A.; Cussó, L.; Desco, M.; et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef]
- Tomas-Ojer, P.; Puthenparampil, M.; Cruciani, C.; Docampo, M.J.; Martin, R.; Sospedra, M. Characterization of Antigen-Induced CD4+ T-Cell Senescence in Multiple Sclerosis. Front. Neurol. 2022, 13, 790884. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef]
- Weyand, C.M.; Brandes, J.C.; Schmidt, D.; Fulbright, J.W.; Goronzy, J.J. Functional properties of CD4+ CD28- T cells in the aging immune system. Mech. Ageing Dev. 1998, 102, 131–147. [Google Scholar] [CrossRef]
- Thewissen, M.; Linsen, L.; Somers, V.; Geusens, P.; Raus, J.; Stinissen, P. Premature immunosenescence in rheumatoid arthritis and multiple sclerosis patients. Ann. N. Y. Acad. Sci. 2005, 1051, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Broux, B.; Markovic-Plese, S.; Stinissen, P.; Hellings, N. Pathogenic features of CD4+CD28- T cells in immune disorders. Trends Mol. Med. 2012, 18, 446–453. [Google Scholar] [CrossRef]
- Haegert, D.G. Multiple sclerosis: A disorder of altered T-cell homeostasis. Mult. Scler. Int. 2011, 2011, 461304. [Google Scholar] [CrossRef] [PubMed]
- Zuroff, L.; Rezk, A.; Shinoda, K.; Espinoza, D.A.; Elyahu, Y.; Zhang, B.; Chen, A.A.; Shinohara, R.T.; Jacobs, D.; Alcalay, R.N.; et al. Immune aging in multiple sclerosis is characterized by abnormal CD4 T cell activation and increased frequencies of cytotoxic CD4 T cells with advancing age. eBioMedicine 2022, 82, 104179. [Google Scholar] [CrossRef]
- Carlson, T.; Kroenke, M.; Rao, P.; Lane, T.E.; Segal, B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 2008, 205, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Town, T.; Tan, J.; Flavell, R.A.; Mullan, M. T-cells in Alzheimer’s disease. Neuromol. Med. 2005, 7, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Togo, T.; Akiyama, H.; Iseki, E.; Kondo, H.; Ikeda, K.; Kato, M.; Oda, T.; Tsuchiya, K.; Kosaka, K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002, 124, 83–92. [Google Scholar] [CrossRef]
- Panossian, L.A.; Porter, V.R.; Valenzuela, H.F.; Zhu, X.; Reback, E.; Masterman, D.; Cummings, J.L.; Effros, R.B. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol. Aging 2003, 24, 77–84. [Google Scholar] [CrossRef]
- Larbi, A.; Pawelec, G.; Witkowski, J.M.; Schipper, H.M.; Derhovanessian, E.; Goldeck, D.; Fulop, T. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Pellicanò, M.; Larbi, A.; Goldeck, D.; Colonna-Romano, G.; Buffa, S.; Bulati, M.; Rubino, G.; Iemolo, F.; Candore, G.; Caruso, C.; et al. Immune profiling of Alzheimer patients. J. Neuroimmunol. 2012, 242, 52–59. [Google Scholar] [CrossRef]
- Serre-Miranda, C.; Roque, S.; Santos, N.C.; Portugal-Nunes, C.; Costa, P.; Palha, J.A.; Sousa, N.; Correia-Neves, M. Effector memory CD4(+) T cells are associated with cognitive performance in a senior population. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e54. [Google Scholar] [CrossRef]
- Hotta, K.; Sho, M.; Fujimoto, K.; Shimada, K.; Yamato, I.; Anai, S.; Konishi, N.; Hirao, Y.; Nonomura, K.; Nakajima, Y. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br. J. Cancer 2011, 105, 1191–1196. [Google Scholar] [CrossRef]
- Sun, L.; Ju, T.; Wang, T.; Zhang, L.; Ding, F.; Zhang, Y.; An, R.; Sun, Y.; Li, Y.; Lu, Y.; et al. Decreased Netrin-1 and Correlated Th17/Tregs Balance Disorder in Aβ(1-42) Induced Alzheimer’s Disease Model Rats. Front. Aging Neurosci. 2019, 11, 124. [Google Scholar] [CrossRef]
- D’Angelo, C.; Goldeck, D.; Pawelec, G.; Gaspari, L.; Di Iorio, A.; Paganelli, R. Exploratory study on immune phenotypes in Alzheimer’s disease and vascular dementia. Eur. J. Neurol. 2020, 27, 1887–1894. [Google Scholar] [CrossRef]
