Allosteric Antagonism of the A2A Adenosine Receptor by a Series of Bitopic Ligands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture, Membrane Preparation and Radioligand Binding
2.3. Fluorescent Ligand Binding to Intact HEK293 Cells Expressing the Human A2AAR
2.4. cAMP Assay
2.5. Simulation of Concentration–Effect Curves Using Wolfram Mathematica
2.6. Statistical Analysis
2.7. Chemical Synthesis
3. Results
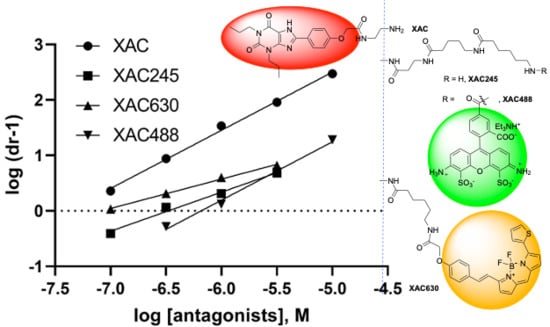
3.1. Functional Antagonism
3.2. Binding
3.3. Mathematical Modeling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobson, K.A.; Tosh, D.K.; Jain, S.; Gao, Z.G. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front. Cell. Neurosci. 2019, 13, 124. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, K.A.; Müller, C.E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016, 104, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Moro, S.; Gao, Z.G.; Jacobson, K.A.; Spalluto, G. Progress in the pursuit of therapeutic adenosine receptor antagonists. Med. Res. Rev. 2006, 26, 131–159. [Google Scholar] [CrossRef]
- Gao, Z.G.; IJzerman, A.P. Allosteric modulation of A2A adenosine receptors by amiloride analogues and sodium ions. Biochem. Pharmacol. 2000, 60, 669–676. [Google Scholar] [CrossRef]
- Liu, W.; Chun, E.; Thompson, A.A.; Chubukov, P.; Xu, F.; Katritch, V.; Han, G.W.; Roth, C.B.; Heitman, L.H.; IJzerman, A.P.; et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 2012, 337, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Eddy, M.T.; Lee, M.Y.; Gao, Z.G.; White, K.L.; Didenko, T.; Horst, R.; Audet, M.; Stanczak, P.; McClary, K.M.; Han, G.W.; et al. Allosteric Coupling of Drug Binding and Intracellular Signaling in the A2A Adenosine Receptor. Cell 2018, 172, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Eddy, M.T.; Gao, Z.G.; Mannes, P.; Patel, N.; Jacobson, K.A.; Katritch, V.; Stevens, R.C.; Wüthrich, K. Extrinsic Tryptophans as NMR Probes of Allosteric Coupling in Membrane Proteins: Application to the A2A Adenosine Receptor. J. Am. Chem. Soc. 2018, 140, 8228–8235. [Google Scholar] [CrossRef]
- White, K.L.; Eddy, M.T.; Gao, Z.G.; Han, G.W.; Lian, T.; Deary, A.; Patel, N.; Jacobson, K.A.; Katritch, V.; Stevens, R.C. Structural Connection between Activation Microswitch and Allosteric Sodium Site in GPCR Signaling. Structure 2018, 26, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-de-Terán, H.; Massink, A.; Rodríguez, D.; Liu, W.; Han, G.W.; Joseph, J.S.; Katritch, I.; Heitman, L.H.; Xia, L.; IJzerman, A.P.; et al. The role of a sodium ion binding site in the allosteric modulation of the A2A adenosine G protein-coupled receptor. Structure 2013, 21, 2175–2185. [Google Scholar] [CrossRef] [Green Version]
