Prediction of Prognosis and Chemotherapeutic Sensitivity Based on Cuproptosis-Associated lncRNAs in Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Public Data Acquisition and Processing
2.2. Development of CRLs Risk Score
2.3. Immune Profile Analysis and Enrichment Analysis
2.4. Drug Therapy Response and Tumor Mutation Burden
2.5. Statistical Analyses
3. Results
3.1. Landscape of Genetic and Transcriptional Variations in CRGs in CESC
3.2. Screening of Biomarkers Associated with Cuproptosis in CESC
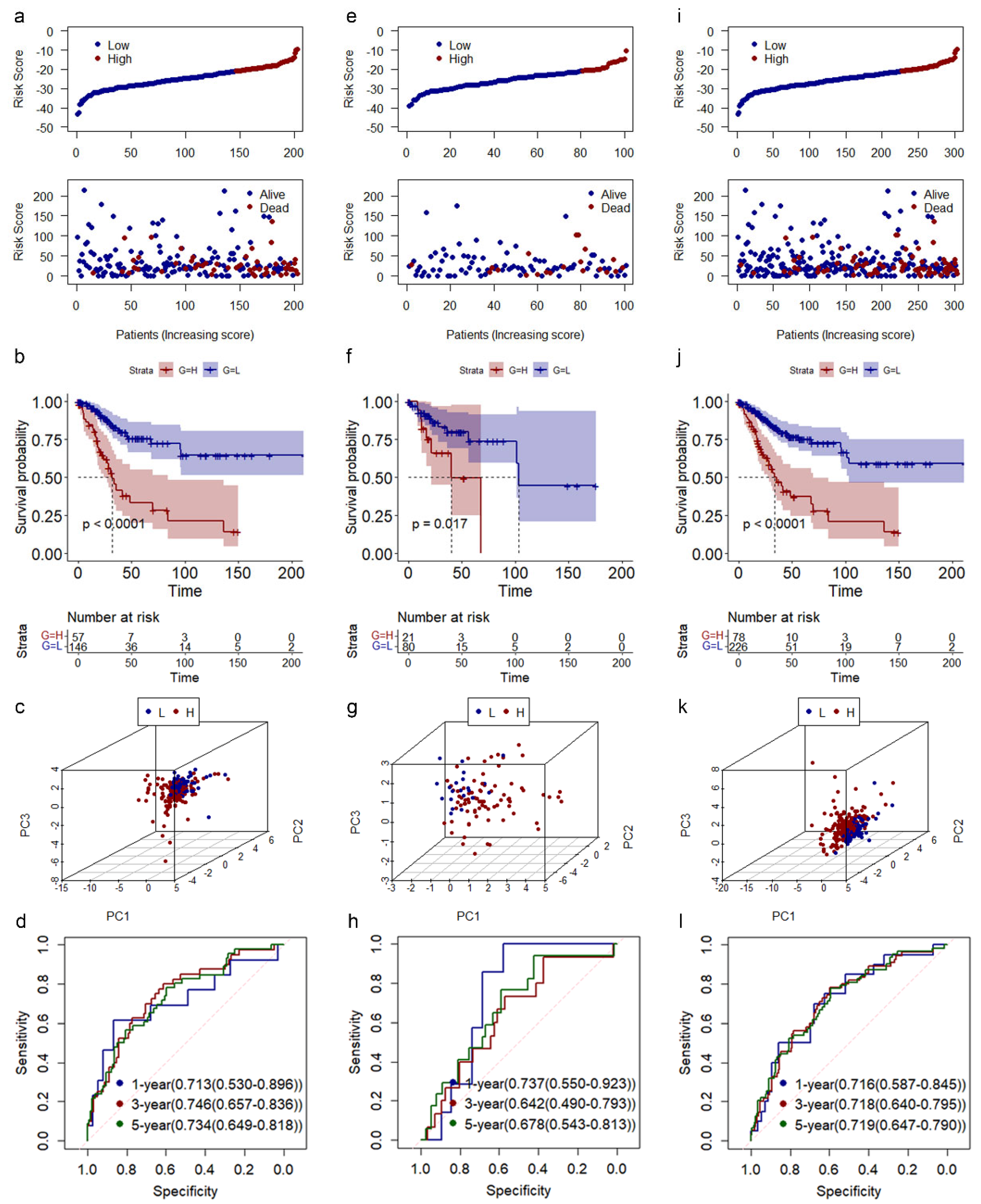
3.3. Construction and Validation of Risk Model in CESC
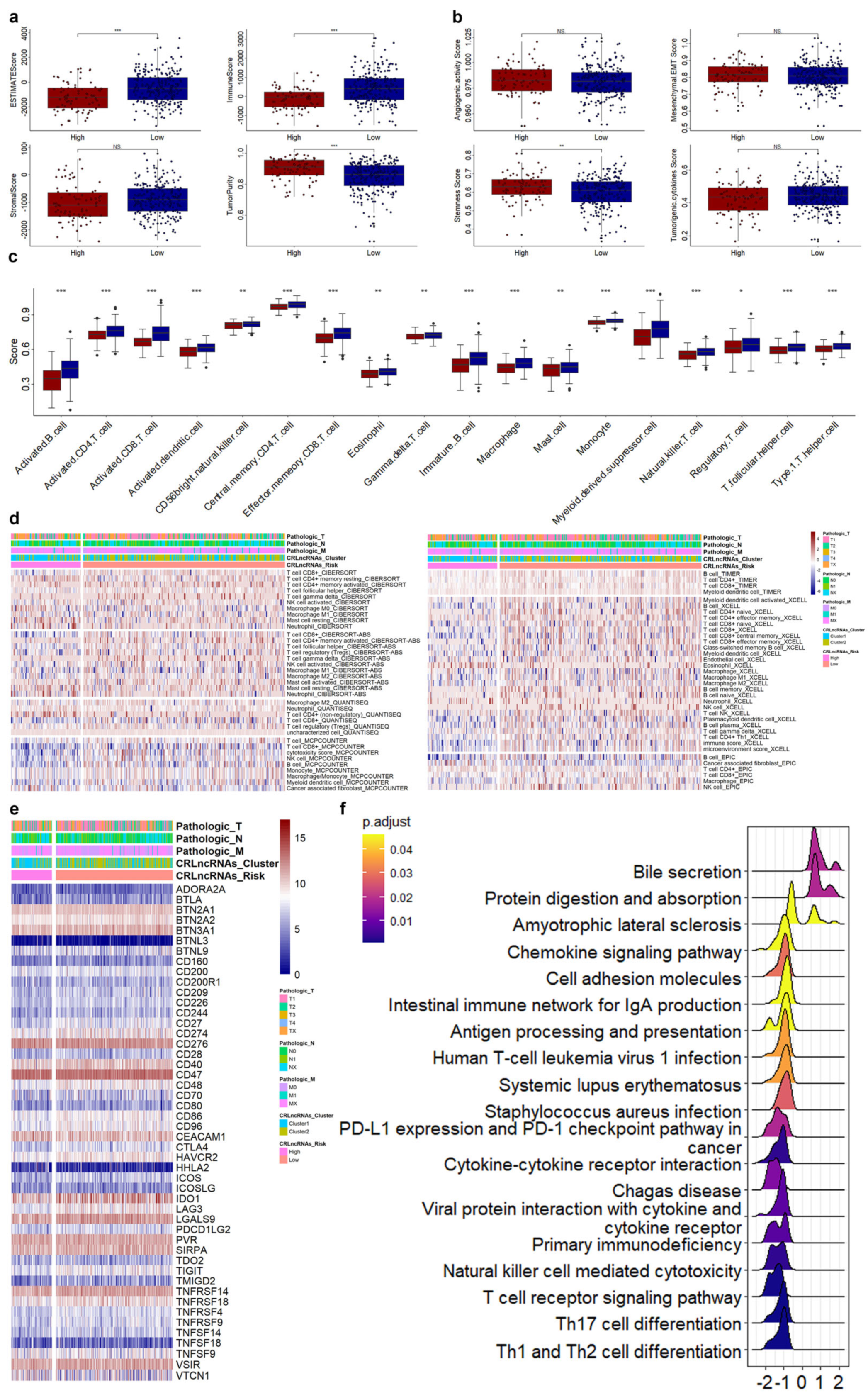
3.4. Correlations of CRLs Risk Score with Immunotherapy, Chemoradiotherapy Sensitivity-Related Genes, and Immune Features in CESC
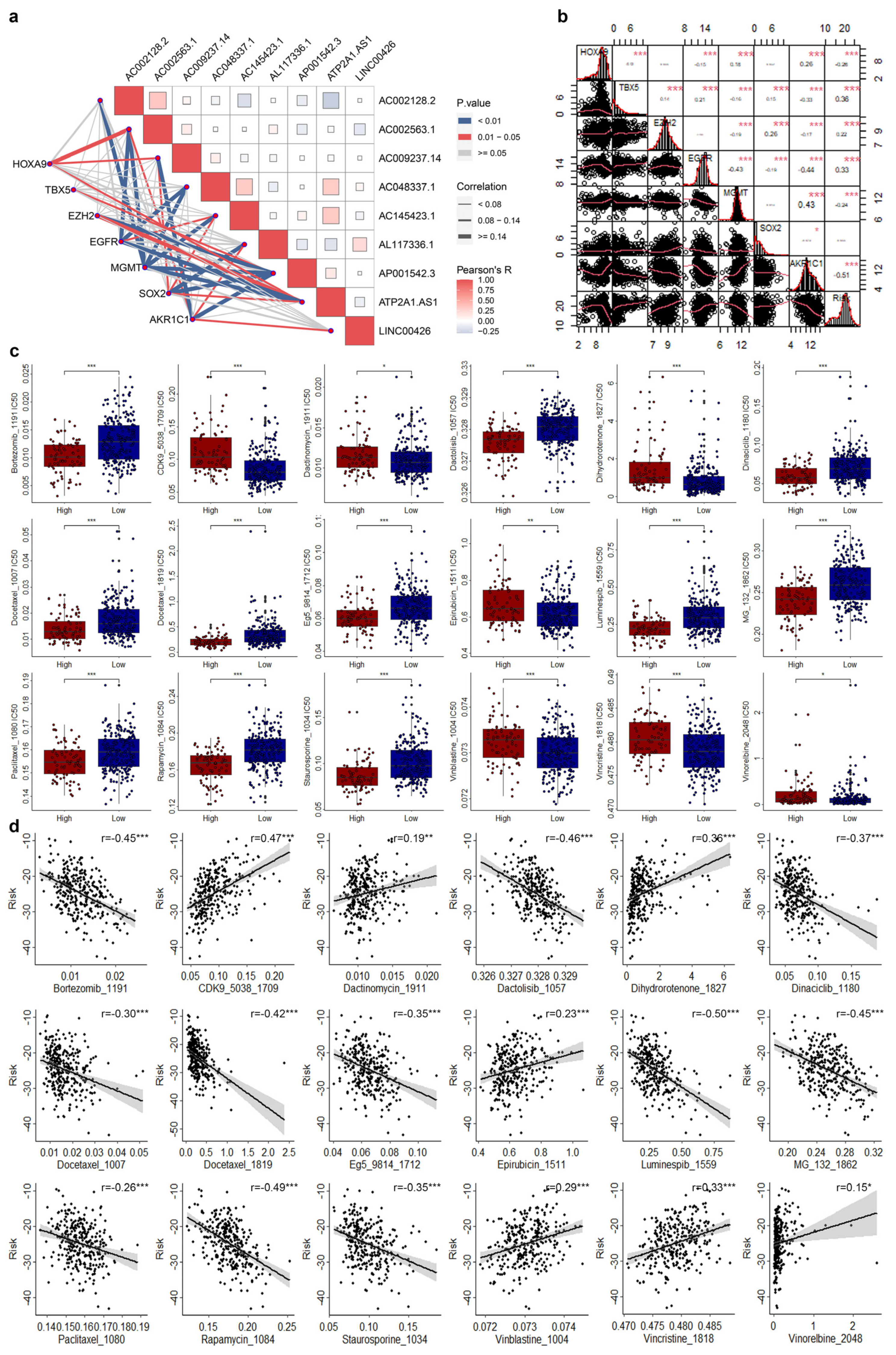