- Ciccocioppo, F.; Lanuti, P.; Pierdomenico, L.; Simeone, P.; Bologna, G.; Ercolino, E.; Buttari, F.; Fantozzi, R.; Thomas, A.; Onofrj, M.; et al. The Characterization of Regulatory T-Cell Profiles in Alzheimer’s Disease and Multiple Sclerosis. Sci. Rep. 2019, 9, 8788. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, C.; Volpicelli, F.; Lippiello, P.; Buono, B.; Raucci, F.; Piccolo, M.; Iqbal, A.J.; Irace, C.; Miniaci, M.C.; Perrone Capano, C.; et al. Neutralization of IL-17 rescues amyloid-β-induced neuroinflammation and memory impairment. Br. J. Pharmacol. 2019, 176, 3544–3557. [Google Scholar] [CrossRef] [PubMed]
- Vellecco, V.; Saviano, A.; Raucci, F.; Casillo, G.M.; Mansour, A.A.; Panza, E.; Mitidieri, E.; Femminella, G.D.; Ferrara, N.; Cirino, G.; et al. Interleukin-17 (IL-17) triggers systemic inflammation, peripheral vascular dysfunction, and related prothrombotic state in a mouse model of Alzheimer’s disease. Pharmacol. Res. 2023, 187, 106595. [Google Scholar] [CrossRef]
- Brigas, H.C.; Ribeiro, M.; Coelho, J.E.; Gomes, R.; Gomez-Murcia, V.; Carvalho, K.; Faivre, E.; Costa-Pereira, S.; Darrigues, J.; de Almeida, A.A.; et al. IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer’s disease. Cell Rep. 2021, 36, 109574. [Google Scholar] [CrossRef]
- Rosenmann, H.; Grigoriadis, N.; Eldar-Levy, H.; Avital, A.; Rozenstein, L.; Touloumi, O.; Behar, L.; Ben-Hur, T.; Avraham, Y.; Berry, E.; et al. A novel transgenic mouse expressing double mutant tau driven by its natural promoter exhibits tauopathy characteristics. Exp. Neurol. 2008, 212, 71–84. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Vom Berg, J.; Prokop, S.; Miller, K.R.; Obst, J.; Kälin, R.E.; Lopategui-Cabezas, I.; Wegner, A.; Mair, F.; Schipke, C.G.; Peters, O.; et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 2012, 18, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; Klegeris, A.; McGeer, P.L. Inflammation, the complement system and the diseases of aging. Neurobiol. Aging 2005, 26 (Suppl. 1), 94–97. [Google Scholar] [CrossRef]
- Chen, J.H.; Ke, K.F.; Lu, J.H.; Qiu, Y.H.; Peng, Y.P. Protection of TGF-β1 against neuroinflammation and neurodegeneration in Aβ1-42-induced Alzheimer’s disease model rats. PLoS ONE 2015, 10, e0116549. [Google Scholar] [CrossRef]
- Zenaro, E.; Pietronigro, E.; Della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Qu, Q.; Zhao, X.; Zhang, J. JKAP, Th1 cells, and Th17 cells are dysregulated and inter-correlated, among them JKAP and Th17 cells relate to cognitive impairment progression in Alzheimer’s disease patients. Ir. J. Med. Sci. 2022, 191, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Yang, X.; Yang, X.; Xue, J.; Yang, Y. Ganoderic Acid A To Alleviate Neuroinflammation of Alzheimer’s Disease in Mice by Regulating the Imbalance of the Th17/Tregs Axis. J. Agric. Food Chem. 2021, 69, 14204–14214. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.K.; Reynolds, A.D.; Mosley, R.L.; Gendelman, H.E. Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid. Redox Signal. 2009, 11, 2151–2166. [Google Scholar] [CrossRef]
- Zoey, F.L.; Palanivel, M.; Padmanabhan, P.; Gulyás, B. Parkinson’s Disease: A Nanotheranostic Approach Targeting Alpha-Synuclein Aggregation. Front. Cell Dev. Biol. 2021, 9, 707441. [Google Scholar] [CrossRef]
- Goedert, M. Neurodegeneration. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Cedillos, R.; Choyke, S.; Lukic, Z.; McGuire, K.; Marvin, S.; Burrage, A.M.; Sudholt, S.; Rana, A.; O’Connor, C.; et al. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS ONE 2013, 8, e62143. [Google Scholar] [CrossRef] [PubMed]