- Massink, A.; Gutiérrez-de-Terán, H.; Lenselink, E.B.; Ortiz Zacarías, N.V.; Xia, L.; Heitman, L.H.; Katritch, V.; Stevens, R.C.; IJzerman, A.P. Sodium ion binding pocket mutations and adenosine A2A receptor function. Mol. Pharmacol. 2015, 87, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Massink, A.; Louvel, J.; Adlere, I.; van Veen, C.; Huisman, B.J.; Dijksteel, G.S.; Guo, D.; Lenselink, E.B.; Buckley, B.J.; Matthews, H.; et al. 5′-Substituted Amiloride Derivatives as Allosteric Modulators Binding in the Sodium Ion Pocket of the Adenosine A2A Receptor. J. Med. Chem. 2016, 59, 4769–4777. [Google Scholar] [CrossRef]
- Sun, B.; Bachhawat, P.; Chu, M.L.; Wood, M.; Ceska, T.; Sands, Z.A.; Mercier, J.; Lebon, F.; Kobilka, T.S.; Kobilka, B.K. Crystal structure of the adenosine A2A receptor bound to an antagonist reveals a potential allosteric pocket. Proc. Natl. Acad. Sci. USA 2017, 114, 2066–2071. [Google Scholar] [CrossRef] [Green Version]
- Köse, M.; Gollos, S.; Karcz, T.; Fiene, A.; Heisig, F.; Behrenswerth, A.; Kieć-Kononowicz, K.; Namasivayam, V.; Müller, C.E. Fluorescent-Labeled Selective Adenosine A2B Receptor Antagonist Enables Competition Binding Assay by Flow Cytometry. J. Med. Chem. 2018, 61, 4301–4316. [Google Scholar] [CrossRef]
- Federico, S.; Margiotta, E.; Moro, S.; Kozma, E.; Gao, Z.G.; Jacobson, K.A.; Spalluto, G. Conjugable A3 adenosine receptor antagonists for the development of functionalized ligands and their use in fluorescent probes. Eur. J. Med. Chem. 2020, 186, 111886. [Google Scholar] [CrossRef]
- Baker, J.G.; Middleton, R.; Adams, L.; May, L.T.; Briddon, S.J.; Kellam, B.; Hill, S.J. Influence of fluorophore and linker composition on the pharmacology of fluorescent adenosine A1 receptor ligands. Br. J. Pharmacol. 2010, 159, 772–786. [Google Scholar] [CrossRef] [Green Version]
- Kecskés, M.; Kumar, T.S.; Yoo, L.; Gao, Z.G.; Jacobson, K.A. Novel Alexa Fluor-488 labeled antagonist of the A2A adenosine receptor: Application to a fluorescence polarization-based receptor binding assay. Biochem. Pharmacol. 2010, 80, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, K.A. Functionalized congener approach to the design of ligands for G protein–coupled receptors (GPCRs). Bioconjugate Chem. 2009, 20, 1816–1835. [Google Scholar] [CrossRef] [Green Version]
- Duroux, R.; Ciancetta, A.; Mannes, P.; Yu, J.; Boyapati, S.; Gizewski, E.; Yous, S.; Ciruela, F.; Auchampach, J.A.; Gao, Z.G.; et al. Bitopic fluorescent antagonists of the A2A adenosine receptor based on pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine functionalized congeners. Medchemcomm 2017, 8, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Tahtaoui, C.; Parrot, I.; Klotz, P.; Guillier, F.; Galzi, J.L.; Hibert, M.; Ilien, B. Fluorescent pirenzepine derivatives as potential bitopic ligands of the human M1 muscarinic receptor. J. Med. Chem. 2004, 47, 4300–4315. [Google Scholar] [CrossRef]
