Manganese Suppresses the Haploinsufficiency of Heterozygous trpy1Δ/TRPY1 Saccharomyces cerevisiae Cells and Stimulates the TRPY1-Dependent Release of Vacuolar Ca2+ under H2O2 Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Media
2.2. Plasmid and Yeast Transformation
2.3. Yeast Cell Growth Assay
2.3.1. Growth in Liquid Media
2.3.2. Growth on Solid Media
2.4. TRPY1 Gene Expression by Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.5. Assay of Cell Mn2+
2.6. Detection of [Ca2+]cyt by Aequorin Bioluminescence Assay
2.7. Statistics
3. Results
3.1. Haploinsufficiency of Yeast Strain Heterozyous for TRPY1 Is Alleviated by Mn2+
3.2. Mn2+ Potentiates the Increase of [Ca2+]cyt under Oxidative Stress in Strain trpy1Δ/TRPY1
3.3. Mn2+ Stimulates TRPY1 to Release Ca2+ into the Cytosol under H2O2 Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsumoto, T.K.; Ellsmore, A.J.; Cessna, S.G.; Low, P.S.; Pardo, J.M.; Bressan, R.A.; Hasegawa, P.M. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 33075–33080. [Google Scholar] [CrossRef] [PubMed]
- Batiza, A.F.; Schulz, T.; Masson, P.H. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 1996, 271, 23357–23362. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, M.; Groppi, S.; Belotti, F.; Ambrosini, R.; Filippi, G.; Martegani, E.; Tisi, R. Hypotonic stress-induced calcium signaling in Saccharomyces cerevisiae involves TRP-like transporters on the endoplasmic reticulum membrane. Cell Calcium 2015, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Denis, V.; Cyert, M.S. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 2002, 156, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Loukin, S.; Zhou, X.; Kung, C. Saimi, Y. A genome-wide survey suggests an osmoprotective role for vacuolar Ca2+ release in cell wall-compromised yeast. FASEB J. 2008, 22, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Iida, H.; Tsuji, F.I.; Isobe, M.; Uozumi, N.; Muto, S. Salicylic acid induces a cytosolic Ca2+ elevation in yeast. Biosci. Biotechnol. Biochem. 1998, 62, 986–989. [Google Scholar] [CrossRef]