- Danzer, K.M.; Haasen, D.; Karow, A.R.; Moussaud, S.; Habeck, M.; Giese, A.; Kretzschmar, H.; Hengerer, B.; Kostka, M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007, 27, 9220–9232. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.D.; Stone, D.K.; Hutter, J.A.; Benner, E.J.; Mosley, R.L.; Gendelman, H.E. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J. Immunol. 2010, 184, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.S.; Cao, S.; Rowse, A.L.; Thome, A.D.; Li, X.; Mangieri, L.R.; Cron, R.Q.; Shacka, J.J.; Raman, C.; Standaert, D.G. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci. 2013, 33, 9592–9600. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, C.; Zhu, J.; Feng, Y.; Chen, W.; Feng, Z.; Wang, D.; Sun, S.; Lin, W.; Wang, Y. Increased Levels of Pro-Inflammatory and Anti-Inflammatory Cellular Responses in Parkinson’s Disease Patients: Search for a Disease Indicator. Med. Sci. Monit. 2017, 23, 2972–2978. [Google Scholar] [CrossRef] [PubMed]
- Fiszer, U.; Mix, E.; Fredrikson, S.; Kostulas, V.; Olsson, T.; Link, H. gamma delta+ T cells are increased in patients with Parkinson’s disease. J. Neurol. Sci. 1994, 121, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.A.; Estes, K.A.; Kosloski, L.M.; Allen, H.E.; Dempsey, K.M.; Torres-Russotto, D.R.; Meza, J.L.; Santamaria, P.M.; Bertoni, J.M.; Murman, D.L.; et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J. Neuroimmune Pharmacol. 2012, 7, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Niwa, F.; Kuriyama, N.; Nakagawa, M.; Imanishi, J. Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson’s disease. Geriatr. Gerontol. Int. 2012, 12, 102–107. [Google Scholar] [CrossRef]
- Uitti, R.J.; Baba, Y.; Whaley, N.R.; Wszolek, Z.K.; Putzke, J.D. Parkinson disease: Handedness predicts asymmetry. Neurology 2005, 64, 1925–1930. [Google Scholar] [CrossRef]
- Bas, J.; Calopa, M.; Mestre, M.; Molleví, D.G.; Cutillas, B.; Ambrosio, S.; Buendia, E. Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J. Neuroimmunol. 2001, 113, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Marxreiter, F.; Krach, F.; Fadler, T.; Grosch, J.; Maroni, M.; Graef, D.; Eberhardt, E.; Riemenschneider, M.J.; Yeo, G.W.; et al. Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease. Cell Stem Cell 2019, 24, 1006. [Google Scholar] [CrossRef] [PubMed]
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef]
- Seo, J.; Park, J.; Kim, K.; Won, J.; Yeo, H.G.; Jin, Y.B.; Koo, B.S.; Lim, K.S.; Jeong, K.J.; Kang, P.; et al. Chronic Infiltration of T Lymphocytes into the Brain in a Non-human Primate Model of Parkinson’s Disease. Neuroscience 2020, 431, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Marin, B.; Fontana, A.; Arcuti, S.; Copetti, M.; Boumédiene, F.; Couratier, P.; Beghi, E.; Preux, P.M.; Logroscino, G. Age-specific ALS incidence: A dose-response meta-analysis. Eur. J. Epidemiol. 2018, 33, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular Mechanisms and Therapeutic Implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Schroth, J.; Tree, T.; Turner, M.R.; Shaw, P.J.; Henson, S.M.; Malaspina, A. Senescent-like Blood Lymphocytes and Disease Progression in Amyotrophic Lateral Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200042. [Google Scholar] [CrossRef]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Huang, A.; Wen, S.; Liao, B.; Appel, S.H. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain 2011, 134 Pt 5, 1293–1314. [Google Scholar] [CrossRef]
- Henkel, J.S.; Beers, D.R.; Wen, S.; Rivera, A.L.; Toennis, K.M.; Appel, J.E.; Zhao, W.; Moore, D.H.; Powell, S.Z.; Appel, S.H. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol. Med. 2013, 5, 64–79. [Google Scholar] [CrossRef]
