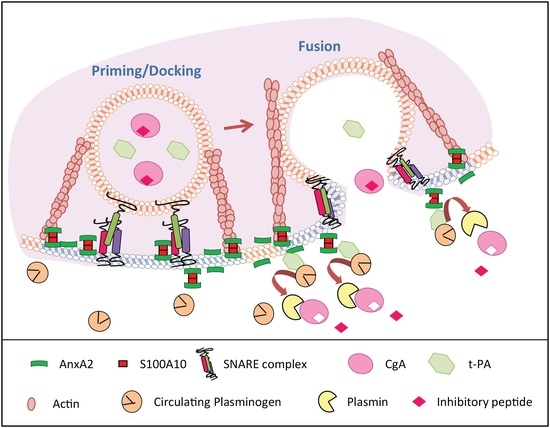
Annexin A2 Egress during Calcium-Regulated Exocytosis in Neuroendocrine Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Chromaffin Cell Culture and Transfection
2.3. Cell Stimulation and Extracellular AnxA2 and t-PA Measurements
2.4. Immunofluorescence and Confocal Microscopy
2.5. Plasma Membrane Sheet Preparation and Transmission Electron Microscopy Observation
2.6. Statistical Analysis
3. Results
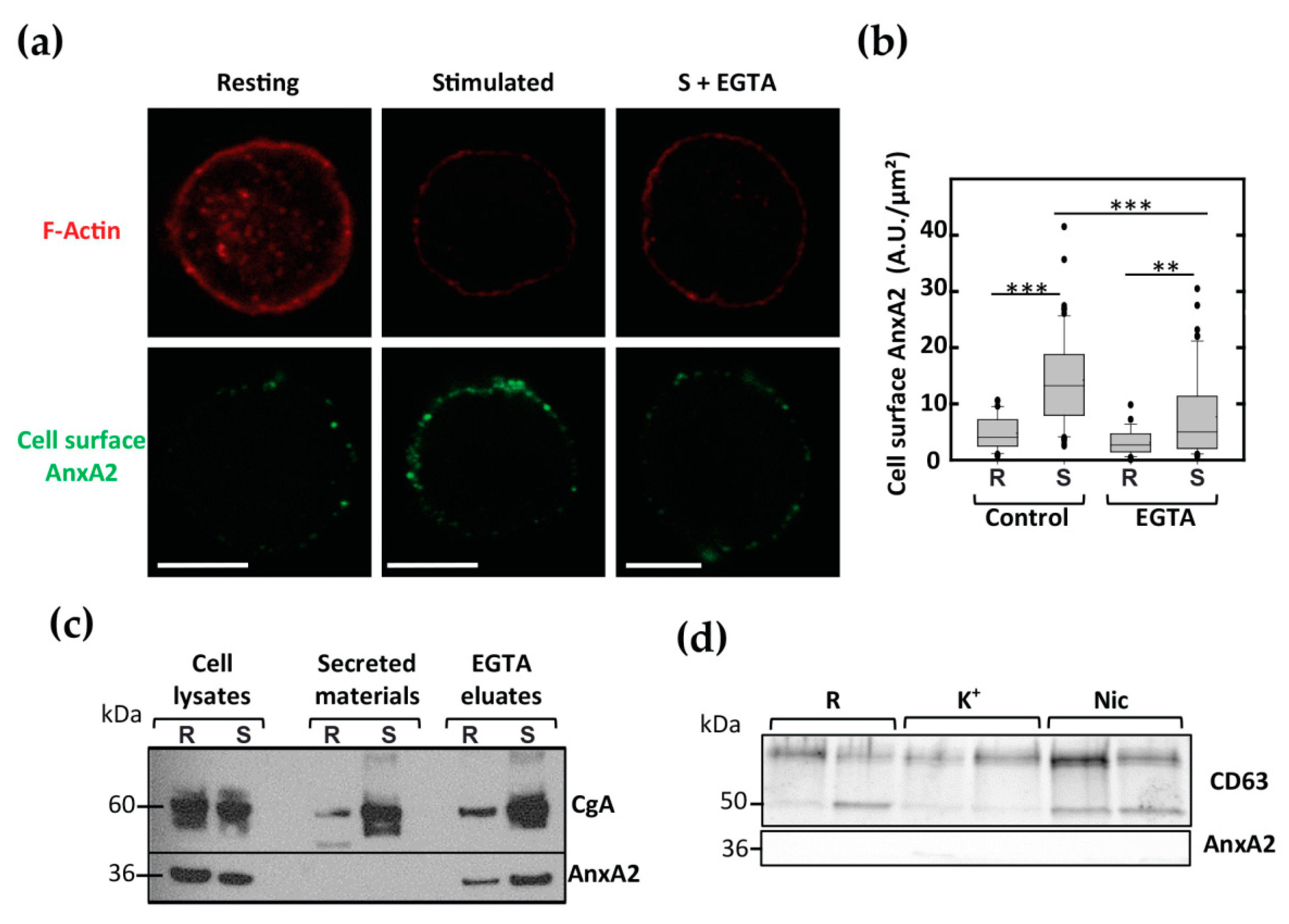
3.1. AnxA2 Crosses the Plasma Membrane in Stimulated Chromaffin Cells in a Ca2+-Dependent Manner and Accumulates on the Extracellular Membrane Leaflet