- Viladevall, L.; Serrano, R.; Ruiz, A.; Domenech, G.; Giraldo, J.; Barcelo, A.; Arino, J. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 43614–43624. [Google Scholar] [CrossRef]
- Peiter, E.; Fischer, M.; Sidaway, K.; Roberts, S.K.; Sanders, D. The Saccharomyces cerevisiae Ca2+ channel Cch1pMid1p is essential for tolerance to cold stress and iron toxicity. FEBS Lett. 2005, 579, 5697–5703. [Google Scholar] [CrossRef]
- Araki, Y.; Wu, H.; Kitagaki, H.; Akao, T.; Takagi, H.; Shimoi, H. Ethanol stress stimulates the Ca2+-mediated calcineurin/Crz1 pathway in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2009, 107, 1–6. [Google Scholar] [CrossRef]
- Courchesne, W.E.; Vlasek, C.; Klukovich, R.; Coffee, S. Ethanol induces calcium influx via the Cch1-Mid1 transporter in Saccharomyces cerevisiae. Arch. Microbiol. 2011, 193, 323–334. [Google Scholar] [CrossRef]
- Gupta, S.S.; Ton, V.K.; Beaudry, V.; Rulli, S.; Cunningham, K.; Rao, R. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J. Biol. Chem. 2003, 278, 28831–28839. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Zhang, Y.Q.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef]
- Hejchman, E.; Ostrowska, K.; Maciejewska, D.; Kossakowski, J.; Courchesne, W.E. Synthesis and antifungal activity of derivatives of 2- and 3-benzofurancarboxylic acids. J. Pharmacol. Exp. Ther. 2012, 343, 380–388. [Google Scholar] [CrossRef]
- Roberts, S.K.; McAinsh, M.; Widdicks, L. Cch1p mediates Ca2+ influx to protect Saccharomyces cerevisiae against eugenol toxicity. PLoS ONE 2012, 7, e43989. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.K.; McAinsh, M.; Cantopher, H.; Sandison, S. Calcium dependence of eugenol tolerance and toxicity in Saccharomyces cerevisiae. PLoS ONE 2014, 9, e102712. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.V.; Lungu, L.; Cristache, L.F.; Ciuculescu, C.; Danet, A.F.; Farcasanu, I.C. Heat shock, visible light or high calcium augment the cytotoxic effects of Ailanthus altissima (Swingle) leaf extracts against Saccharomyces cerevisiae cells. Nat. Prod. Res. 2015, 29, 1744–1747. [Google Scholar] [CrossRef]