- Saresella, M.; Piancone, F.; Tortorella, P.; Marventano, I.; Gatti, A.; Caputo, D.; Lunetta, C.; Corbo, M.; Rovaris, M.; Clerici, M. T helper-17 activation dominates the immunologic milieu of both amyotrophic lateral sclerosis and progressive multiple sclerosis. Clin. Immunol. 2013, 148, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Zhao, W.; Liao, B.; Kano, O.; Wang, J.; Huang, A.; Appel, S.H.; Henkel, J.S. Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain Behav. Immun. 2011, 25, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Zhao, W.; Wang, J.; Zhang, X.; Wen, S.; Neal, D.; Thonhoff, J.R.; Alsuliman, A.S.; Shpall, E.J.; Rezvani, K.; et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight 2017, 2, e89530. [Google Scholar] [CrossRef] [PubMed]
- Rashid Chehreh Bargh, S.; Tafakhori, A.; Masoumi, F.; Rahmani, F.; Ahmadi, M.; Namdar, A.; Azimi, M.; Tavasolian, P.; Habibi, S.; Zamani, B.; et al. Evaluation of regulatory T lymphocytes and IL2Ra and FOXP3 gene expression in peripheral mononuclear cells from patients with amyotrophic lateral sclerosis. Ir. J. Med. Sci. 2018, 187, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Miyara, M.; Sakaguchi, S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol. Med. 2007, 13, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kipnis, J.; Avidan, H.; Caspi, R.R.; Schwartz, M. Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: A dialogue with microglia. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. 2), 14663–14669. [Google Scholar] [CrossRef]
- Zufiría, M.; Gil-Bea, F.J.; Fernández-Torrón, R.; Poza, J.J.; Muñoz-Blanco, J.L.; Rojas-García, R.; Riancho, J.; López de Munain, A. ALS: A bucket of genes, environment, metabolism and unknown ingredients. Prog. Neurobiol. 2016, 142, 104–129. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.F.; Josephs, K.A.; Petrucelli, L. Review: Transactive response DNA-binding protein 43 (TDP-43): Mechanisms of neurodegeneration. Neuropathol. Appl. Neurobiol. 2010, 36, 97–112. [Google Scholar] [CrossRef]
- Lindestam Arlehamn, C.S.; Pham, J.; Alcalay, R.N.; Frazier, A.; Shorr, E.; Carpenter, C.; Sidney, J.; Dhanwani, R.; Agin-Liebes, J.; Garretti, F.; et al. Widespread Tau-Specific CD4 T Cell Reactivity in the General Population. J. Immunol. 2019, 203, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lindestam Arlehamn, C.S.; Dhanwani, R.; Pham, J.; Kuan, R.; Frazier, A.; Rezende Dutra, J.; Phillips, E.; Mallal, S.; Roederer, M.; Marder, K.S.; et al. α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 2020, 11, 1875. [Google Scholar] [CrossRef]
- Sulzer, D.; Alcalay, R.N.; Garretti, F.; Cote, L.; Kanter, E.; Agin-Liebes, J.; Liong, C.; McMurtrey, C.; Hildebrand, W.H.; Mao, X.; et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 2017, 546, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Rotunno, M.S.; Bosco, D.A. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front. Cell Neurosci. 2013, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.X.; et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef]
- Zaccai, S.; Nemirovsky, A.; Lerner, L.; Alfahel, L.; Eremenko, E.; Israelson, A.; Monsonego, A. CD4 T-cell aging exacerbates neuroinflammation in a late-onset mouse model of amyotrophic lateral sclerosis. J. Neuroinflamm. 2024, 21, 17. [Google Scholar] [CrossRef]
- Lowther, D.E.; Hafler, D.A. Regulatory T cells in the central nervous system. Immunol. Rev. 2012, 248, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Ye, M.; Kang, G.H.; Lee, C.; Lee, G.; Choi, D.B.; Jung, J.; Kim, H.; Lee, S.; Kim, J.S.; et al. Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer’s disease model. Oncotarget 2016, 7, 69347–69357. [Google Scholar] [CrossRef]
- Thonhoff, J.R.; Simpson, E.P.; Appel, S.H. Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr. Opin. Neurol. 2018, 31, 635–639. [Google Scholar] [CrossRef]
- Thonhoff, J.R.; Beers, D.R.; Zhao, W.; Pleitez, M.; Simpson, E.P.; Berry, J.D.; Cudkowicz, M.E.; Appel, S.H. Expanded autologous regulatory T-lymphocyte infusions in ALS: A phase I, first-in-human study. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e465. [Google Scholar] [CrossRef]
