The Hippo Pathway Effector YAP1 Regulates Intestinal Epithelial Cell Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, shRNAs and Lentiviral Infection
2.2. Tissues
2.3. Antibodies
2.4. Western Blot Analysis
2.5. RNA Extraction, Reverse Transcriptase and Quantitative RT-PCR
2.6. Indirect Immunofluorescence Staining and Confocal Imaging
2.7. Transmission Electron Microscopy (TEM)
2.8. Statistical Analysis
3. Results
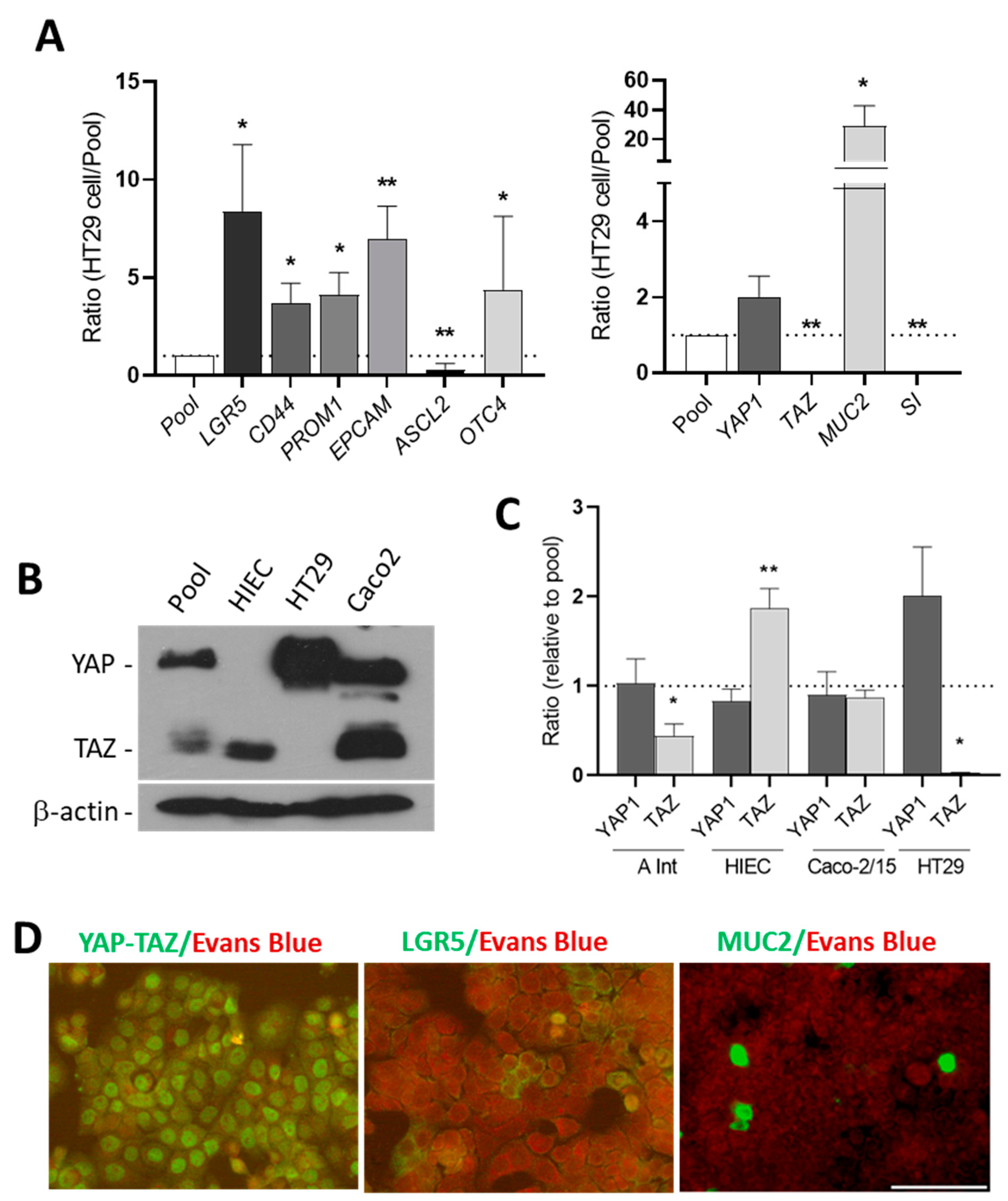
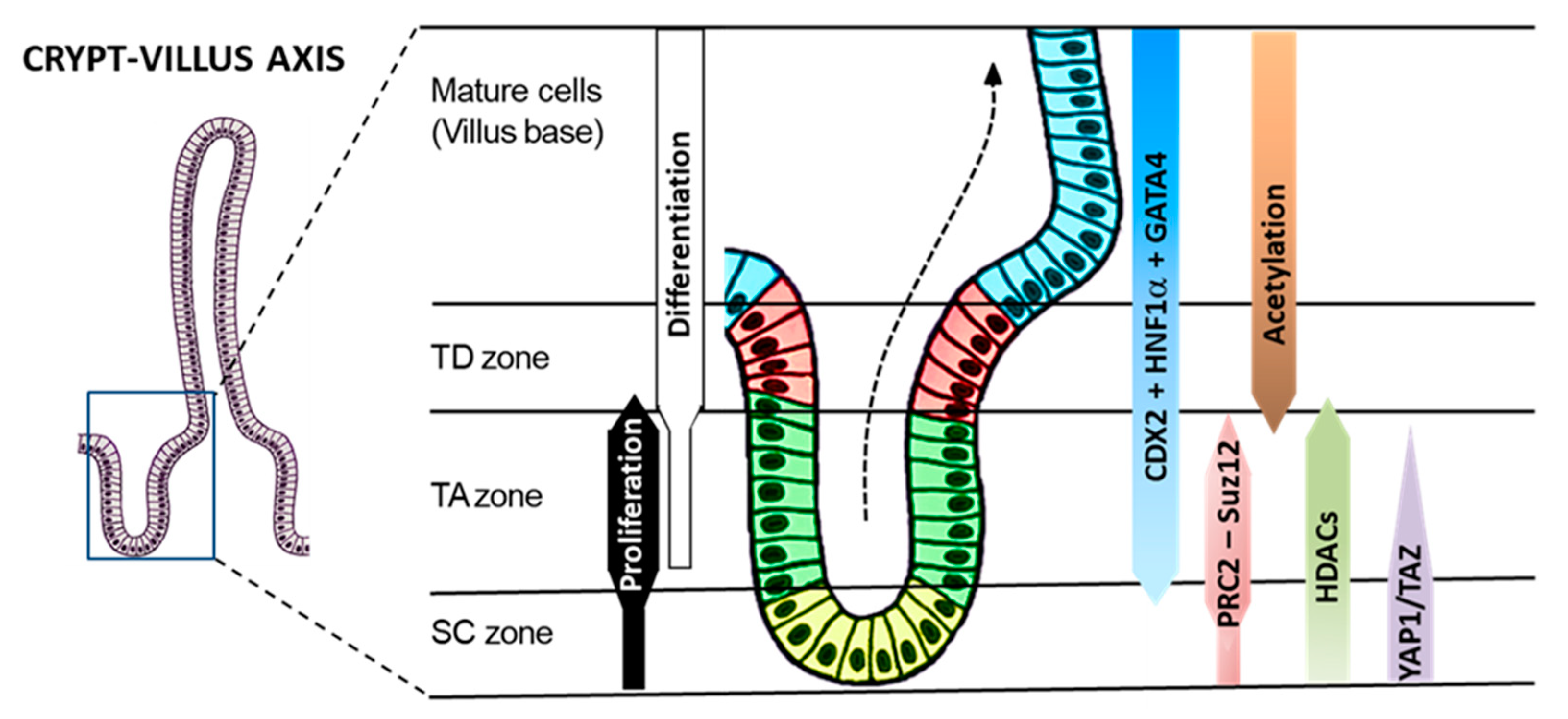
3.1. The Expression of YAP1/TAZ Protein in Human Intestinal Crypt Cells
3.2. HT29 Cells Express Stem Cell Markers and YAP1
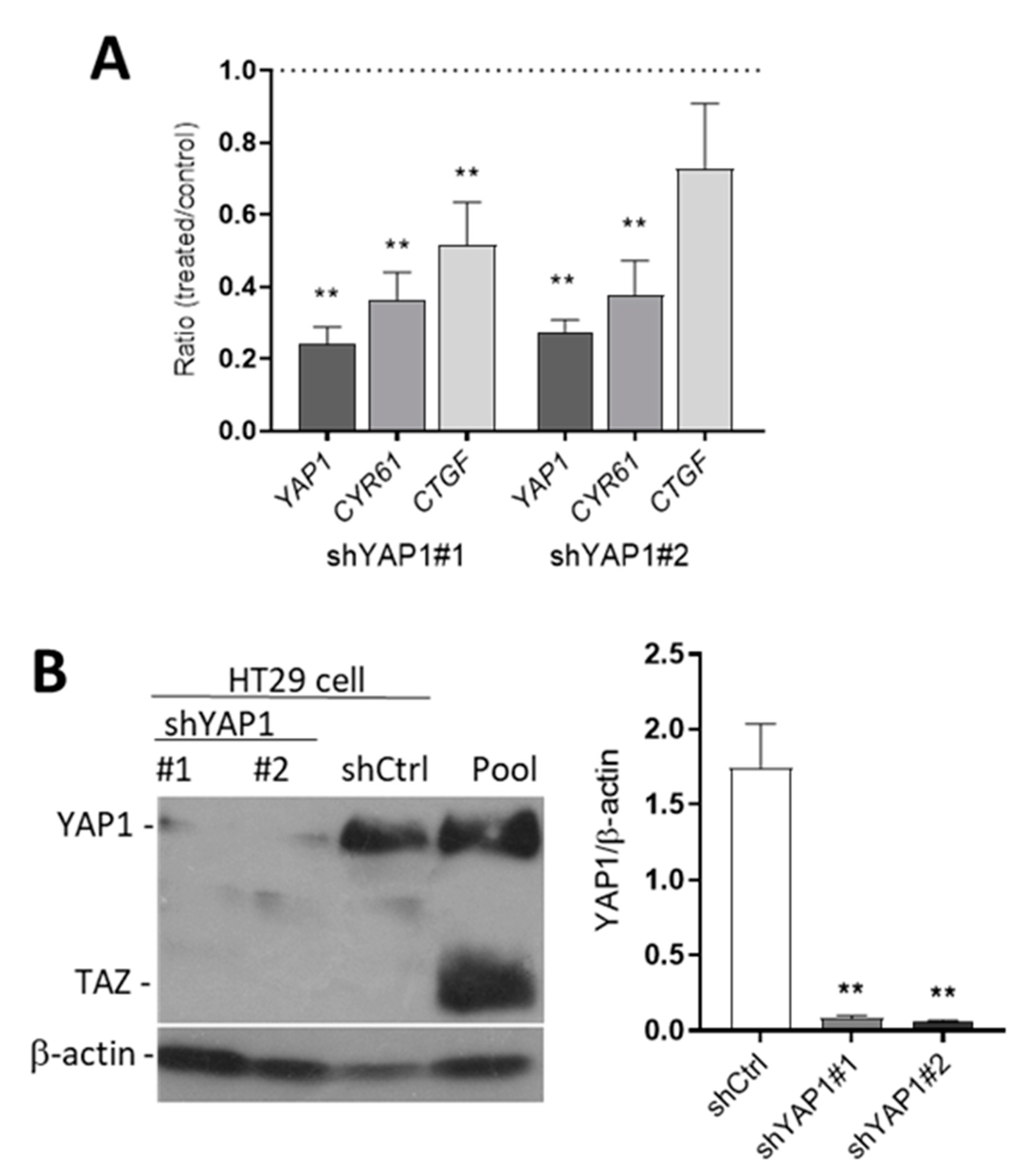
3.3. Knockdown YAP1 Expression by shRNAs
3.4. Effect of YAP1 Knockdown on Intestinal Stem Cell Marker Expression
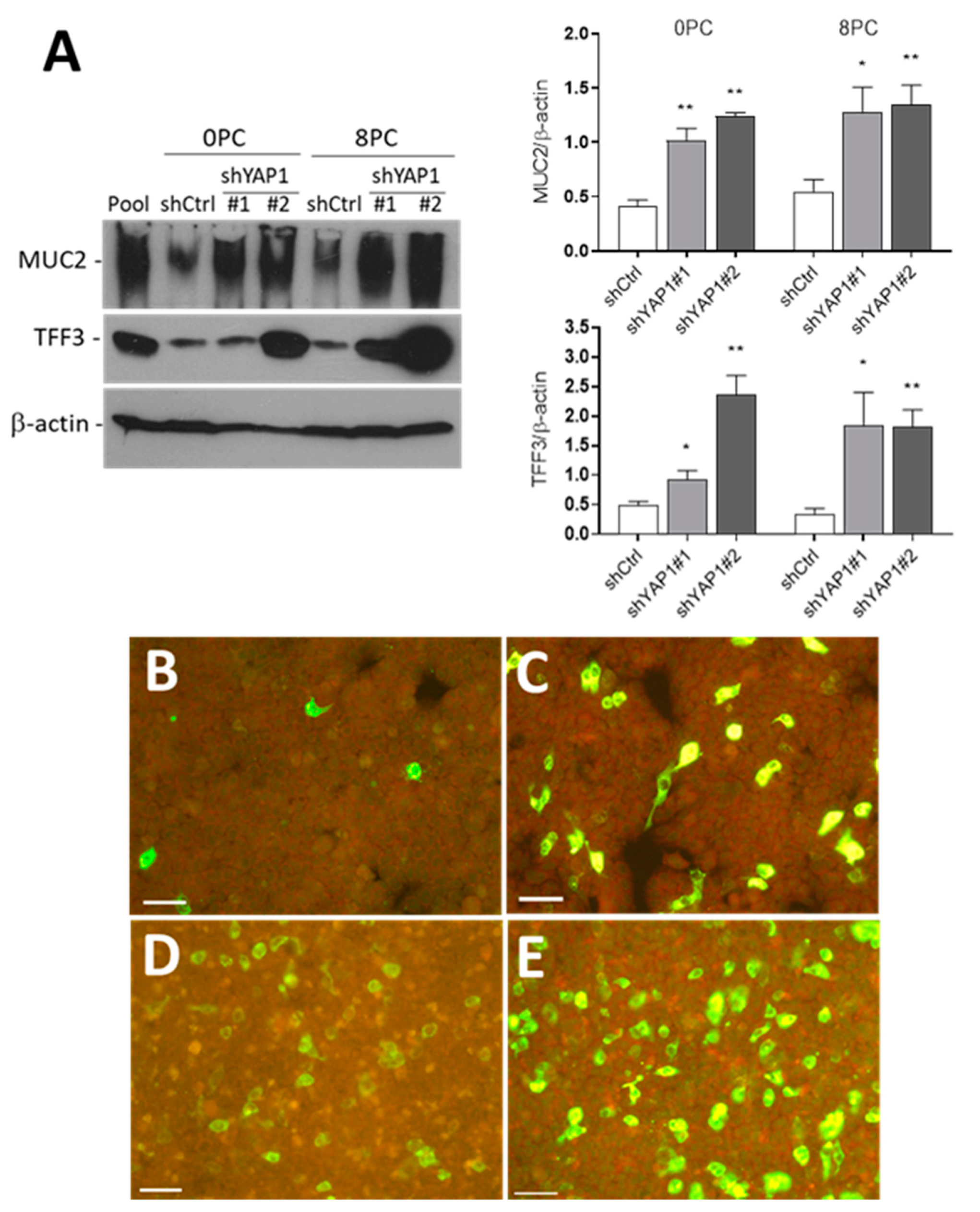
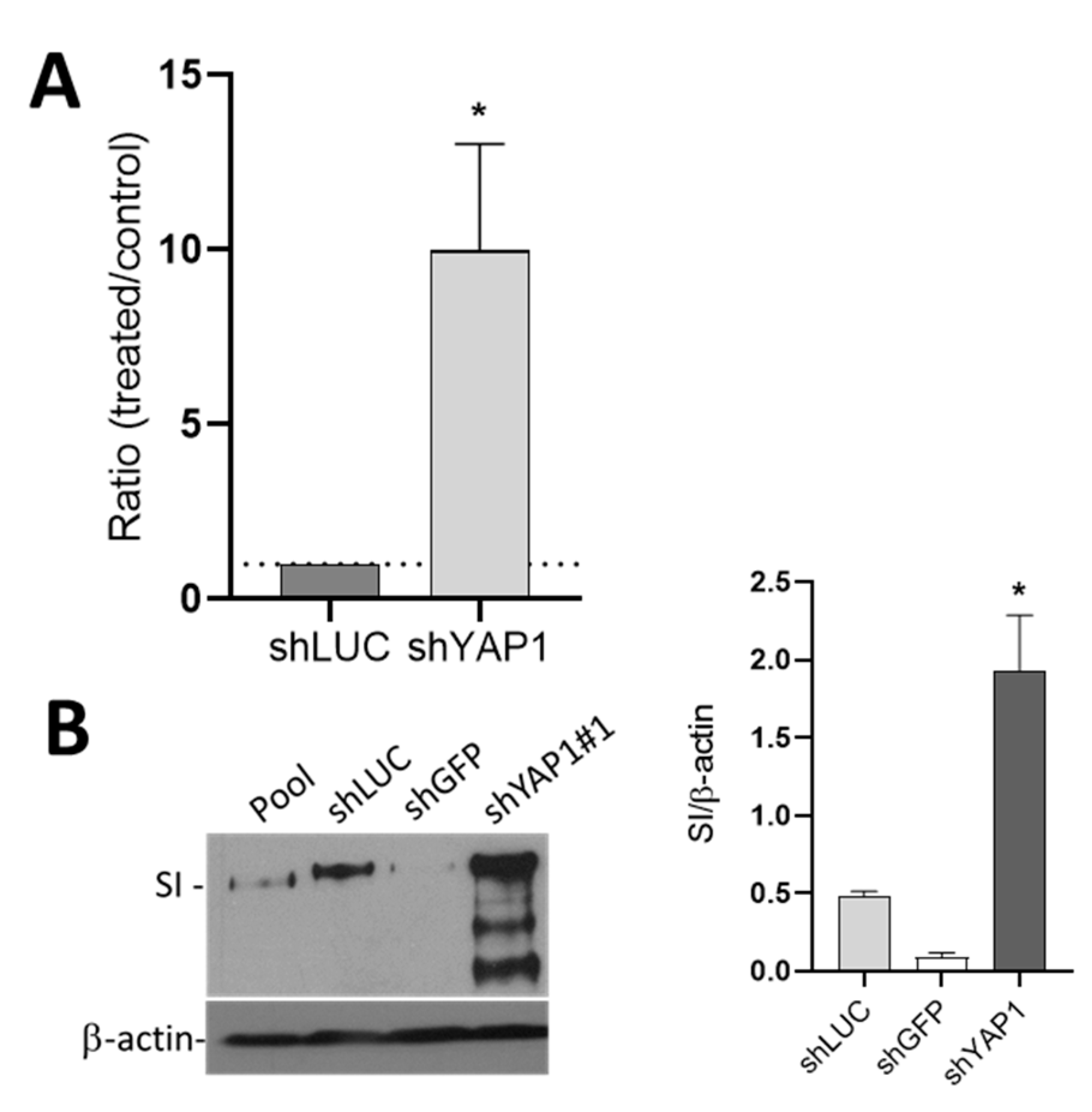
3.5. The Effect of YAP1 Knockdown on Intestinal Epithelial Cell Differentiation
3.6. Influence of YAP1 on Expression of Intestinal Differentiation-Regulating Transcription Factors
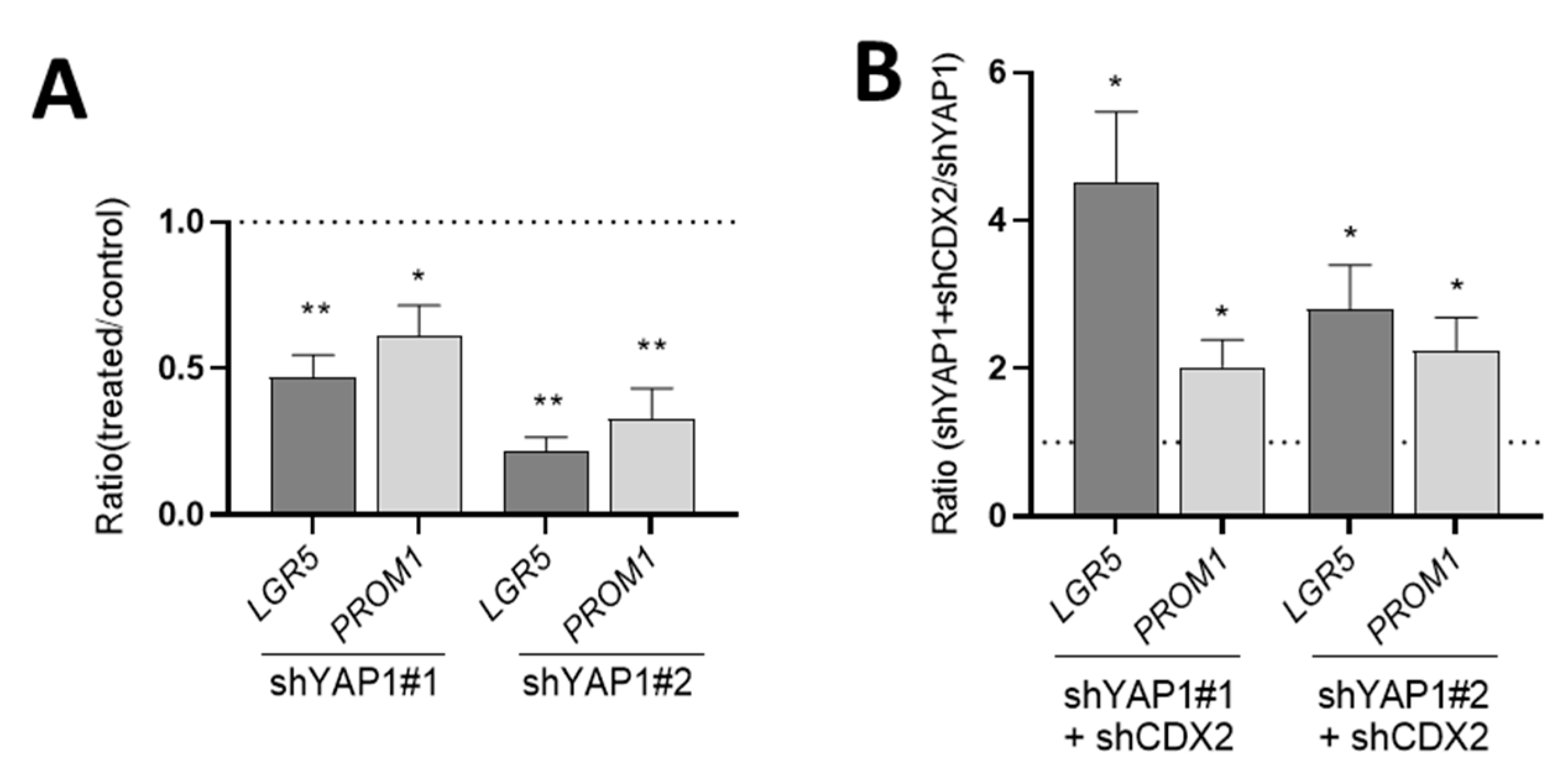
3.7. YAP1 Controls Differentiation of Intestinal Absorptive and Goblet Cells through CDX2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fallah, S.; Sénicourt, B.; Beaulieu, J.-F. Proliferation in the Gastrointestinal Epithelium. In Encyclopedia of Gastroenterology, 2nd ed.; Kuipers, E.J., Ed.; Academic Press: Oxford, UK, 2020; pp. 304–310. [Google Scholar]
- Van der Heijden, M.; Vermeulen, L. Stem cells in homeostasis and cancer of the gut. Mol. Cancer 2019, 18, 66. [Google Scholar] [CrossRef]
- Spit, M.; Koo, B.K.; Maurice, M.M. Tales from the crypt: Intestinal niche signals in tissue renewal, plasticity and cancer. Open Biol. 2018, 8, 180120. [Google Scholar] [CrossRef] [Green Version]
- Andersson-Rolf, A.; Zilbauer, M.; Koo, B.K.; Clevers, H. Stem Cells in Repair of Gastrointestinal Epithelia. Physiology 2017, 32, 278–289. [Google Scholar] [CrossRef]
- Sei, Y.; Feng, J.; Chow, C.C.; Wank, S.A. Asymmetric cell division-dominant neutral drift model for normal intestinal stem cell homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G64–G74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, A.M.; Graham, T.A. Revealing human intestinal stem cell and crypt dynamics. Mol. Cell Oncol. 2014, 1, e970069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjerknes, M.; Cheng, H. Gastrointestinal stem cells. II. Intestinal stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G381–G387. [Google Scholar] [CrossRef] [PubMed]