- Vilanova, C.; Hueso, A.; Palanca, C.; Marco, G.; Pitarch, M.; Otero, E.; Crespo, J.; Szablowski, J.; Rivera, S.; Domínguez-Escribà, L.; et al. Aequorin-expressing yeast emits light under electric control. J. Biotechnol. 2011, 152, 93–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinontoan, R.; Krystofova, S.; Kawano, T.; Mori, I.C.; Tsuji, F.I.; Iida, H.; Muto, S. Phenylethylamine induces an increase in cytosolic Ca2+ in yeast. Biosci. Biotechnol. Biosci. 2002, 66, 1069–1074. [Google Scholar] [CrossRef]
- Popa, C.V.; Dumitru, I.; Ruta, L.L.; Danet, A.F.; Farcasanu, I.C. Exogenous oxidative stress induces Ca2+ release in the yeast Saccharomyces cerevisiae. FEBS J. 2010, 277, 4027–4038. [Google Scholar] [CrossRef]
- Ruta, L.L.; Popa, C.V.; Nicolau, I.; Farcasanu, I.C. Calcium signaling and copper toxicity in Saccharomyces cerevisiae cells. Environ. Sci. Pollut. Res. Int. 2016, 23, 24514–24526. [Google Scholar] [CrossRef]
- Ruta, L.L.; Popa, V.C.; Nicolau, I.; Danet, A.F.; Iordache, V.; Neagoe, A.D.; Farcasanu, I.C. Calcium signaling mediates the response to cadmium toxicity in Saccharomyces cerevisiae cells. FEBS Lett. 2014, 588, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.D.; Ruta, L.L.; Nicolau, I.; Popa, C.V.; Iordache, V.; Neagoe, A.D.; Farcasanu, I.C. Interaction between lanthanide ions and Saccharomyces cerevisiae cells. J. Biol. Inorg. Chem. 2015, 20, 1097–1107. [Google Scholar] [CrossRef]
- Bootman, M.D.; Berridge, M.J.; Putney, J.W.; Llewelyn Roderick, H. Calcium Signaling; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011; 449p, ISBN 978-0-87969-903-1. [Google Scholar]
- Palmer, C.P.; Zhou, X.; Lin, J.; Loukin, S.H.; Kung, C.; Saimi, Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 7801–7805. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.W. Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium 2011, 50, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.W.; Fink, G.R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell Biol. 1996, 16, 2226–2237. [Google Scholar] [CrossRef]
- Miseta, A.; Kellermayer, R.; Aiello, D.P.; Fu, L.; Bedwell, D.M. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999, 451, 132–136. [Google Scholar] [CrossRef]
