Novel Insights into Beta 2 Adrenergic Receptor Function in the rd10 Model of Retinitis Pigmentosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Treatments
2.3. Quantitative Real Time PCR
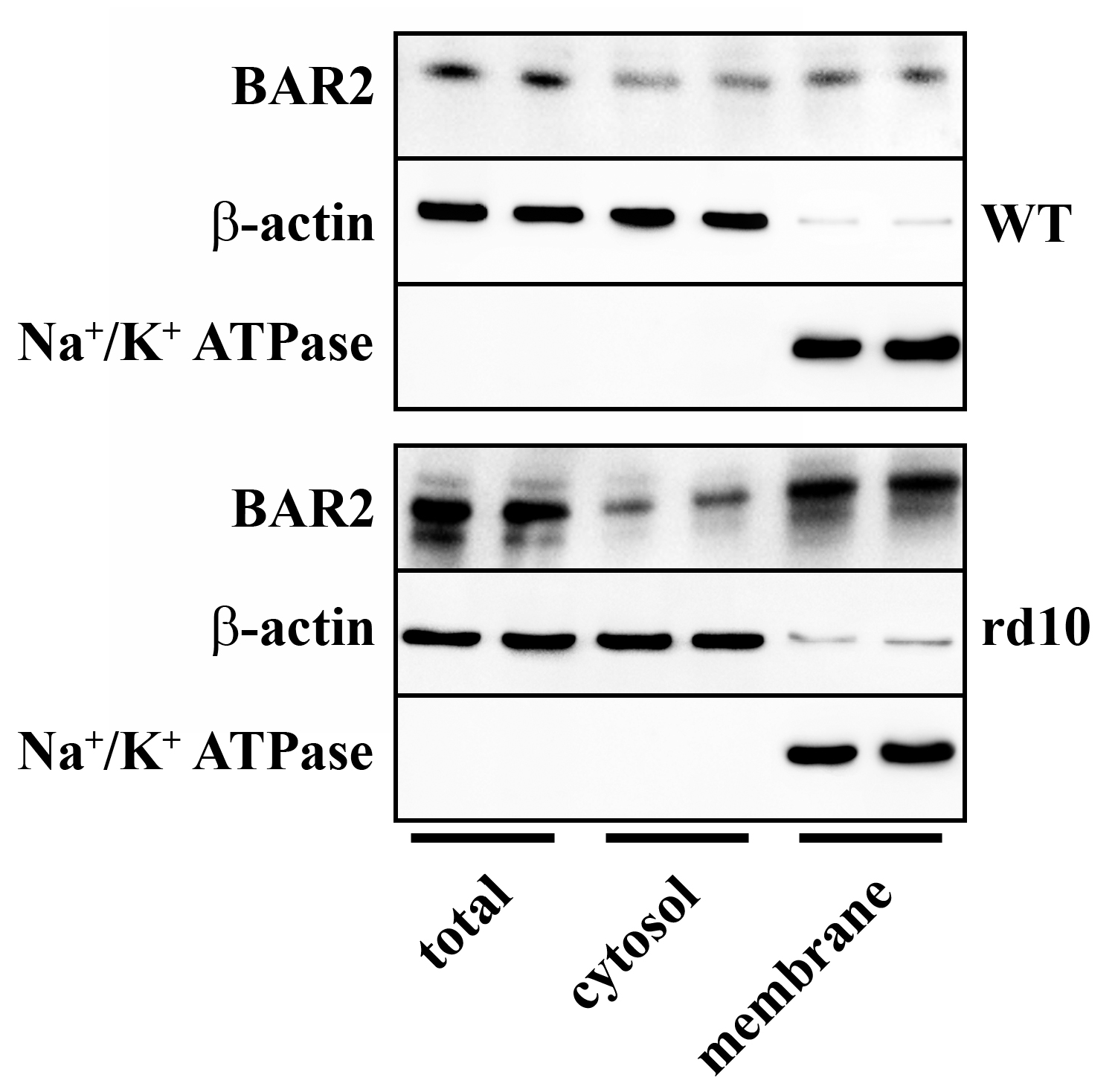
2.4. Western Blotting
2.5. Measurement of NE Levels
2.6. Immunohistochemistry and Quantitative Analysis
2.7. Measurement of Scotopic and Photopic Electroretinogram
2.8. Data Analysis
3. Results
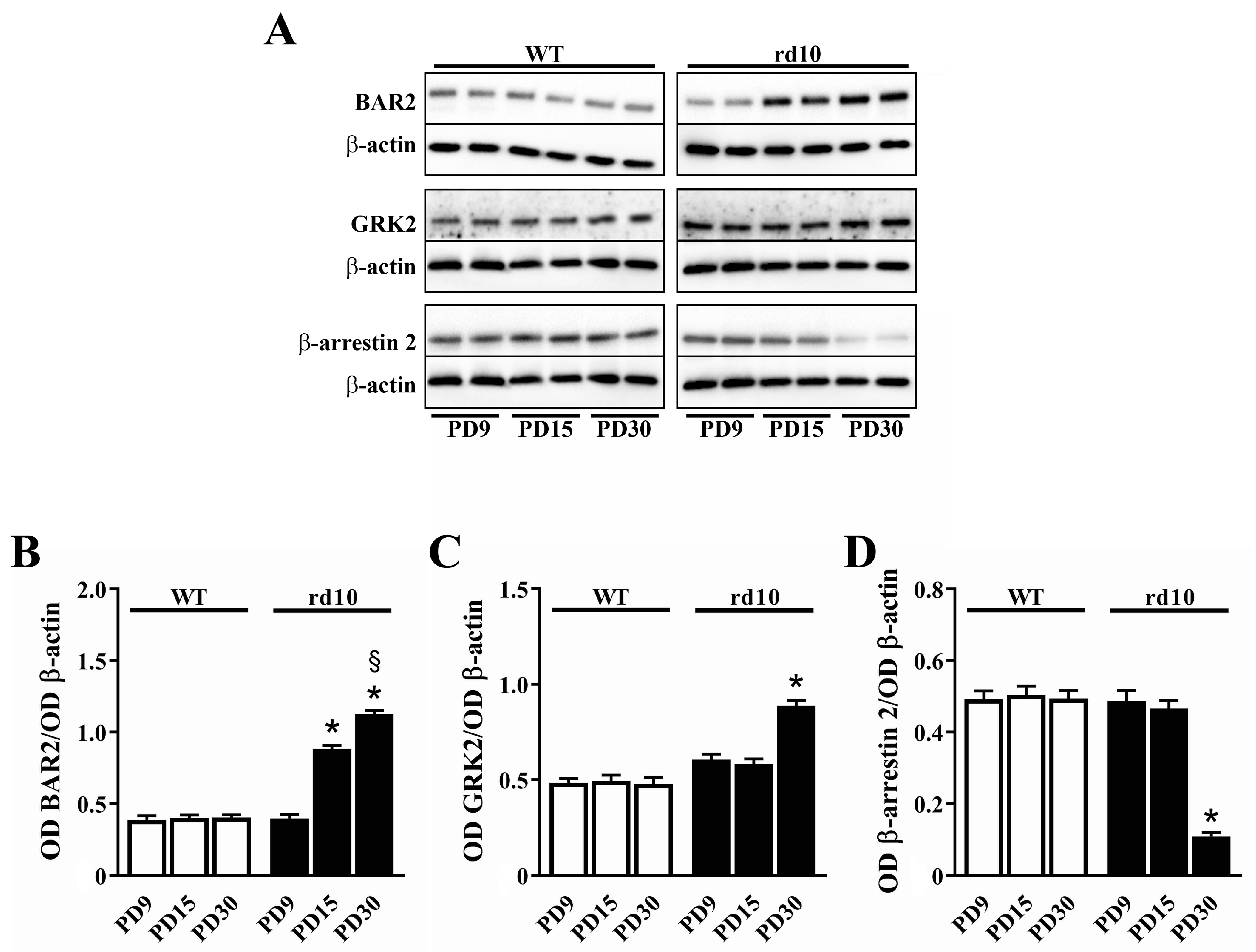
3.1. Sympathetic Overdrive in the rd10 Retina: Desensitization Defects
3.2. HIF-1 Regulation of BAR2 Expression
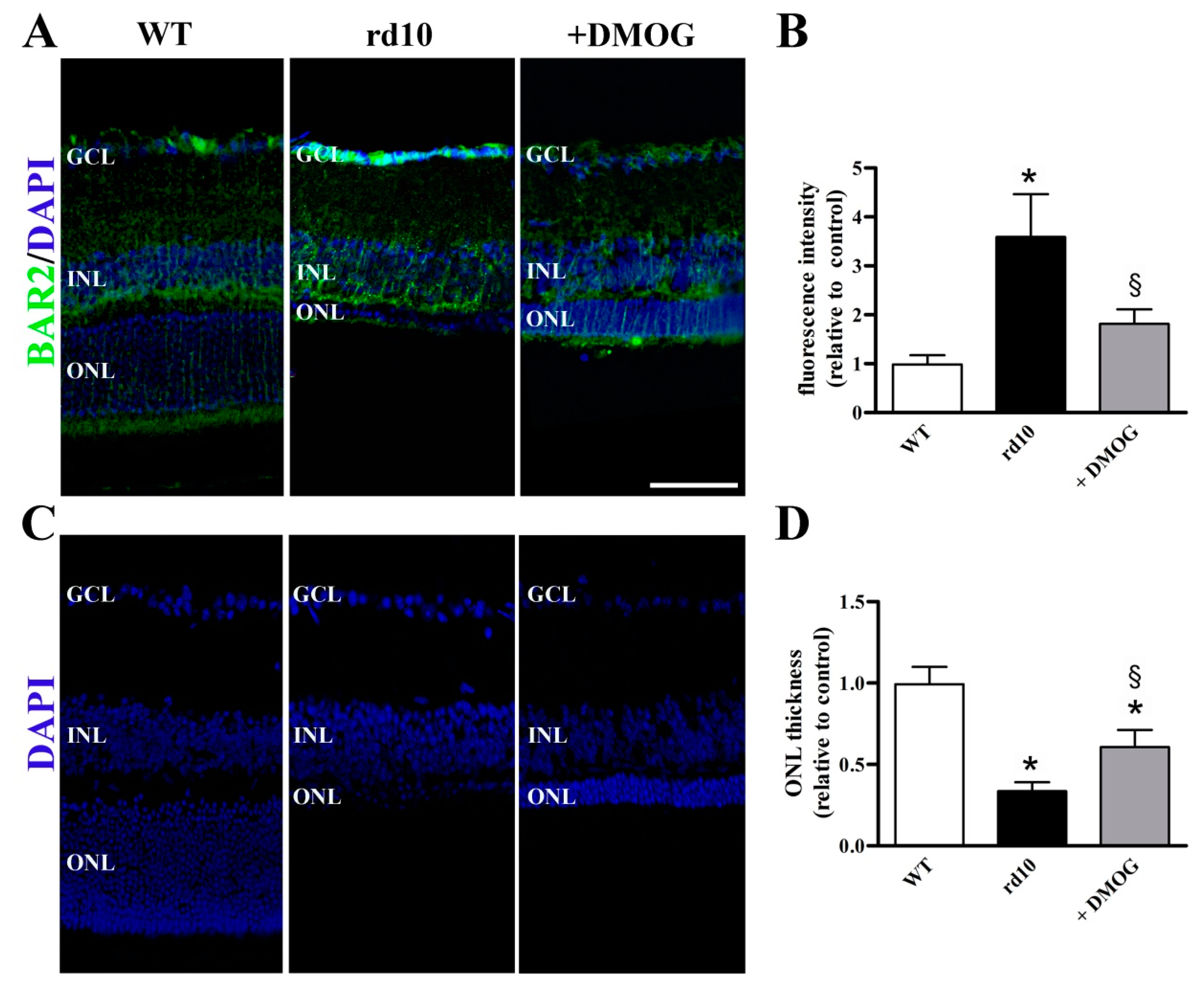
3.3. HIF-1α Stabilization Partially Prevents Reactive Gliosis, Microglial Activation and Cone Loss
3.4. Effects of HIF-1α Stabilization on ERG
4. Discussion
4.1. Overactivation of the Sympathetic System
4.2. HIF-1α Stabilization Reduces BAR2 Upregulation and Restores the Desensitization Cascade
4.3. HIF-1α Stabilization Restores the Neuroinflammatory Cascade and Prevents Cone Loss
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.; Valter, K.; Walsh, N.; Lee, D.; Stone, J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2013–2019. [Google Scholar] [CrossRef]
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp. Eye Res. 2006, 83, 473–483. [Google Scholar] [CrossRef]
- Cammalleri, M.; Dal Monte, M.; Locri, F.; Pecci, V.; De Rosa, M.; Pavone, V.; Bagnoli, P. The urokinase-type plasminogen activator system as drug target in retinitis pigmentosa: New pre-clinical evidence in the rd10 mouse model. J. Cell. Mol. Med. 2019, 23, 5176–5192. [Google Scholar] [CrossRef]
- Olivares-González, L.; Martínez-Fernández de la Cámara, C.; Hervás, D.; Millán, J.M.; Rodrigo, R. HIF-1α stabilization reduces retinal degeneration in a mouse model of retinitis pigmentosa. FASEB J. 2018, 32, 2438–2451. [Google Scholar] [CrossRef] [Green Version]
- Strowitzki, M.J.; Cummins, E.P.; Taylor, C.T. Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous? Cells 2019, 26, 384. [Google Scholar] [CrossRef] [Green Version]