- Graber, D.J.; Cook, W.J.; Sentman, M.L.; Murad-Mabaera, J.M.; Sentman, C.L. Human CD4+CD25+ T cells expressing a chimeric antigen receptor against aberrant superoxide dismutase 1 trigger antigen-specific immunomodulation. Cytotherapy 2023, 26, P126–P135. [Google Scholar] [CrossRef]
- Smith, J.A.; Tso, J.Y.; Clark, M.R.; Cole, M.S.; Bluestone, J.A. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J. Exp. Med. 1997, 185, 1413–1422. [Google Scholar] [CrossRef]
- Penaranda, C.; Tang, Q.; Bluestone, J.A. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J. Immunol. 2011, 187, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- De Mercanti, S.; Rolla, S.; Cucci, A.; Bardina, V.; Cocco, E.; Vladic, A.; Soldo-Butkovic, S.; Habek, M.; Adamec, I.; Horakova, D.; et al. Alemtuzumab long-term immunologic effect: Treg suppressor function increases up to 24 months. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e194. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef] [PubMed]
- Dos Passos, G.R.; Sato, D.K.; Becker, J.; Fujihara, K. Th17 Cells Pathways in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders: Pathophysiological and Therapeutic Implications. Mediat. Inflamm. 2016, 2016, 5314541. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm. IGF Res. 2009, 19, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W.; Brief, S.; Westly, H.J.; Novakofski, J.; Bechtel, P.J.; Simon, J.; Walker, E.B. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc. Natl. Acad. Sci. USA 1986, 83, 5663–5667. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Peterson, C.A.; Dahly, E.M.; Ney, D.M. IGF-I alters lymphocyte survival and regeneration in thymus and spleen after dexamethasone treatment. Am. J. Physiol. 1998, 274, R912–R920. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Panoskaltsis-Mortari, A.; Kuro, O.M.; Holländer, G.A.; Blazar, B.R.; Weinberg, K.I. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood 2007, 109, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Velardi, E.; Tsai, J.J.; van den Brink, M.R.M. T cell regeneration after immunological injury. Nat. Rev. Immunol. 2021, 21, 277–291. [Google Scholar] [CrossRef]
- Butler, T.; Goldberg, J.D.; Galvin, J.E.; Maloney, T.; Ravdin, L.; Glodzik, L.; de Leon, M.J.; Hochman, T.; Bowen, R.L.; Atwood, C.S. Rationale, study design and implementation of the LUCINDA Trial: Leuprolide plus Cholinesterase Inhibition to reduce Neurologic Decline in Alzheimer’s. Contemp. Clin. Trials 2021, 107, 106488. [Google Scholar] [CrossRef]
- Casadesus, G.; Webber, K.M.; Atwood, C.S.; Pappolla, M.A.; Perry, G.; Bowen, R.L.; Smith, M.A. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim. Biophys. Acta 2006, 1762, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, R.; Pido-Lopez, J.; Imami, N.; Henson, S.M.; Ngom, P.T.; Morre, M.; Niphuis, H.; Remarque, E.; Rosenwirth, B.; Heeney, J.L. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007, 10, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Schluns, K.S.; Kieper, W.C.; Jameson, S.C.; Lefrançois, L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 2000, 1, 426–432. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; Jenq, R.R.; Young, L.F.; Ghosh, A.; Singer, N.V.; West, M.L.; Smith, O.M.; Holland, A.M.; Tsai, J.J.; et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science 2012, 336, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy. Cell Metab. 2017, 26, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Lee, C.; Longo, V.D. Nutrition and fasting mimicking diets in the prevention and treatment of autoimmune diseases and immunosenescence. Mol. Cell Endocrinol. 2017, 455, 4–12. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Alexaki, A.; Brinia, M.E.; Anagnostouli, M.; Stefanis, L.; Stathopoulos, P. The mTOR Signaling Pathway in Multiple Sclerosis; from Animal Models to Human Data. Int. J. Mol. Sci. 2022, 23, 8077. [Google Scholar] [CrossRef]