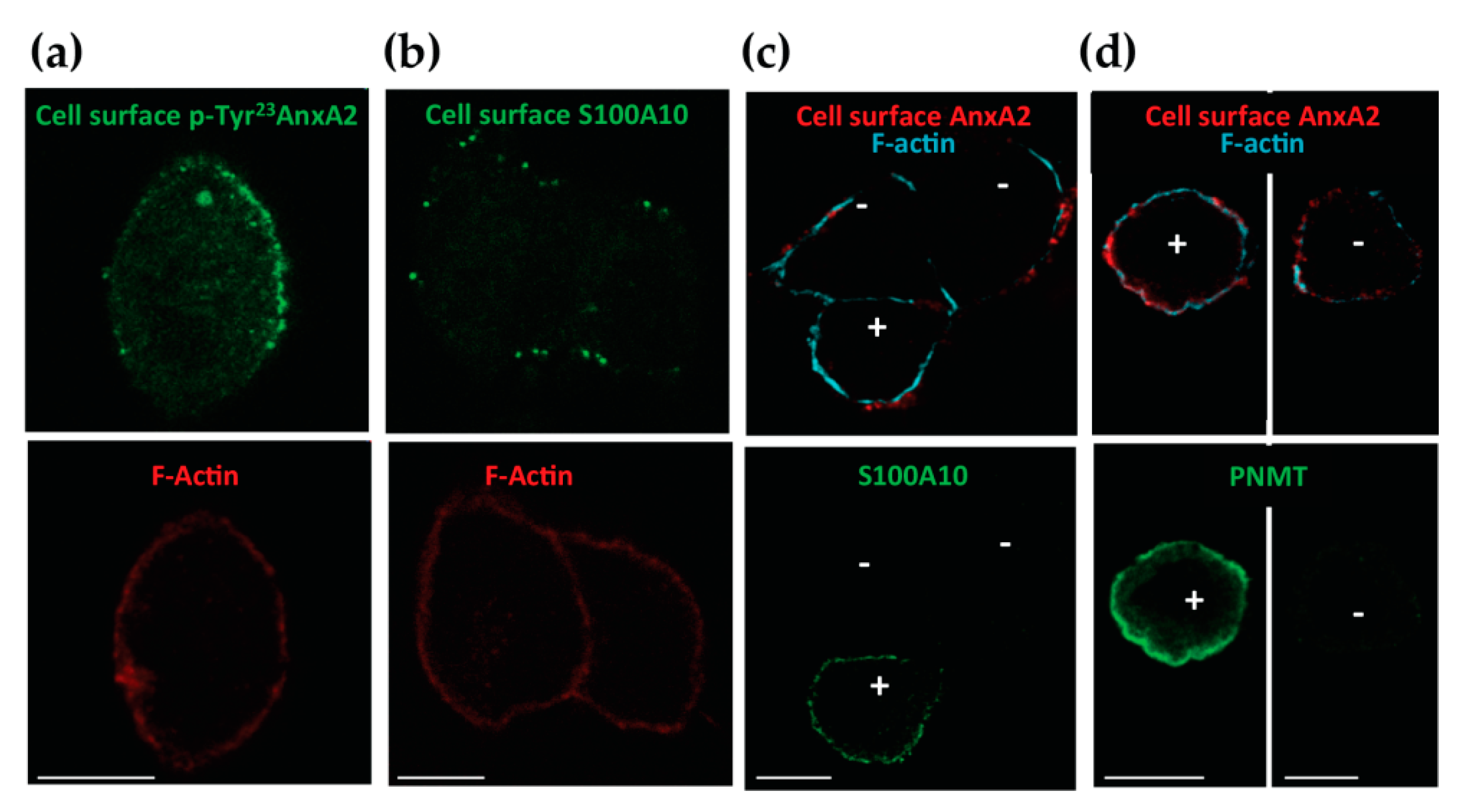
3.2. Tyr 23 Phosphorylated AnxA2 Tetramer Binds the External Face of the Plasma Membrane
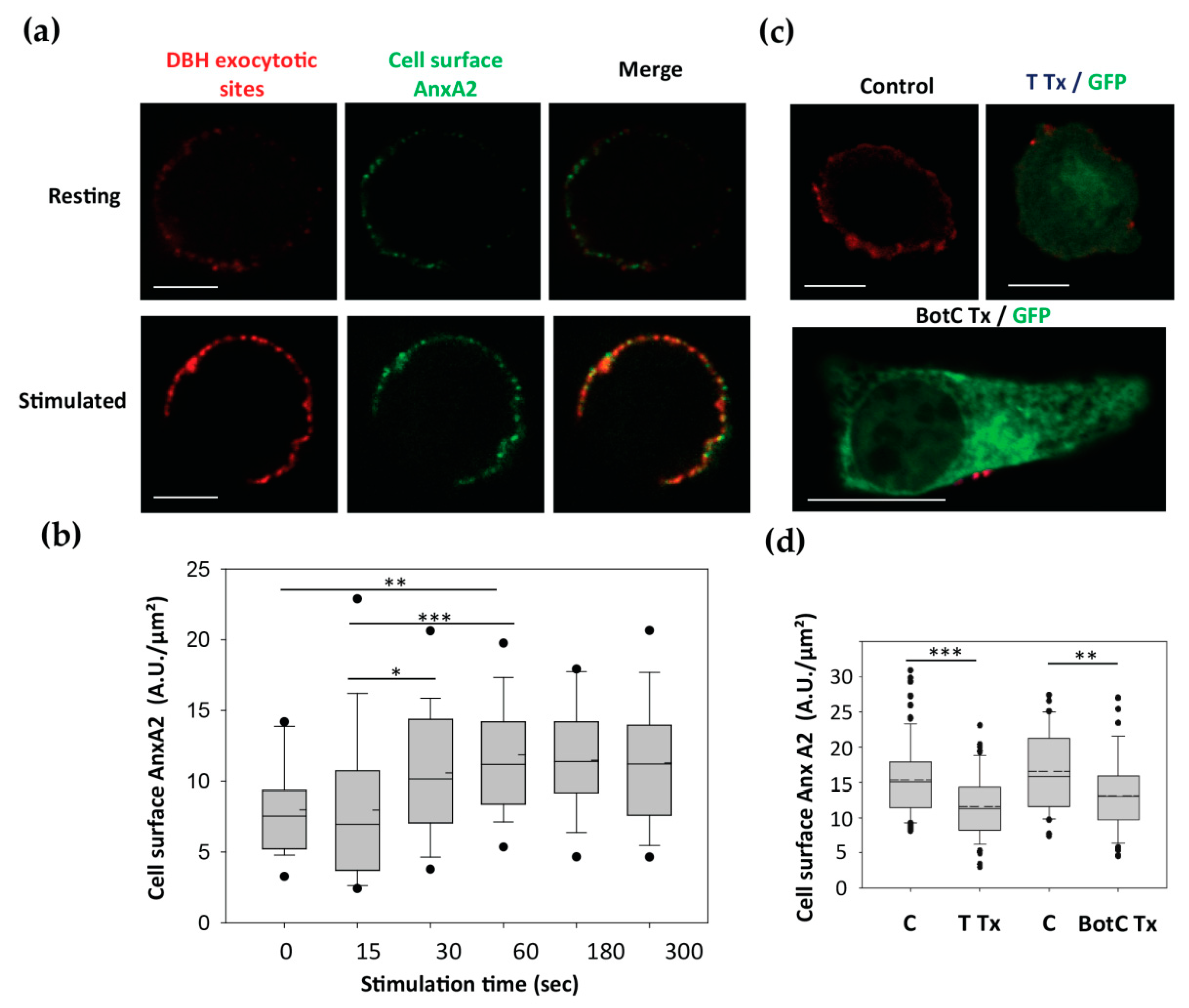
3.3. The Egress of AnxA2 is Linked to Exocytosis
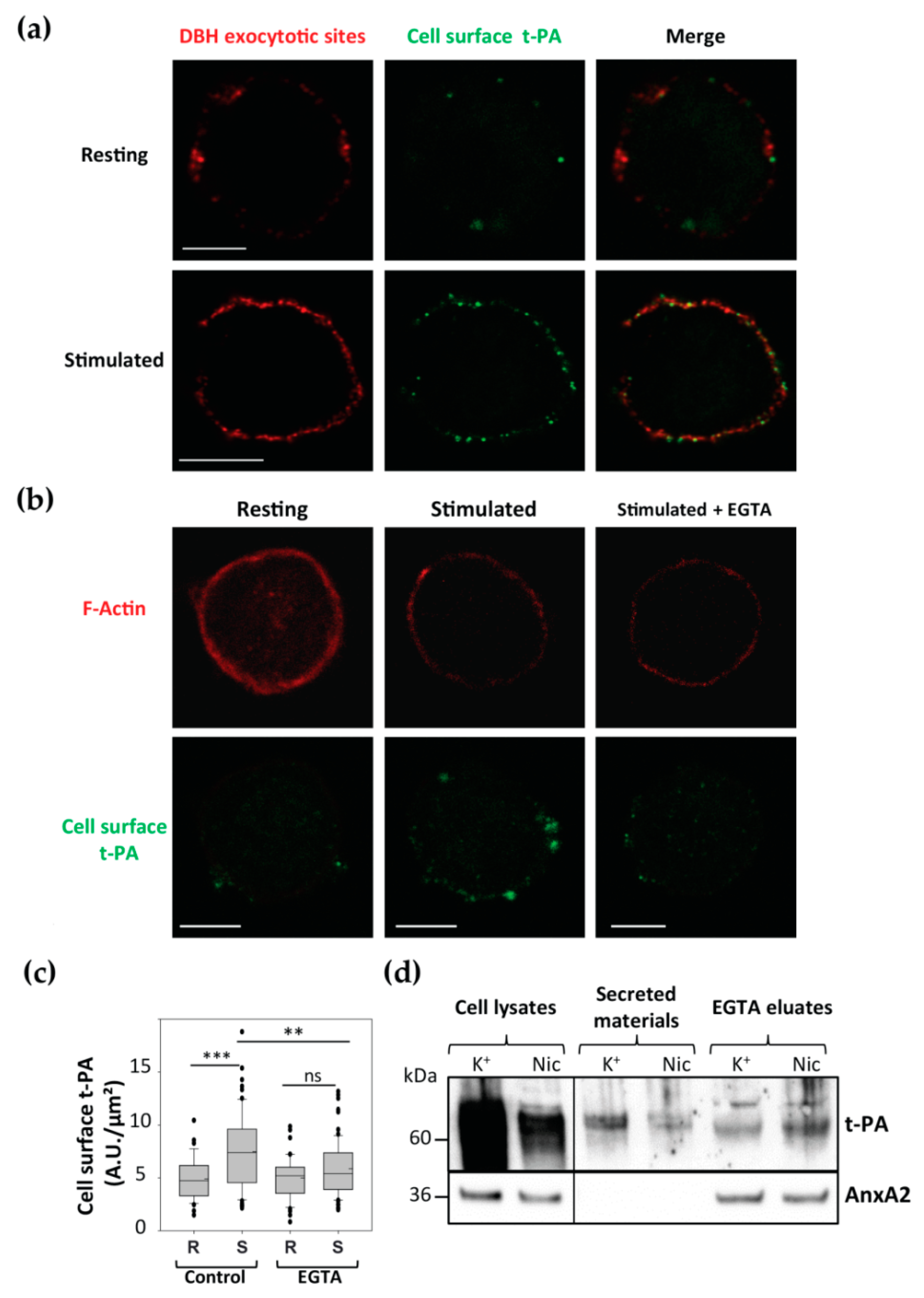
3.4. Cell Surface Membrane-Associated t-PA is Present Close to Exocytotic Sites
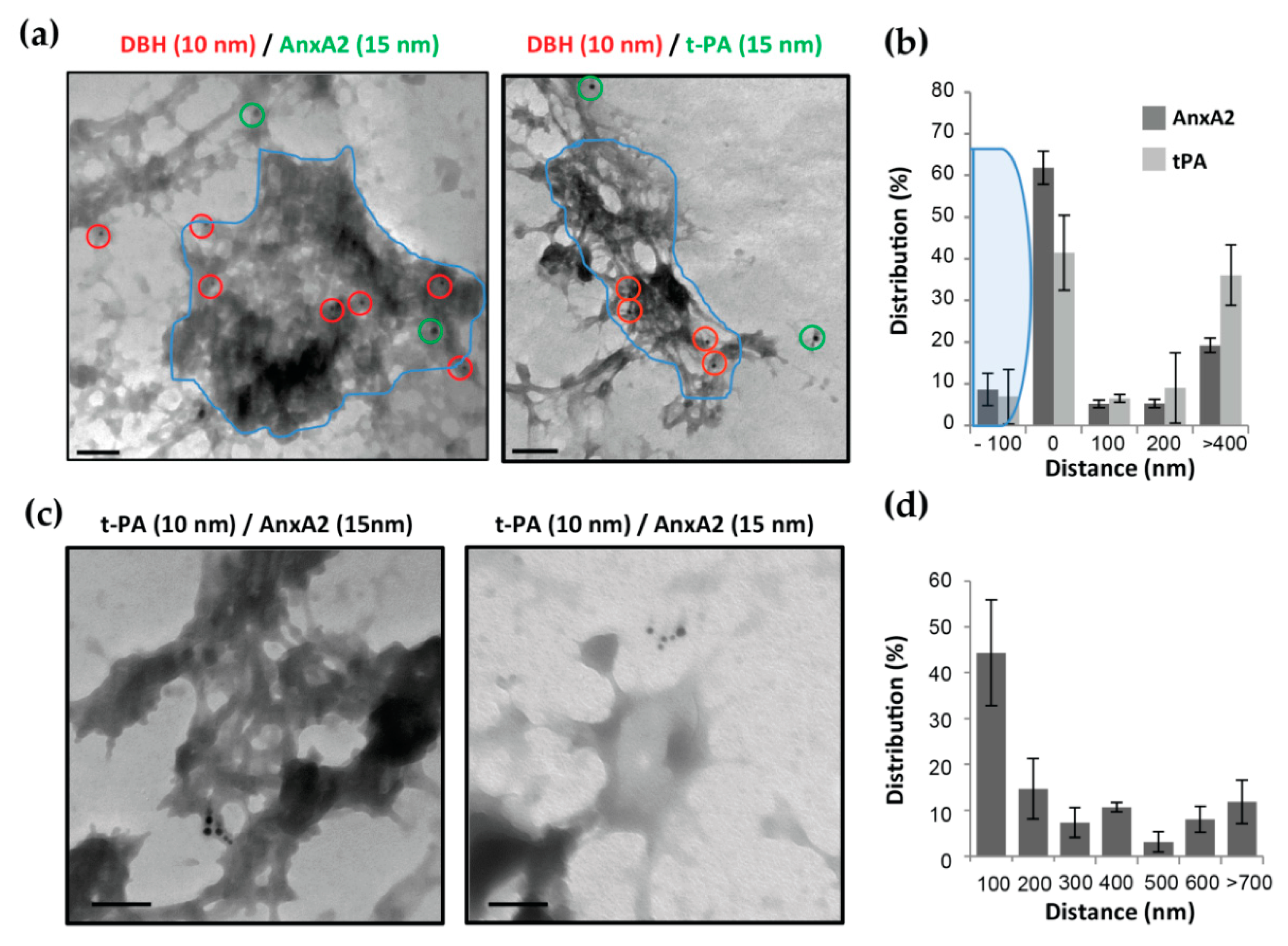
3.5. AnxA2 and t-PA are Side by Side at Cell Surface of Stimulated Chromaffin Cells
4. Discussion
4.1. By Which Mechanism is AnxA2 Translocated to the Cell Surface in Chromaffin Cells?
4.2. What Might be the Role of Cell Surface AnxA2?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanguy, E.; Carmon, O.; Wang, Q.; Jeandel, L.; Chasserot-Golaz, S.; Montero-Hadjadje, M.; Vitale, N. Lipids implicated in the journey of a secretory granule: From biogenesis to fusion. J. Neurochem. 2016, 137, 904–912. [Google Scholar] [CrossRef]
- Bombardier, J.P.; Munson, M. Three steps forward, two steps back: Mechanistic insights into the assembly and disassembly of the SNARE complex. Curr. Opin. Chem. Biol. 2015, 29, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, A.; Bydoun, M.; Holloway, R.; Waisman, D. Annexin A2 heterotetramer: Structure and function. Int. J. Mol. Sci. 2013, 14, 6259–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabel, M.; Chasserot-Golaz, S. Annexin A2, an essential partner of the exocytotic process in chromaffin cells. J. Neurochem. 