- Donato, L.; D’Angelo, R.; Alibrandi, S.; Rinaldi, C.; Sidoti, A.; Scimone, C. Effects of A2E-Induced Oxidative Stress on Retinal Epithelial Cells: New Insights on Differential Gene Response and Retinal Dystrophies. Antioxidants 2020, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Collin, G.B.; Gogna, N.; Chang, B.; Damkham, N.; Pinkney, J.; Hyde, L.F.; Stone, L.; Naggert, J.K.; Nishina, P.M.; Krebs, M.P. Mouse Models of Inherited Retinal Degeneration with Photoreceptor Cell Loss. Cells 2020, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.B.; D’Amore, P.A.; Connor, K.M. Revisiting the mouse model of oxygen-induced retinopathy. Eye Brain. 2016, 8, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Kanan, Y.; Khan, M.; Lorenc, V.E.; Long, D.; Chadha, R.; Sciamanna, J.; Green, K.; Campochiaro, P.A. Metipranolol promotes structure and function of retinal photoreceptors in the rd10 mouse model of human retinitis pigmentosa. J. Neurochem. 2019, 148, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammalleri, M.; Locri, F.; Catalani, E.; Filippi, L.; Cervia, D.; Dal Monte, M.; Bagnoli, P. The Beta Adrenergic Receptor Blocker Propranolol Counteracts Retinal Dysfunction in a Mouse Model of Oxygen Induced Retinopathy: Restoring the Balance between Apoptosis and Autophagy. Front. Cell. Neurosci. 2017, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Casini, G.; La Marca, G.; Isacchi, B.; Filippi, L.; Bagnoli, P. Eye drop propranolol administration promotes the recovery of oxygen-induced retinopathy in mice. Exp. Eye Res. 2013, 111, 27–35. [Google Scholar] [CrossRef]
- Ristori, C.; Filippi, L.; Dal Monte, M.; Martini, D.; Cammalleri, M.; Fortunato, P.; la Marca, G.; Fiorini, P.; Bagnoli, P. Role of the adrenergic system in a mouse model of oxygen-induced retinopathy: Antiangiogenic effects of beta-adrenoreceptor blockade. Invest. Ophthalmol. Vis. Sci. 2011, 52, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Baker, G. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br. J. Pharmacol. 2005, 144, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilcíková, L.; Bauer, V.; Kolena, J. The action of adrenoceptor agonists and antagonists on the guinea pig and dog trachea. Gen. Physiol. Biophys. 1987, 6, 87–101. [Google Scholar] [PubMed]