- Roostaee, A.; Benoit, Y.D.; Boudjadi, S.; Beaulieu, J.F. Epigenetics in Intestinal Epithelial Cell Renewal. J. Cell Physiol. 2016, 231, 2361–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gericke, B.; Schecker, N.; Amiri, M.; Naim, H.Y. Structure-function analysis of human sucrase-isomaltase identifies key residues required for catalytic activity. J. Biol. Chem. 2017, 292, 11070–11078. [Google Scholar] [CrossRef] [Green Version]
- Lammi, C.; Bollati, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Soybean- and Lupin-Derived Peptides Inhibit DPP-IV Activity on In Situ Human Intestinal Caco-2 Cells and Ex Vivo Human Serum. Nutrients 2018, 10, 1082. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 1997, 31, 1–78. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergstrom, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schutte, A.; van der Post, S.; Svensson, F.; Rodriguez-Pineiro, A.M.; Nystrom, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanahan, M.T.; Carroll, I.M.; Gulati, A.S. Critical design aspects involved in the study of Paneth cells and the intestinal microbiota. Gut Microbes. 2014, 5, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawardene, A.R.; Corfe, B.M.; Staton, C.A. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 2011, 92, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, B.S.; He, X.C.; Li, L. The regulatory niche of intestinal stem cells. J. Physiol. 2016, 594, 4827–4836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Biehs, B.; Chiu, C.; Siebel, C.W.; Wu, Y.; Costa, M.; de Sauvage, F.J.; Klein, O.D. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep. 2015, 11, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Demitrack, E.S.; Samuelson, L.C. Notch regulation of gastrointestinal stem cells. J. Physiol. 2016, 594, 4791–4803. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H.C.; Bevins, C.L. Paneth cells: Maestros of the small intestinal crypts. Annu. Rev. Physiol. 2013, 75, 289–311. [Google Scholar] [CrossRef]
- Traber, P.G.; Silberg, D.G. Intestine-specific gene transcription. Annu. Rev. Physiol. 1996, 58, 275–297. [Google Scholar] [CrossRef]