- Daval, S.B.; Valant, C.; Bonnet, D.; Kellenberger, E.; Hibert, M.; Galzi, J.L.; Ilien, B. Fluorescent derivatives of AC-42 to probe bitopic orthosteric/allosteric binding mechanisms on muscarinic M1 receptors. J. Med. Chem. 2012, 55, 2125–2143. [Google Scholar] [CrossRef]
- Kumar, V.; Moritz, A.E.; Keck, T.M.; Bonifazi, A.; Ellenberger, M.P.; Sibley, C.D.; Free, R.B.; Shi, L.; Lane, J.R.; Sibley, D.R.; et al. Synthesis and Pharmacological Characterization of Novel trans-Cyclopropylmethyl-Linked Bivalent Ligands That Exhibit Selectivity and Allosteric Pharmacology at the Dopamine D3 Receptor (D3R). J. Med. Chem. 2017, 60, 1478–1494. [Google Scholar] [CrossRef] [Green Version]
- Fronik, P.; Gaiser, B.I.; Sejer Pedersen, D. Bitopic Ligands and Metastable Binding Sites: Opportunities for G Protein-Coupled Receptor (GPCR) Medicinal Chemistry. J. Med. Chem. 2017, 60, 4126–4134. [Google Scholar] [CrossRef]
- Gaiser, B.I.; Danielsen, M.; Marcher-Rørsted, E.; Røpke Jørgensen, K.; Wróbel, T.M.; Frykman, M.; Johansson, H.; Bräuner-Osborne, H.; Gloriam, D.E.; Mathiesen, J.M.; et al. Probing the Existence of a Metastable Binding Site at the β2-Adrenergic Receptor with Homobivalent Bitopic Ligands. J. Med. Chem. 2019, 62, 7806–7839. [Google Scholar] [CrossRef]
- Mohr, K.; Tränkle, C.; Kostenis, E.; Barocelli, E.; De Amici, M.; Holzgrabe, U. Rational design of dualsteric GPCR ligands: Quests and promise. Br. J. Pharmacol. 2010, 159, 997–1008. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.P.; Wakelin, L.P.; Denny, W.A.; Finch, A.M. Homobivalent Conjugation Increases the Allosteric Effect of 9-aminoacridine at the α1-Adrenergic Receptors. Mol. Pharmacol. 2017, 91, 135–144. [Google Scholar] [CrossRef]
- Hall, D.A. Modeling the functional effects of allosteric modulators at pharmacological receptors: An extension of the two-state model of receptor activation. Mol. Pharmacol. 2000, 58, 1412–1423. [Google Scholar] [CrossRef]
- Vernall, A.J.; Stoddart, L.A.; Briddon, S.J.; Ng, H.W.; Laughton, C.A.; Doughty, S.W.; Hill, S.J.; Kellam, B. Conversion of a non-selective adenosine receptor antagonist into A3-selective high affinity fluorescent probes using peptide-based linkers. Org. Biomol. Chem. 2013, 11, 5673–5682. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.G.; Jacobson, K.A. Distinct Signaling Patterns of Allosteric Antagonism at the P2Y1 Receptor. Mol. Pharmacol. 2017, 92, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Doré, A.S.; Robertson, N.; Errey, J.C.; Ng, I.; Hollenstein, K.; Tehan, B.; Hurrell, E.; Bennett, K.; Congreve, M.; Magnani, F.; et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 2011, 19, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ahn, S.; Kahsai, A.W.; Meng, K.C.; Latorraca, N.R.; Pani, B.; Venkatakrishnan, A.J.; Masoudi, A.; Weis, W.I.; Dror, R.O.; et al. Mechanism of intracellular allosteric β2AR antagonist revealed by X-ray crystal structure. Nature 2017, 548, 480–484. [Google Scholar] [CrossRef]