- Casini, G.; Dal Monte, M.; Fornaciari, I.; Filippi, L.; Bagnoli, P. The β-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog. Retin. Eye Res. 2014, 42, 103–129. [Google Scholar] [CrossRef]
- Martini, D.; Dal Monte, M.; Ristori, C.; Cupisti, E.; Mei, S.; Fiorini, P.; Filippi, L.; Bagnoli, P. Antiangiogenic effects of β2 -adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J. Neurochem. 2011, 119, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.J.; Steinle, J.J. Role of β-Adrenergic Receptors in Inflammatory Marker Expression in Müller Cells. Invest. Ophthalmol. Vis. Sci. 2007, 48, 5276–5281. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.L.; Theilade, J.; Aplin, M.; Sheikh, S.P. Role of G-protein-coupled receptor kinase 2 in the heart—Do regulatory mechanisms open novel therapeutic perspectives? Trends Cardiovasc. Med. 2006, 16, 169–177. [Google Scholar] [CrossRef]
- Pfleger, J.; Gresham, K.; Koch, W.J. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat. Rev. Cardiol. 2019, 16, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Carion, T.W.; Jiang, Y.; Chahine, A.; Steinle, J.J.; Berger, E.A. A regulatory role for β-adrenergic receptors regarding the resolvin D1 (RvD1) pathway in the diabetic retina. PLoS ONE 2017, 12, e0185383. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vision Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, B.; Sampath, P.; Seshadri, V.; Maitra, R.K.; DiCorleto, P.E.; Fox, P.L. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell 2003, 115, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.D.; Schwarz, M.A. Adapted approach to profile genes while reconciling Vegf-a mRNA expression in the developing and injured lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1202–L1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, B.; Hawes, N.L.; Pardue, M.T.; German, A.M.