- Sorin, A.; Rosas, G.; Rao, R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 1997, 272, 9895–9901. [Google Scholar] [CrossRef]
- Strayle, J.; Pozzan, T.; Rudolph, H.K. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 mM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 1999, 18, 4733–4743. [Google Scholar] [CrossRef]
- Hamamoto, S.; Mori, Y.; Yabe, I.; Uozumi, N. In vitro and in vivo characterization of modulation of the vacuolar cation channel TRPY1 from Saccharomyces cerevisiae. FEBS J. 2018, 285, 1146–1161. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Batiza, A.F.; Loukin, S.H.; Palmer, C.P.; Kung, C.; Saimi, Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc. Natl. Acad. Sci. USA 2003, 100, 7105–7110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Schlenstedt, G.; Flockerzi, V.; Beck, A. Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett. 2010, 584, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Pir, P.; Gutteridge, A.; Wu, J.; Rash, B.; Kell, D.B.; Zhang, N.; Oliver, S.G. The genetic control of growth rate: A systems biology study in yeast. BMC Syst. Biol. 2012, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, C.B.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Sherman, F. Getting started with yeast. Methods Enzymol. 2002, 350, 3–41. [Google Scholar] [PubMed]
- Nakajima-Shimada, J.; Iida, H.; Tsuji, F.I.; Anraku, Y. Monitoring of intracellular calcium in Saccharomyces cerevisiae with an apoaequorine cDNA expression system. Proc. Natl. Acad. Sci. USA 1991, 88, 6878–6882. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Engelberg, D. Commonly used Saccharomyces cerevisiae strains (e.g. BY4741, W303) are growth sensitive on synthetic complete medium due to poor leucine uptake. FEMS Microbiol. Lett. 2007, 273, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Tisi, R.; Baldassa, S.; Belotti, F.; Martegani, E. Phospholipase C is required for glucose-induced calcium influx in budding yeast. FEBS Lett. 2002, 520, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Dohmen, R.J.; Strasser, A.W.M.; Honer, C.B.; Hollenberg, C.P. An efficient transformation procedure enabling long-term storage of competent cells of various yeast genera. Yeast 1991, 7, 691–692. [Google Scholar] [CrossRef]