2016, 137, 890–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabel, M.; Delavoie, F.; Demais, V.; Royer, C.; Bailly, Y.; Vitale, N.; Bader, M.F.; Chasserot-Golaz, S. Annexin A2-dependent actin bundling promotes secretory granule docking to the plasma membrane and exocytosis. J. Cell Biol. 2015, 210, 785–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabel, M.; Delavoie, F.; Royer, C.; Tahouly, T.; Gasman, S.; Bader, M.F.; Vitale, N.; Chasserot-Golaz, S. Phosphorylation cycling of Annexin A2 Tyr23 is critical for calcium-regulated exocytosis in neuroendocrine cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1207–1217. [Google Scholar] [CrossRef]
- Deora, A.B.; Kreitzer, G.; Jacovina, A.T.; Hajjar, K.A. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J. Biol. Chem. 2004, 279, 43411–43418. [Google Scholar] [CrossRef] [Green Version]
- Valapala, M.; Vishwanatha, J.K. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J. Biol. Chem. 2011, 286, 30911–30925. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.Q.; Lu, B. Expression of annexin A2 in GABAergic interneurons in the normal rat brain. J. Neurochem. 2007, 100, 1211–1223. [Google Scholar] [CrossRef]
- Calabresi, P.; Napolitano, M.; Centonze, D.; Marfia, G.A.; Gubellini, P.; Teule, M.A.; Berretta, N.; Bernardi, G.; Frati, L.; Tolu, M.; et al. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur. J. Neurosci. 2000, 12, 1002–1012. [Google Scholar] [CrossRef]
- Tsirka, S.E. Tissue plasminogen activator as a modulator of neuronal survival and function. Biochem. Soc. Trans. 2002, 30, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.V.; Migne, C.; Devilliers, G.; Ayala-Sanmartin, J. Annexin 2 “secretion” accompanying exocytosis of chromaffin cells: Possible mechanisms of annexin release. Exp. Cell Res. 2002, 276, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chasserot-Golaz, S.; Vitale, N.; Umbrecht-Jenck, E.; Knight, D.; Gerke, V.; Bader, M.F. Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol. Biol. Cell 2005, 16, 1108–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chasserot-Golaz, S.; Vitale, N.; Sagot, I.; Delouche, B.; Dirrig, S.; Pradel, L.A.; Henry, J.P.; Aunis, D.; Bader, M.F. Annexin II in exocytosis: Catecholamine secretion requires the translocation of p36 to the subplasmalemmal region in chromaffin cells. J. Cell Biol. 1996, 133, 1217–1236. [Google Scholar] [CrossRef]
- McMahon, H.T.; Ushkaryov, Y.A.; Edelmann, L.; Link, E.; Binz, T.; Niemann, H.; Jahn, R.; Sudhof, T.C. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 1993, 364, 346–349. [Google Scholar] [CrossRef]
- Lam, A.D.; Tryoen-Toth, P.; Tsai, B.; Vitale, N.; Stuenkel, E.L. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol. Biol. Cell 2008, 19, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Bader, M.F.; Thierse, D.; Aunis, D.; Ahnert-Hilger, G.; Gratzl, M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J. Biol. Chem. 1986, 261, 5777–5783. [Google Scholar]
- de Chaumont, F.; Dallongeville, S.; Chenouard, N.; Herve, N.; Pop, S.; Provoost, T.; Meas-Yedid, V.; Pankajakshan, P.; Lecomte, T.; Le Montagner, Y.; et al. Icy: An open bioimage informatics platform for extended reproducible research. Nat. Methods 2012, 9, 690–696. [Google Scholar] [CrossRef]
- Umbrecht-Jenck, E.; Demais, V.; Calco, V.; Bailly, Y.; Bader, M.F.; Chasserot-Golaz, S. S100A10-mediated translocation of annexin-A2 to SNARE proteins in adrenergic chromaffin cells undergoing exocytosis. Traffic 2010, 11, 958–971. [Google Scholar] [CrossRef]