- Boudreau, F.; Rings, E.H.; van Wering, H.M.; Kim, R.K.; Swain, G.P.; Krasinski, S.D.; Moffett, J.; Grand, R.J.; Suh, E.R.; Traber, P.G. Hepatocyte nuclear factor-1α, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription implication for the developmental regulation of the sucrase-isomaltase gene. J. Biol. Chem. 2002, 277, 31909–31917. [Google Scholar] [CrossRef] [Green Version]
- Benoit, Y.D.; Pare, F.; Francoeur, C.; Jean, D.; Tremblay, E.; Boudreau, F.; Escaffit, F.; Beaulieu, J.F. Cooperation between HNF-1alpha, Cdx2, and GATA-4 in initiating an enterocytic differentiation program in a normal human intestinal epithelial progenitor cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G504–G517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandberg, T.P.; Sweere, I.; van Pelt, G.W.; Putter, H.; Vermeulen, L.; Kuppen, P.J.; Tollenaar, R.; Mesker, W.E. Prognostic value of low CDX2 expression in colorectal cancers with a high stromal content—A short report. Cell Oncol. 2019, 42, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Liu, D.; Sun, X.; Yang, K.; Yao, J.; Cheng, C.; Wang, C.; Zheng, J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/beta-catenin signaling via transactivation of GSK-3beta and Axin2 expression. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Lussier, C.R.; Brial, F.; Roy, S.A.; Langlois, M.J.; Verdu, E.F.; Rivard, N.; Perreault, N.; Boudreau, F. Loss of hepatocyte-nuclear-factor-1alpha impacts on adult mouse intestinal epithelial cell growth and cell lineages differentiation. PLoS ONE 2010, 5, e12378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Lee, C.S.; Labosky, P.A.; Yang, V.W.; Kaestner, K.H. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002, 129, 2619–2628. [Google Scholar]
- Yu, T.; Chen, X.; Zhang, W.; Li, J.; Xu, R.; Wang, T.C.; Ai, W.; Liu, C. Kruppel-like factor 4 regulates intestinal epithelial cell morphology and polarity. PLoS ONE 2012, 7, e32492. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.X.; Meng, Z.; Plouffe, S.W.; Guan, K.L. Hippo pathway regulation of gastrointestinal tissues. Annu. Rev. Physiol. 2015, 77, 201–227. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Khan, S.K.; Gvozdenovic-Jeremic, J.; Kim, Y.; Dahlman, J.; Kim, H.; Park, O.; Ishitani, T.; Jho, E.H.; Gao, B.; et al. Hippo signaling interactions with Wnt/beta-catenin and Notch signaling repress liver tumorigenesis. J. Clin. Investig. 2017, 127, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.S.; Jeon, Y.; Kim, S.M.; Jang, J.Y.; Park, M.K.; Kim, I.H.; Hwang, D.S.; Lim, D.S.; Lee, H. Depletion of MOB1A/B causes intestinal epithelial degeneration by suppressing Wnt activity and activating BMP/TGF-beta signaling. Cell Death Dis. 2018, 9, 1083. [Google Scholar] [CrossRef]