- Ruta, L.L.; Kissen, R.; Nicolau, I.; Neagoe, A.D.; Petrescu, A.J.; Bones, A.M.; Farcasanu, I.C. Heavy metal accumulation by Saccharomyces cerevisiae cells armed with metal binding hexapeptides targeted to the inner face of the plasma membrane. Appl. Microbiol. Biotechnol. 2017, 101, 5749–5763. [Google Scholar] [CrossRef] [PubMed]
- Tisi, R.; Martegani, E.; Brandão, R.L. Monitoring yeast intracellular Ca2+ levels using an in vivo bioluminescence assay. Cold Spring Harb. Protoc. 2015, 2015, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, K.; Pfeifer, M.; Parker, C.N.; Schuierer, S.; Tallarico, J.; Hoepfner, D.; Movva, N.R.; Scheel, G.; Helliwell, S.B. Two low complexity ultra-high throughput methods to identify diverse chemically bioactive molecules using Saccharomyces cerevisiae. Microbiol. Res. 2017, 199, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.; Das, K.K.; Bachhawat, A.K. Glutathione depletion activates the yeast vacuolar transient receptor potential channel, Yvc1p, by reversible glutathionylation of specific cysteines. Mol. Biol. Cell 2016, 27, 3913–3925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John Haynes, W.; Zhou, X.L.; Su, Z.W.; Loukin, S.H.; Saimi, Y.; Kung, C. Indole and other aromatic compounds activate the yeast TRPY1channel. FEBS Lett. 2008, 582, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Supek, F.; Supekova, L.; Nelson, H.; Nelson, N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl. Acad. Sci. USA 1996, 93, 5105–5110. [Google Scholar] [CrossRef] [PubMed]
- Dürr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef]
- Mandal, D.; Woolf, T.B.; Rao, R. Manganese selectivity of Pmr1, the yeast secretory pathway ion pump, is defined by residue Gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J. Biol. Chem. 2000, 275, 23933–23938. [Google Scholar] [CrossRef]
- Lapinskas, P.J.; Cunningham, K.W.; Liu, X.F.; Fink, G.R.; Culotta, V.C. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 1995, 15, 1382–1388. [Google Scholar] [CrossRef]