- Stauch, B.; Johansson, L.C.; McCorvy, J.D.; Patel, N.; Han, G.W.; Huang, X.P.; Gati, C.; Batyuk, A.; Slocum, S.T.; Ishchenko, A.; et al. Structural basis of ligand recognition at the human MT1 melatonin receptor. Nature 2019, 569, 284–288. [Google Scholar] [CrossRef]
- Briddon, S.J.; Middleton, R.J.; Cordeaux, Y.; Flavin, F.M.; Weinstein, J.A.; George, M.W.; Kellam, B.; Hill, S.J. Quantitative analysis of the formation and diffusion of A1-adenosine receptor-antagonist complexes in single living cells. Proc. Natl. Acad. Sci. USA 2004, 101, 4673–4678. [Google Scholar] [CrossRef] [Green Version]
- Bouzo-Lorenzo, M.; Stoddart, L.A.; Xia, L.; IJzerman, A.P.; Heitman, L.H.; Briddon, S.J.; Hill, S.J. A live cell NanoBRET binding assay allows the study of ligand-binding kinetics to the adenosine A3 receptor. Purinergic Signal. 2019, 15, 139–153. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.G.; Verzijl, D.; Zweemer, A.; Ye, K.; Göblyös, A.; IJzerman, A.P.; Jacobson, K.A. Functionally biased modulation of A3 adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem. Pharmacol. 2011, 82, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, J.; van der Mey, D.; Bermudez, M.; Klöckner, J.; Schrage, R.; Kostenis, E.; Tränkle, C.; Wolber, G.; Mohr, K.; Holzgrabe, U. Dualsteric muscarinic antagonists-orthosteric binding pose controls allosteric subtype selectivity. J. Med. Chem. 2014, 57, 6739–6750. [Google Scholar] [CrossRef]
- Daval, S.B.; Kellenberger, E.; Bonnet, D.; Utard, V.; Galzi, J.L.; Ilien, B. Exploration of the orthosteric/allosteric interface in human M1 muscarinic receptors by bitopic fluorescent ligands. Mol. Pharmacol. 2013, 84, 71–85. [Google Scholar] [CrossRef]
- Shao, Z.; Yan, W.; Chapman, K.; Ramesh, K.; Ferrell, A.J.; Yin, J.; Wang, X.; Xu, Q.; Rosenbaum, D.M. Structure of an allosteric modulator bound to the CB1 cannabinoid receptor. Nat. Chem. Biol. 2019, 15, 1199–1205. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Z.G.; Zhang, K.; Kiselev, E.; Crane, S.; Wang, J.; Paoletta, S.; Yi, C.; Ma, L.; Zhang, W.; et al. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 2015, 520, 317–321. [Google Scholar] [CrossRef]
- Heitman, L.H.; Ye, K.; Oosterom, J.; IJzerman, A.P. Amiloride derivatives and a nonpeptidic antagonist bind at two distinct allosteric sites in the human gonadotropin-releasing hormone receptor. Mol. Pharmacol. 2008, 73, 1808–1815. [Google Scholar] [CrossRef] [Green Version]
- Comeo, E.; Kindon, N.D.; Soave, M.; Stoddart, L.A.; Kilpatrick, L.E.; Scammells, P.J.; Hill, S.J.; Kellam, B. Subtype-Selective Fluorescent Ligands as Pharmacological Research Tools for the Human Adenosine A2A Receptor. J. Med. Chem. 2020, 63, 2656–2672. [Google Scholar] [CrossRef] [Green Version]
- Kirtley, M.E.; Koshland, D.E., Jr. Models for cooperative effects in proteins containing subunits. Effects of two interacting ligands. J. Biol. Chem. 1967, 242, 4192–4205. [Google Scholar]