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Rengarajan, K.; Boyd, A.P.; Sidney, S.S.; et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007, 47, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Gargini, C.; Terzibasi, E.; Mazzoni, F.; Strettoi, E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 2007, 500, 222–238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, Z.M. Oxygen-induced retinopathy in mice with retinal photoreceptor cell degeneration. Life Sci. 2014, 102, 28–35. [Google Scholar] [CrossRef]
- Lahdenranta, J.; Pasqualini, R.; Schlingemann, R.O.; Hagedorn, M.; Stallcup, W.B.; Bucana, C.D.; Sidman, R.L.; Arap, W. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc. Natl. Acad. Sci. USA 2001, 98, 10368–10373. [Google Scholar] [CrossRef] [Green Version]
- Roche, S.L.; Ruiz-Lopez, A.M.; Moloney, J.N.; Byrne, A.M.; Cotter, T.G. Microglial-induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa. Glia 2018, 66, 295–310. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Müller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Chan, W.Y.; Kuang, G.B.; Wei, H.; Shum, A.S.; Yew, D.T. Differential expression of glial fibrillary acidic protein (GFAP) in the retinae and visual cortices of rats with experimental renal hypertension. Neurosci. Lett. 1995, 198, 165–168. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Makita, N.; Kabasawa, Y.; Otani, Y.; Junichiro Sato, F.; Hashimoto, M.; Nakaya, M.; Nishihara, H.; Nangaku, M.; Kurose, H.; Ohwada, T.; et al. Attenuated desensitization of β-adrenergic receptor by water-soluble N-nitrosamines that induce S-nitrosylation without NO release. Circ. Res. 2013, 112, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef] [Green Version]
- Lechtenberg, K.J.; Meyer, S.T.; Doyle, J.B.; Peterson, T.C.; Buckwalter, M.S. Augmented β2-adrenergic signaling dampens the neuroinflammatory response following ischemic stroke and increases stroke size. J. Neuroinflammation 2019, 16, 112. [Google Scholar] [CrossRef] [Green Version]