- Hong, A.W.; Meng, Z.; Guan, K.L. The Hippo pathway in intestinal regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Ohgushi, M.; Minaguchi, M.; Sasai, Y. Rho-Signaling-Directed YAP/TAZ Activity Underlies the Long-Term Survival and Expansion of Human Embryonic Stem Cells. Cell Stem Cell 2015, 17, 448–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.R.P.; Rundsten, C.F.; et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 2018, 22, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panciera, T.; Azzolin, L.; Fujimura, A.; Di Biagio, D.; Frasson, C.; Bresolin, S.; Soligo, S.; Basso, G.; Bicciato, S.; Rosato, A.; et al. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell Stem Cell 2016, 19, 725–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorieff, A.; Liu, Y.; Inanlou, M.R.; Khomchuk, Y.; Wrana, J.L. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 2015, 526, 715–718. [Google Scholar] [CrossRef]
- Rosado-Olivieri, E.A.; Anderson, K.; Kenty, J.H.; Melton, D.A. YAP inhibition enhances the differentiation of functional stem cell-derived insulin-producing beta cells. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Takahashi, T.; Shiraishi, A. Stem Cell Signaling Pathways in the Small Intestine. Int. J. Mol. Sci. 2020, 21, 2032. [Google Scholar] [CrossRef] [Green Version]
- Barry, E.R.; Morikawa, T.; Butler, B.L.; Shrestha, K.; de la Rosa, R.; Yan, K.S.; Fuchs, C.S.; Magness, S.T.; Smits, R.; Ogino, S.; et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 2013, 493, 106–110. [Google Scholar] [CrossRef]
- Imajo, M.; Ebisuya, M.; Nishida, E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat. Cell Biol. 2015, 17, 7–19. [Google Scholar] [CrossRef]
- Vázquez-Iglesias, L.; Barcia-Castro, L.; Rodríguez-Quiroga, M.; Páez de la Cadena, M.; Rodríguez-Berrocal, J.; Cordero, O.J. Surface expression marker profile in colon cancer cell lines and sphere-derived cells suggests complexity in CD26(+) cancer stem cells subsets. Biol. Open 2019, 8, bio041673. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: New York, NY, USA, 2015; pp. 113–124. [Google Scholar]
- Kitamura, H.; Cho, M.; Lee, B.H.; Gum, J.R.; Siddiki, B.B.; Ho, S.B.; Toribara, N.W.; Lesuffleur, T.; Zweibaum, A.; Kitamura, Y.; et al. Alteration in mucin gene expression and Biological properties of HT29 colon cancer cell subpopulations. Eur. J. Cancer 1996, 32A, 1788–1796. [Google Scholar] [CrossRef]