- Kenakin, T.P. The classification of drugs and drug receptors in isolated tissues. Pharmacol. Rev. 1984, 36, 165–222. [Google Scholar]
- Colquhoun, D. Molecular pharmacology: Structure and function of acetylcholine-receptor ion channels. Nature 1986, 321, 382–383. [Google Scholar] [CrossRef]
- Wyllie, D.J.; Chen, P.E. Taking the time to study competitive antagonism. Br. J. Pharmacol. 2007, 150, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Kenakin, T. What is pharmacological ‘affinity’? Relevance to biased agonism and antagonism. Trends Pharmacol. Sci. 2014, 35, 434–441. [Google Scholar] [CrossRef]
- Kenakin, T. The Quantitative Characterization of Functional Allosteric Effects. Curr. Protoc. Pharmacol. 2017, 76, 9–22. [Google Scholar] [CrossRef]
- Arunlakshana, O.; Schild, H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959, 14, 48–58. [Google Scholar] [CrossRef]
- Ye, L.; Van Eps, N.; Zimmer, M.; Ernst, O.P.; Prosser, R.S. Activation of the A2A adenosine G-protein-coupled receptor by conformational selection. Nature 2016, 533, 265–268. [Google Scholar] [CrossRef]
- Gao, Z.G.; Melman, N.; Erdmann, A.; Kim, S.G.; Müller, C.E.; IJzerman, A.P.; Jacobson, K.A. Differential allosteric modulation by amiloride analogues of agonist and antagonist binding at A1 and A3 adenosine receptors. Biochem. Pharmacol. 2003, 65, 525–534. [Google Scholar] [CrossRef]
- Ehlert, F.J.; Griffin, M.T. Two-state models and the analysis of the allosteric effect of gallamine at the M2 muscarinic receptor. J. Pharmacol. Exp. Ther. 2008, 325, 1039–1060. [Google Scholar] [CrossRef] [Green Version]
- López-Giménez, J.F.; Villazón, M.; Brea, J.; Loza, M.I.; Palacios, J.M.; Mengod, G.; Vilaró, M.T. Multiple conformations of native and recombinant human 5-hydroxytryptamine2a receptors are labeled by agonists and discriminated by antagonists. Mol. Pharmacol. 2001, 60, 690–699. [Google Scholar]
- Bennett, K.A.; Tehan, B.; Lebon, G.; Tate, C.G.; Weir, M.; Marshall, F.H.; Langmead, C.J. Pharmacology and structure of isolated conformations of the adenosine A₂A receptor define ligand efficacy. Mol. Pharmacol. 2013, 83, 949–958. [Google Scholar] [CrossRef] [Green Version]
- Sušac, L.; Eddy, M.T.; Didenko, T.; Stevens, R.C.; Wüthrich, K. A2A adenosine receptor functional states characterized by 19F-NMR. Proc. Natl. Acad. Sci. USA 2018, 115, 12733–12738. [Google Scholar] [CrossRef] [Green Version]
- Valant, C.; May, L.T.; Aurelio, L.; Chuo, C.H.; White, P.J.; Baltos, J.A.; Sexton, P.M.; Scammells, P.J.; Christopoulos, A. Separation of on-target efficacy from adverse effects through rational design of a bitopic adenosine receptor agonist. Proc. Natl. Acad. Sci. USA 2014, 111, 4614–4619. [Google Scholar] [CrossRef] [Green Version]