- Bader, M.F.; Holz, R.W.; Kumakura, K.; Vitale, N. Exocytosis: The chromaffin cell as a model system. Ann. N. Y. Acad. Sci. 2002, 971, 178–183. [Google Scholar] [CrossRef]
- Livett, B.G.; Kozousek, V.; Mizobe, F.; Dean, D.M. Substance P inhibits nicotinic activation of chromaffin cells. Nature 1979, 278, 256–257. [Google Scholar] [CrossRef]
- Trifaro, J.M.; Lee, R.W. Morphological characteristics and stimulus-secretion coupling in bovine adcrenal chromaffin cell cultures. Neuroscience 1980, 5, 1533–1546. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhofstad, A.A.; Coupland, R.E.; Parker, T.R.; Goldstein, M. Immunohistochemical and biochemical study on the development of the noradrenaline- and adrenaline-storing cells of the adrenal medulla of the rat. Cell Tissue Res. 1985, 242, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Ahnert-Hilger, G.; Bigalke, H. Molecular aspects of tetanus and botulinum neurotoxin poisoning. Prog. Neurobiol. 1995, 46, 83–96. [Google Scholar] [CrossRef]
- Kim, J.; Hajjar, K.A. Annexin II: A plasminogen-plasminogen activator co-receptor. Front. Biosci. 2002, 7, d341–d348. [Google Scholar] [CrossRef] [PubMed]
- Santell, L.; Marotti, K.R.; Levin, E.G. Targeting of tissue plasminogen activator into the regulated secretory pathway of neuroendocrine cells. Brain Res. 1999, 816, 258–265. [Google Scholar] [CrossRef]
- Parmer, R.J.; Mahata, M.; Gong, Y.; Mahata, S.K.; Jiang, Q.; O’Connor, D.T.; Xi, X.P.; Miles, L.A. Processing of chromogranin A by plasmin provides a novel mechanism for regulating catecholamine secretion. J. Clin. Investig. 