- Robine, S.; Sahuquillo-Merino, C.; Louvard, D.; Pringault, E. Regulatory sequences on the human villin gene trigger the expression of a reporter gene in a differentiating HT29 intestinal cell line. J. Biol. Chem. 1993, 268, 11426–11434. [Google Scholar] [PubMed]
- Beaulieu, J.F.; Quaroni, A. Clonal analysis of sucrase-isomaltase expression in the human colon adenocarcinoma Caco-2 cells. Biochem. J. 1991, 280, 599–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pageot, L.P.; Perreault, N.; Basora, N.; Francoeur, C.; Magny, P.; Beaulieu, J.F. Human cell models to study small intestinal functions: Recapitulation of the crypt-villus axis. Microsc. Res. Tech. 2000, 49, 394–406. [Google Scholar] [CrossRef]
- Whitehead, R.; Watson, N. Gastrointestinal cell lines as a model for differentiation. In The Gut as a Model in Cell and Molecular Biology; Halter, F., Winton, D., Wright, N.A., Eds.; Kluwer: Dordrecht, The Netherlands, 1997; pp. 275–290. [Google Scholar]
- Tremblay, E.; Auclair, J.; Delvin, E.; Levy, E.; Menard, D.; Pshezhetsky, A.V.; Rivard, N.; Seidman, E.G.; Sinnett, D.; Vachon, P.H.; et al. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: A comparison with the Caco-2 cell model. J. Cell Biochem. 2006, 99, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.F.; Menard, D. Isolation, characterization, and culture of normal human intestinal crypt and villus cells. Methods Mol. Biol. 2012, 806, 157–173. [Google Scholar] [CrossRef]
- Rosenbluh, J.; Nijhawan, D.; Cox, A.G.; Li, X.; Neal, J.T.; Schafer, E.J.; Zack, T.I.; Wang, X.; Tsherniak, A.; Schinzel, A.C.; et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 2012, 151, 1457–1473. [Google Scholar] [CrossRef] [Green Version]
- Groulx, J.F.; Giroux, V.; Beausejour, M.; Boudjadi, S.; Basora, N.; Carrier, J.C.; Beaulieu, J.F. Integrin alpha6A splice variant regulates proliferation and the Wnt/beta-catenin pathway in human colorectal cancer cells. Carcinogenesis 2014, 35, 1217–1227. [Google Scholar] [CrossRef] [Green Version]
- Benoit, Y.D.; Lepage, M.B.; Khalfaoui, T.; Tremblay, E.; Basora, N.; Carrier, J.C.; Gudas, L.J.; Beaulieu, J.F. Polycomb repressive complex 2 impedes intestinal cell terminal differentiation. J. Cell Sci. 2012, 125, 3454–3463. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.F.; Nichols, B.; Quaroni, A. Posttranslational regulation of sucrase-isomaltase expression in intestinal crypt and villus cells. J. Biol. Chem. 1989, 264, 20000–20011. [Google Scholar]