- Almeida, L.; Lochner, M.; Berod, L.; Sparwasser, T. Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016, 28, 514–524. [Google Scholar] [CrossRef]
- Peng, M.; Yin, N.; Chhangawala, S.; Xu, K.; Leslie, C.S.; Li, M.O. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 2016, 354, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhou, H.; Yu, H.; Yang, W.; Wang, B.; Qian, F.; Cheng, Y.; He, S.; Zhao, X.; Zhu, L.; et al. miR-99a regulates CD4(+) T cell differentiation and attenuates experimental autoimmune encephalomyelitis by mTOR-mediated glycolysis. Mol. Ther. Nucleic Acids 2021, 26, 1173–1185. [Google Scholar] [CrossRef]
- Jin, J.; Kim, C.; Xia, Q.; Gould, T.M.; Cao, W.; Zhang, H.; Li, X.; Weiskopf, D.; Grifoni, A.; Sette, A.; et al. Activation of mTORC1 at late endosomes misdirects T cell fate decision in older individuals. Sci. Immunol. 2021, 6, eabg0791. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Pennypacker, J.K. Drugs that modulate aging: The promising yet difficult path ahead. Transl. Res. 2014, 163, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Walters, H.E.; Cox, L.S. mTORC Inhibitors as Broad-Spectrum Therapeutics for Age-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2325. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.; Pearce, E.L. Targeting T cell metabolism for therapy. Trends Immunol. 2015, 36, 71–80. [Google Scholar] [CrossRef]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.C.; Zhang, J.Q.; Zou, J.Y.; Zhang, X.; Chen, W.M.; Gu, Y.; Hong, H. The effect of metformin on senescence of T lymphocytes. Immun. Ageing 2023, 20, 73. [Google Scholar] [CrossRef]
- Sukumar, M.; Liu, J.; Ji, Y.; Subramanian, M.; Crompton, J.G.; Yu, Z.; Roychoudhuri, R.; Palmer, D.C.; Muranski, P.; Karoly, E.D.; et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Investig. 2013, 123, 4479–4488. [Google Scholar] [CrossRef]
- Balint, B.; Haas, J.; Schwarz, A.; Jarius, S.; Fürwentsches, A.; Engelhardt, K.; Bussmann, C.; Ebinger, F.; Fritzsching, B.; Paul, F.; et al. T-cell homeostasis in pediatric multiple sclerosis: Old cells in young patients. Neurology 2013, 81, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Markovic-Plese, S.; Cortese, I.; Wandinger, K.P.; McFarland, H.F.; Martin, R. CD4+CD28- costimulation-independent T cells in multiple sclerosis. J. Clin. Investig. 2001, 108, 1185–1194. [Google Scholar] [CrossRef]
- Williams, G.P.; Schonhoff, A.M.; Jurkuvenaite, A.; Gallups, N.J.; Standaert, D.G.; Harms, A.S. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 2021, 144, 2047–2059. [Google Scholar] [CrossRef]
- Thonhoff, J.R.; Berry, J.D.; Macklin, E.A.; Beers, D.R.; Mendoza, P.A.; Zhao, W.; Thome, A.D.; Triolo, F.; Moon, J.J.; Paganoni, S.; et al. Combined Regulatory T-Lymphocyte and IL-2 Treatment Is Safe, Tolerable, and Biologically Active for 1 Year in Persons With Amyotrophic Lateral Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200019. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Lu, Y.; Liang, X.; Zhao, M.; Yu, X.; Fu, H.; Yang, W. CD4+ T-Cell Senescence in Neurodegenerative Disease: Pathogenesis and Potential Therapeutic Targets. Cells 2024, 13, 749. https://doi.org/10.3390/cells13090749
Gao Y, Lu Y, Liang X, Zhao M, Yu X, Fu H, Yang W. CD4+ T-Cell Senescence in Neurodegenerative Disease: Pathogenesis and Potential Therapeutic Targets. Cells. 2024; 13(9):749. https://doi.org/10.3390/cells13090749
Chicago/Turabian StyleGao, Yan, Yaoping Lu, Xiaojing Liang, Mengwei Zhao, Xinyue Yu, Haiying Fu, and Wei Yang. 2024. "CD4+ T-Cell Senescence in Neurodegenerative Disease: Pathogenesis and Potential Therapeutic Targets" Cells 13, no. 9: 749. https://doi.org/10.3390/cells13090749