- Fasciani, I.; Pietrantoni, I.; Rossi, M.; Mannoury la Cour, C.; Aloisi, G.; Marampon, F.; Scarselli, M.; Millan, M.J.; Maggio, R. Distinctive binding properties of the negative allosteric modulator, [3H]SB269,652, at recombinant dopamine D3 receptors. Eur. J. Pharmacol. 2018, 819, 181–189. [Google Scholar] [CrossRef] [Green Version]










| Binding (Ki, nM) | Functional Antagonism | ||||
|---|---|---|---|---|---|
| MRS7416 | [3H]ZM241385 | [3H]CGS21680 | KB (nM) | Slope | |
| XAC | 50.7 ± 9.3 | 32.1 ± 5.9 | 38.3 ± 6.7 | 46.6 ± 12.3 | 1.06 ± 0.06 |
| XAC245 | 152 ± 29 | 198 ± 33 | 220 ± 36 | 280 ± 33 | 0.68 ± 0.04 |
| XAC630 | 692 ± 173 | 36.6 ± 10.1 | 29.0 ± 5.7 | 51.1 ± 12.2 | 0.47 ± 0.04 |
| XAC488 | NA | 388 ± 62 | 438 ± 110 | 650 ± 120 | 1.03 ± 0.05 |
| SCH442416 | 7.31 ± 2.22 | 4.35 ± 0.89 | 5.01 ± 0.44 | 7.43 ± 1.85 | 1.05 ± 0.04 |
| MRS7352 | 5.28 ± 1.31 | 6.24 ± 2.42 | 4.98 ± 1.13 | 6.61 ± 0.88 | 0.88 ± 0.06 |
| MRS7396 | 256 ± 33 | 2.34 ± 0.33 | 2.83 ± 0.20 | 1.70 ± 0.34 | 0.42 ± 0.02 |
| MRS7416 | 17.6 ± 4.1 a | 23.8 ± 5.6 | 16.4 ± 1.6 | 9.11 ± 1.77 | 0.68 ± 0.03 |
| MRS7322 | ND | 108 ± 33 | ND | 52.5 ± 13.7 | 0.54 ± 0.09 |
| MRS7395 | ND | 295 ± 176 c | ND | 182 ± 38.6 | 0.91 ± 0.07 |
| ZM241385 | 1.83 ± 0.47 | 1.57 ± 0.32 b | 1.86 ± 0.53 | 1.35 ± 0.41 | 0.995 ± 0.055 |
| HMA | 18500 ± 2800 | 3050 ± 1210 | 2120 ± 510 | 3810 ± 450 | 0.59 ± 0.05 |
| AF488-amide | ND | ND | 3.7 ± 1.2% | NA | NA |
| BY630-amide | ND | ND | 2.6 ± 1.5% | NA | NA |
| CGS21680 | 224 ± 23 | 68.6 ± 13.1 | 16.6 ± 3.2 | NA | NA |
| NECA | 377 ± 128 | 58.8 ± 12.2 | 23.7 ± 5.5 | NA | NA |
| UK432097 | 23.3 ± 3.9 | ND | 12.1 ± 4.0 | NA | NA |
| LUF5834 | 23.0 ± 4.6 | 13.3 ± 6.5 | ND | NA | NA |
| Antagonist | L | K | M | A | β | γ | δ | [R] |
|---|---|---|---|---|---|---|---|---|
| MRS7396 | 0.015 | 1 × 107 | 1 × 108 | 1000 | 1 | 0.025 | 1 | 1 |
| MRS7416 | 0.015 | 1 × 107 | 2 × 107 | 1000 | 1 | 0.007 | 1 | 1 |
| XAC245 | 0.015 | 1 × 107 | 1 × 106 | 1000 | 1 | 0.3 | 1 | 1 |
| XAC630 | 0.015 | 1 × 107 | 1.5 × 107 | 1000 | 1 | 0.1 | 1 | 1 |
| HMA | 0.015 | 1 × 107 | 1 × 105 | 1000 | 1 | 0.3 | 1 | 1 |
| MRS7322 | 0.015 | 1 × 107 | 2 × 106 | 1000 | 1 | 0.03 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.-G.; Toti, K.S.; Campbell, R.; Suresh, R.R.; Yang, H.; Jacobson, K.A. Allosteric Antagonism of the A2A Adenosine Receptor by a Series of Bitopic Ligands. Cells 2020, 9, 1200. https://doi.org/10.3390/cells9051200
Gao Z-G, Toti KS, Campbell R, Suresh RR, Yang H, Jacobson KA. Allosteric Antagonism of the A2A Adenosine Receptor by a Series of Bitopic Ligands. Cells. 2020; 9(5):1200. https://doi.org/10.3390/cells9051200
Chicago/Turabian StyleGao, Zhan-Guo, Kiran S. Toti, Ryan Campbell, R. Rama Suresh, Huijun Yang, and Kenneth A. Jacobson. 2020. "Allosteric Antagonism of the A2A Adenosine Receptor by a Series of Bitopic Ligands" Cells 9, no. 5: 1200. https://doi.org/10.3390/cells9051200