- Konieczka, K.; Flammer, A.J.; Todorova, M.; Meyer, P.; Flammer, J. Retinitis pigmentosa and ocular blood flow. EPMA J. 2012, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Mildenberger, E.; Siegel, G.; Versmold, H.T. Locally released norepinephrine in the oxygen-dependent regulation of vascular tone of human umbilical vein. Pediatr. Res. 2004, 55, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Osborne, N.N.; Wood, J.P.M. Metipranolol blunts nitric oxide-induced lipid peroxidation and death of retinal photoreceptors: A comparison with other anti-glaucoma drugs. Invest. Ophthalmol. Vis. Sci. 2004, 45, 3787–3795. [Google Scholar] [CrossRef]
- Melena, J.; Osborne, N.N. Metipranolol attenuates lipid peroxidation in rat brain: A comparative study with other antiglaucoma drugs. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Wu, B.; Tan, C.; He, X.; Xu, B.; Yu, G.; Wang, H. β-Arrestin2 Inhibits Expression of Inflammatory Cytokines in BEAS-2B Lung Epithelial Cells Treated with Cigarette Smoke Condensate via Inhibition of Autophagy. Cell. Physiol. Biochem. 2018, 50, 1270–1285. [Google Scholar] [CrossRef]
- Azzi, M.; Charest, P.G.; Angers, S.; Rousseau, G.; Kohout, T.; Bouvier, M.; Piñeyro, G. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 11406–11411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Xue, M.; Zhang, S.; Cheng, L.; Qian, W.; Duan, W.; Shen, X. Resveratrol inhibits the growth of tumor cells under chronic stress via the ADRB-2-HIF-1α axis. Oncol. Rep. 2019, 41, 1051–1058. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.; Huo, Z.; Liu, Y.; Lin, X.; Li, J.; Peng, L.; Zhao, H.; Zhou, Z.N.; Liang, X.; Liu, Y.; et al. Prolyl hydroxylase 2: A novel regulator of β2 -adrenoceptor internalization. J. Cell. Mol. Med. 2011, 15, 2712–2722. [Google Scholar] [CrossRef] [Green Version]
- Bok, D.; Yasumura, D.; Matthes, M.T.; Ruiz, A.; Duncan, J.L.; Chappelow, A.V.; Zolutukhin, S.; Hauswirth, W.; LaVail, M.M. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp. Eye Res. 2002, 74, 719–735. [Google Scholar] [CrossRef]