2000, 106, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Ceridono, M.; Ory, S.; Momboisse, F.; Chasserot-Golaz, S.; Houy, S.; Calco, V.; Haeberle, A.M.; Demais, V.; Bailly, Y.; Bader, M.F.; et al. Selective recapture of secretory granule components after full collapse exocytosis in neuroendocrine chromaffin cells. Traffic 2011, 12, 72–88. [Google Scholar] [CrossRef]
- Dziduszko, A.; Ozbun, M.A. Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes. J. Virol. 2013, 87, 7502–7515. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Schekman, R. Cell biology. Unconventional secretion, unconventional solutions. Science 2013, 340, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hajjar, K.A. Annexin A2 system in human biology: Cell surface and beyond. Semin. Thromb. Hemost. 2013, 39, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-phospholipid interactions. Functional implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popa, S.J.; Stewart, S.E.; Moreau, K. Unconventional secretion of annexins and galectins. Semin. Cell Dev. Biol. 2018, 83, 42–50. [Google Scholar] [CrossRef]
- Stewart, S.E.; Ashkenazi, A.; Williamson, A.; Rubinsztein, D.C.; Moreau, K. Transbilayer phospholipid movement facilitates the translocation of annexin across membranes. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabouille, C.; Malhotra, V.; Nickel, W. Diversity in unconventional protein secretion. J. Cell Sci. 2012, 125, 5251–5255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirov, A.; Al-Hashimi, H.; Solomon, P.; Mazur, C.; Thorpe, P.E.; Sims, P.J.; Tarantini, F.; Kumar, T.K.; Prudovsky, I. Phosphatidylserine externalization and membrane blebbing are involved in the nonclassical export of FGF1. J. Cell Biochem. 2012, 113, 956–966. [Google Scholar] [CrossRef] [Green Version]