- Daniele, B.; Quaroni, A. Polarized secretion of diamine oxidase by intestinal epithelial cells and its stimulation by heparin. Gastroenterology 1990, 99, 1675–1687. [Google Scholar] [CrossRef]
- Rasband, W.S.; Image, J.U.S. National Institutes of Health. Available online: https://imagej.nih.gov/ij/ (accessed on 28 June 2015).
- Dydensborg, A.B.; Herring, E.; Auclair, J.; Tremblay, E.; Beaulieu, J.F. Normalizing genes for quantitative RT-PCR in differentiating human intestinal epithelial cells and adenocarcinomas of the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1067–G1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Vachon, P.H.; Beaulieu, J.F. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology 1992, 103, 414–423. [Google Scholar] [CrossRef]
- Wang, L.; Shi, S.; Guo, Z.; Zhang, X.; Han, S.; Yang, A.; Wen, W.; Zhu, Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS ONE 2013, 8, e65539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, Y.; Wu, H.; Barry, E.; Yin, Y.; Lawrence, E.; Dawson, D.; Willis, J.E.; Markowitz, S.D.; Camargo, F.D.; et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl. Acad. Sci. USA 2011, 108, E1312–E1320. [Google Scholar] [CrossRef] [Green Version]
- Yuen, H.F.; McCrudden, C.M.; Huang, Y.H.; Tham, J.M.; Zhang, X.; Zeng, Q.; Zhang, S.D.; Hong, W. TAZ expression as a prognostic indicator in colorectal cancer. PLoS ONE 2013, 8, e54211. [Google Scholar] [CrossRef] [Green Version]
- Camargo, F.D.; Gokhale, S.; Johnnidis, J.B.; Fu, D.; Bell, G.W.; Jaenisch, R.; Brummelkamp, T.R. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 2007, 17, 2054–2060. [Google Scholar] [CrossRef] [Green Version]
- Suh, E.; Traber, P.G. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol. Cell Biol. 1996, 16, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Boyd, M.; Hansen, M.; Jensen, T.G.; Perearnau, A.; Olsen, A.K.; Bram, L.L.; Bak, M.; Tommerup, N.; Olsen, J.; Troelsen, J.T. Genome-wide analysis of CDX2 binding in intestinal epithelial cells (Caco-2). J. Biol. Chem. 2010, 285, 25115–25125. [Google Scholar] [CrossRef] [Green Version]