- Spencer, B.; Agarwala, S.; Gentry, L.; Brandt, C.R. HSV-1 vector-delivered FGF2 to the retina is neuroprotective but does not preserve functional responses. Mol. Ther. 2001, 3, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Miyoshi, H.; Verma, I.M.; Gage, F.H. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J. Virol. 1999, 73, 7812–7816. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tao, W.; Luo, L.; Huang, D.; Kauper, K.; Stabila, P.; LaVail, M.M.; Laties, A.M.; Wen, R. CNTF Induces Regeneration of Cone Outer Segments in a Rat Model of Retinal Degeneration. PLoS ONE 2010, 5, e9495. [Google Scholar] [CrossRef] [Green Version]
- Donato, L.; Scimone, C.; Alibrandi, S.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Transcriptome Analyses of lncRNAs in A2E-Stressed Retinal Epithelial Cells Unveil Advanced Links between Metabolic Impairments Related to Oxidative Stress and Retinitis Pigmentosa. Antioxidants 2020, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Donato, L.; Scimone, C.; Alibrandi, S.; Nicocia, G.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Discovery of GLO1 New Related Genes and Pathways by RNA-Seq on A2E-Stressed Retinal Epithelial Cells Could Improve Knowledge on Retinitis Pigmentosa. Antioxidants 2020, 9, 416. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammalleri, M.; Dal Monte, M.; Amato, R.; Lapi, D.; Bagnoli, P. Novel Insights into Beta 2 Adrenergic Receptor Function in the rd10 Model of Retinitis Pigmentosa. Cells 2020, 9, 2060. https://doi.org/10.3390/cells9092060
Cammalleri M, Dal Monte M, Amato R, Lapi D, Bagnoli P. Novel Insights into Beta 2 Adrenergic Receptor Function in the rd10 Model of Retinitis Pigmentosa. Cells. 2020; 9(9):2060. https://doi.org/10.3390/cells9092060
Chicago/Turabian StyleCammalleri, Maurizio, Massimo Dal Monte, Rosario Amato, Dominga Lapi, and Paola Bagnoli. 2020. "Novel Insights into Beta 2 Adrenergic Receptor Function in the rd10 Model of Retinitis Pigmentosa" Cells 9, no. 9: 2060. https://doi.org/10.3390/cells9092060