- Vitale, N.; Caumont, A.S.; Chasserot-Golaz, S.; Du, G.; Wu, S.; Sciorra, V.A.; Morris, A.J.; Frohman, M.A.; Bader, M.F. Phospholipase D1: A key factor for the exocytotic machinery in neuroendocrine cells. EMBO J. 2001, 20, 2424–2434. [Google Scholar] [CrossRef]
- Ory, S.; Ceridono, M.; Momboisse, F.; Houy, S.; Chasserot-Golaz, S.; Heintz, D.; Calco, V.; Haeberle, A.M.; Espinoza, F.A.; Sims, P.J.; et al. Phospholipid scramblase-1-induced lipid reorganization regulates compensatory endocytosis in neuroendocrine cells. J. Neurosci. 2013, 33, 3545–3556. [Google Scholar] [CrossRef] [Green Version]
- Danielsen, E.M.; van Deurs, B.; Hansen, G.H. “Nonclassical” secretion of annexin A2 to the lumenal side of the enterocyte brush border membrane. Biochemistry 2003, 42, 14670–14676. [Google Scholar] [CrossRef]
- Parmer, R.J.; Mahata, S.K.; Jiang, Q.; Taupenot, L.; Gong, Y.; Mahata, M.; O’Connor, D.T.; Miles, L.A. Tissue plasminogen activator and chromaffin cell function. Adv. Exp. Med. Biol. 2000, 482, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.N.; Anantharam, A.; Bittner, M.A.; Axelrod, D.; Holz, R.W. Lumenal protein within secretory granules affects fusion pore expansion. Biophys. J. 2014, 107, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.L.; Sui, G.; Xiong, H.; Broekman, M.J.; Huang, B.; Marcus, A.J.; Hajjar, K.A. Feedback regulation of endothelial cell surface plasmin generation by PKC dependent phosphorylation of annexin A2. J. Biol. Chem. 2010. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Yasothornsrikul, S.; Taupenot, L.; Miles, L.A.; Parmer, R.J. The local chromaffin cell plasminogen/plasmin system and the regulation of catecholamine secretion. Ann. N. Y. Acad. Sci. 2002, 971, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Menke, M.; Gerke, V.; Steinem, C. Phosphatidylserine membrane domain clustering induced by annexin A2/S100A10 heterotetramer. Biochemistry 2005, 44, 15296–15303. [Google Scholar] [CrossRef] [PubMed]
- van Genderen, H.O.; Kenis, H.; Hofstra, L.; Narula, J.; Reutelingsperger, C.P. Extracellular annexin A5: Functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim. Biophys. Acta 2008, 1783, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Wang, J.; Song, J.; Shiozawa, Y.; Wang, J.; Havens, A.; Wang, Z.; Sun, Y.X.; Emerson, S.G.; Krebsbach, P.H.; et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood 2007, 110, 82–90. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabel, M.; Royer, C.; Thahouly, T.; Calco, V.; Gasman, S.; Bader, M.-F.; Vitale, N.; Chasserot-Golaz, S. Annexin A2 Egress during Calcium-Regulated Exocytosis in Neuroendocrine Cells. Cells 2020, 9, 2059. https://doi.org/10.3390/cells9092059
Gabel M, Royer C, Thahouly T, Calco V, Gasman S, Bader M-F, Vitale N, Chasserot-Golaz S. Annexin A2 Egress during Calcium-Regulated Exocytosis in Neuroendocrine Cells. Cells. 2020; 9(9):2059. https://doi.org/10.3390/cells9092059
Chicago/Turabian StyleGabel, Marion, Cathy Royer, Tamou Thahouly, Valérie Calco, Stéphane Gasman, Marie-France Bader, Nicolas Vitale, and Sylvette Chasserot-Golaz. 2020. "Annexin A2 Egress during Calcium-Regulated Exocytosis in Neuroendocrine Cells" Cells 9, no. 9: 2059. https://doi.org/10.3390/cells9092059