- Hinoi, T.; Loda, M.; Fearon, E.R. Silencing of CDX2 expression in colon cancer via a dominant repression pathway. J. Biol. Chem. 2003, 278, 44608–44616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasinski, S.D.; Van Wering, H.M.; Tannemaat, M.R.; Grand, R.J. Differential activation of intestinal gene promoters: Functional interactions between GATA-5 and HNF-1 alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G69–G84. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Bai, Y.Q.; Yuasa, Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem. Biophys. Res. Commun. 2003, 300, 813–818. [Google Scholar] [CrossRef]
- Mesquita, P.; Jonckheere, N.; Almeida, R.; Ducourouble, M.P.; Serpa, J.; Silva, E.; Pigny, P.; Silva, F.S.; Reis, C.; Silberg, D.; et al. Human MUC2 mucin gene is transcriptionally regulated by Cdx homeodomain proteins in gastrointestinal carcinoma cell lines. J. Biol. Chem. 2003, 278, 51549–51556. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.; Koike, T.; Yamagata, M.; Yoneda, M.; Hiraishi, H. Regulation of TFF3 expression by homeodomain protein CDX2. Regul. Pept. 2007, 140, 81–87. [Google Scholar] [CrossRef]
- Erickson, R.H.; Lai, R.S.; Kim, Y.S. Role of hepatocyte nuclear factor 1alpha and 1beta in the transcriptional regulation of human dipeptidyl peptidase IV during differentiation of Caco-2 cells. Biochem. Biophys. Res. Commun. 2000, 270, 235–239. [Google Scholar] [CrossRef]
- Erickson, R.H.; Lai, R.S.; Lotterman, C.D.; Kim, Y.S. Identification of upstream stimulatory factor as an activator of the human dipeptidyl peptidase IV gene in Caco-2 cells. Gene 2000, 258, 77–84. [Google Scholar] [CrossRef]
- Escaffit, F.; Boudreau, F.; Beaulieu, J.F. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J. Cell. Physiol. 2005, 203, 15–26. [Google Scholar] [CrossRef]
- San Roman, A.K.; Tovaglieri, A.; Breault, D.T.; Shivdasani, R.A. Distinct Processes and Transcriptional Targets Underlie CDX2 Requirements in Intestinal Stem Cells and Differentiated Villus Cells. Stem Cell Rep. 2015, 5, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.; Davidsen, J.; Dahlgaard, K.; Pedersen, O.B.; Troelsen, J.T. HNF4alpha and CDX2 Regulate Intestinal YAP1 Promoter Activity. Int. J. Mol. Sci. 2019, 20, 2981. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Freund, J.N.; Muller, M.; Ravey, J.; Nicolas, J.P.; Gueant, J.L.; Namour, F. Differential regulation of CDX1 and CDX2 gene expression by deficiency in methyl group donors. Biochimie 2008, 90, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Graule, J.; Uth, K.; Fischer, E.; Centeno, I.; Galvan, J.A.; Eichmann, M.; Rau, T.T.; Langer, R.; Dawson, H.; Nitsche, U.; et al. CDX2 in colorectal cancer is an independent prognostic factor and regulated by promoter methylation and histone deacetylation in tumors of the serrated pathway. Clin. Epigenetics 2018, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, H.; Toyooka, Y.; Shimosato, D.; Strumpf, D.; Takahashi, K.; Yagi, R.; Rossant, J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 2005, 123, 917–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, K.; Zhou, G.; Zhang, Q.; Li, J.; Zhang, C. Expression of hippo pathway in colorectal cancer. Saudi J. Gastroenterol. 2014, 20, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Pan, Q.; Chen, L.; Ye, J.; Zhong, X.; Li, X.; Meng, L.; Guo, J.; Tian, Y.; He, Y.; et al. Achaete scute-like 2 suppresses CDX2 expression and inhibits intestinal neoplastic epithelial cell differentiation. Oncotarget 2015, 6, 30993–31006. [Google Scholar] [CrossRef] [Green Version]
- Benahmed, F.; Gross, I.; Guenot, D.; Jehan, F.; Martin, E.; Domon-Dell, C.; Brabletz, T.; Kedinger, M.; Freund, J.N.; Duluc, I. The microenvironment controls CDX2 homeobox gene expression in colorectal cancer cells. Am. J. Pathol. 2007, 170, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Qualtrough, D.; Hinoi, T.; Fearon, E.; Paraskeva, C. Expression of CDX2 in normal and neoplastic human colon tissue and during differentiation of an in vitro model system. Gut 2002, 51, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Roostaee, A.; Guezguez, A.; Beausejour, M.; Simoneau, A.; Vachon, P.H.; Levy, E.; Beaulieu, J.F. Histone deacetylase inhibition impairs normal intestinal cell proliferation and promotes specific gene expression. J. Cell Biochem. 2015, 116, 2695–2708. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallah, S.; Beaulieu, J.-F. The Hippo Pathway Effector YAP1 Regulates Intestinal Epithelial Cell Differentiation. Cells 2020, 9, 1895. https://doi.org/10.3390/cells9081895
Fallah S, Beaulieu J-F. The Hippo Pathway Effector YAP1 Regulates Intestinal Epithelial Cell Differentiation. Cells. 2020; 9(8):1895. https://doi.org/10.3390/cells9081895
Chicago/Turabian StyleFallah, Sepideh, and Jean-François Beaulieu. 2020. "The Hippo Pathway Effector YAP1 Regulates Intestinal Epithelial Cell Differentiation" Cells 9, no. 8: 1895. https://doi.org/10.3390/cells9081895