- Su, Z.; Zhou, X.; Loukin, S.H.; Saimi, Y.; Kung, C. Mechanical force and cytoplasmic Ca2+ activate yeast TRPY1 in parallel. J. Membr. Biol. 2009, 227, 141–150. [Google Scholar] [CrossRef]
- Loukin, S.; Kung, C. Manganese effectively supports yeast cell-cycle progression in place of calcium. J. Cell Biol. 1995, 131, 1025–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]

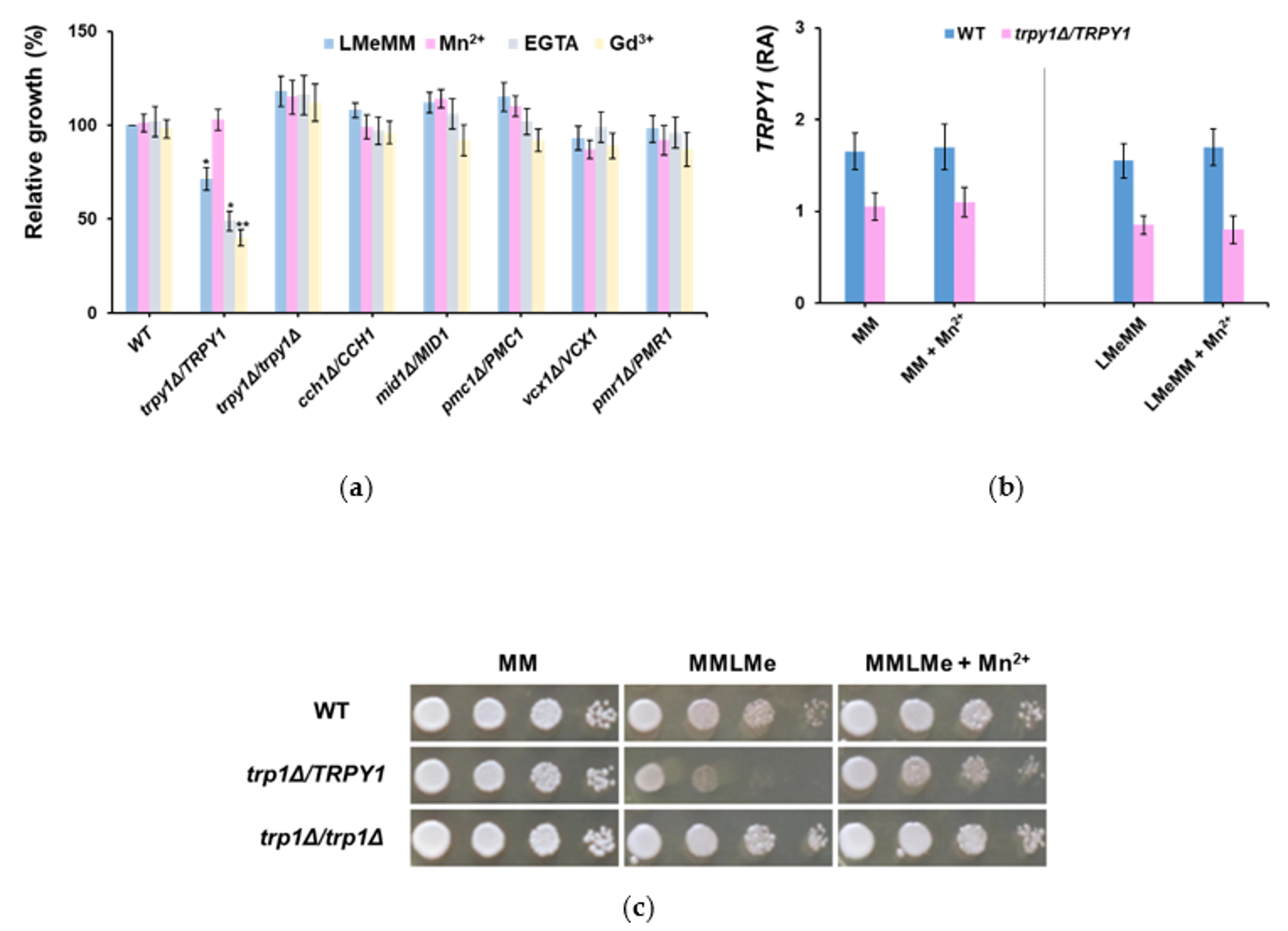

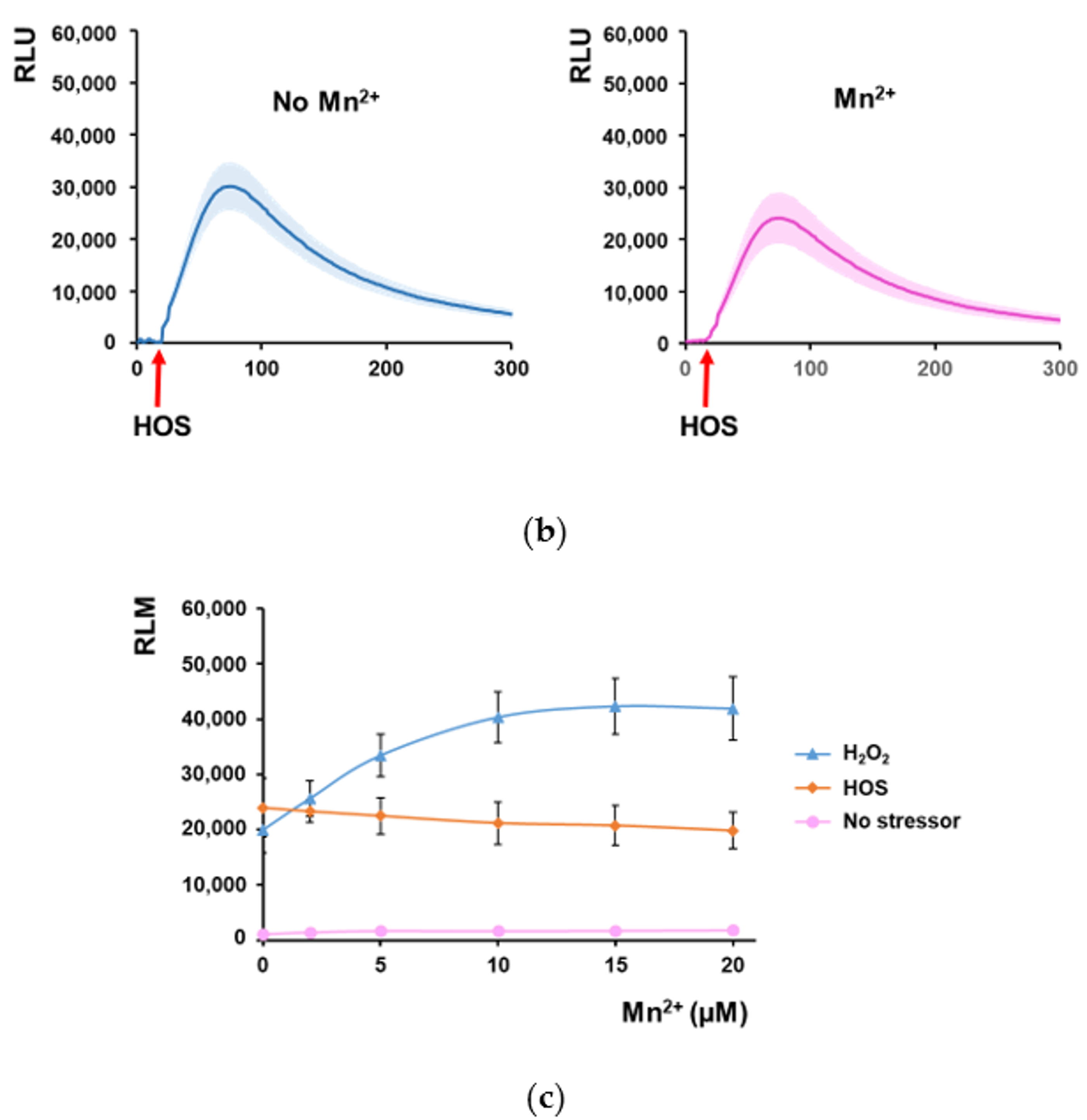
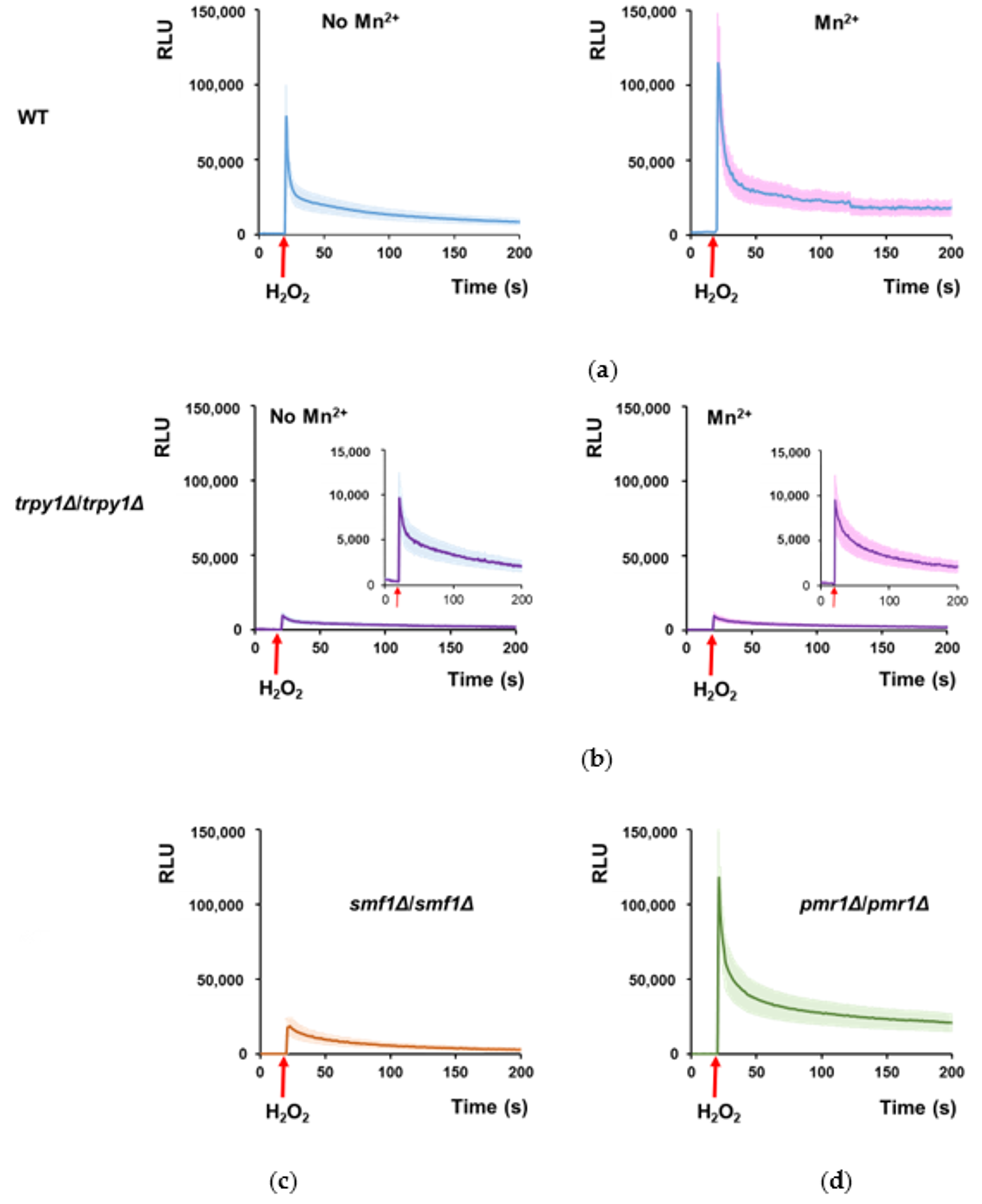

| Substance Tested 1 | Concentration Range | Effect on trpy1Δ/TRPY1 Haploinsufficiency |
|---|---|---|
| CaCl2 | 2–10 mM | No |
| CuCl2 | 0.5–50 µM | No |
| FeCl3 | 1–50 µM | No |
| MnCl2 | 1–50 µM | Alleviation |
| ZnCl2 | 1–50 µM | No |
| EGTA | 0.1–2 mM | Augmentation |
| GdCl3 | 0.1–1 mM | Augmentation |
| Ascorbate | 1–10 mM | No |
| Glutathione 2 | 1–10 mM | No |
| Indole 3 | 1–10 mM | No |
| Strain | Surplus Mn2+ | ||
|---|---|---|---|
| 0 | 10 µM | 50 µM | |
| WT | 0.12 ± 0.12 | 0.64 ± 0.2 | 0.92 ± 0.3 |
| trpy1Δ/TRPY1 | 0.11 ± 0.14 | 0.72 ± 0.1 | 0.98 ± 0.2 |
| trpy1Δ/trpy1Δ | 0.12 ± 0.2 | 0.7 ± 0.2 | 0.84 ± 0.2 |
| smf1Δ/smf1Δ | 0.01 ± 0.014 | 0.1 ± 0.2 | 0.72 ± 0.2 |
| pmr1Δ/pmr1Δ | 0.7 ± 0.22 | 0.84 ± 0.2 | 8.4 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruta, L.L.; Nicolau, I.; Popa, C.V.; Farcasanu, I.C. Manganese Suppresses the Haploinsufficiency of Heterozygous trpy1Δ/TRPY1 Saccharomyces cerevisiae Cells and Stimulates the TRPY1-Dependent Release of Vacuolar Ca2+ under H2O2 Stress. Cells 2019, 8, 79. https://doi.org/10.3390/cells8020079
Ruta LL, Nicolau I, Popa CV, Farcasanu IC. Manganese Suppresses the Haploinsufficiency of Heterozygous trpy1Δ/TRPY1 Saccharomyces cerevisiae Cells and Stimulates the TRPY1-Dependent Release of Vacuolar Ca2+ under H2O2 Stress. Cells. 2019; 8(2):79. https://doi.org/10.3390/cells8020079
Chicago/Turabian StyleRuta, Lavinia L., Ioana Nicolau, Claudia V. Popa, and Ileana C. Farcasanu. 2019. "Manganese Suppresses the Haploinsufficiency of Heterozygous trpy1Δ/TRPY1 Saccharomyces cerevisiae Cells and Stimulates the TRPY1-Dependent Release of Vacuolar Ca2+ under H2O2 Stress" Cells 8, no. 2: 79. https://doi.org/10.3390/cells8020079