3.5. Correlations of CRLs Risk Score with CRSGs and Chemotherapeutic Sensitivity in CESC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Zhang, J.; Tan, J.; Li, J.; Song, Z. Development and Validation of a Combined Ferroptosis and Immune Prognostic Classifier for Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2020, 8, 596679. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Warburton, A.; Markowitz, T.E.; Katz, J.P.; Pipas, J.M.; McBride, A.A. Recurrent integration of human papillomavirus genomes at transcriptional regulatory hubs. NPJ Genom. Med. 2021, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Li, H.; Li, H.; Dai, J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncol. Lett. 2015, 10, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Orbegoso, C.; Murali, K.; Banerjee, S. The current status of immunotherapy for cervical cancer. Rep. Pract. Oncol. Radiother. 2018, 23, 580–588. [Google Scholar] [CrossRef]
- Potikanond, S.; Sookkhee, S.; Na Takuathung, M.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia parviflora Extract Exhibits Anti-cancer Activity against HeLa Cervical Cancer Cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef]
- van Meir, H.; Kenter, G.G.; Burggraaf, J.; Kroep, J.R.; Welters, M.J.; Melief, C.J.; van der Burg, S.H.; van Poelgeest, M.I. The need for improvement of the treatment of advanced and metastatic cervical cancer, the rationale for combined chemo-immunotherapy. Anticancer Agents Med. Chem. 2014, 14, 190–203. [Google Scholar] [CrossRef]
- Deng, Y.; Song, Z.; Huang, L.; Guo, Z.; Tong, B.; Sun, M.; Zhao, J.; Zhang, H.; Zhang, Z.; Li, G. Tumor purity as a prognosis and immunotherapy relevant feature in cervical cancer. Aging 2021, 13, 24768–24785. [Google Scholar] [CrossRef]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, J.; Zhang, X. A novel Cuproptosis-related LncRNA signature to predict prognosis in hepatocellular carcinoma. Sci. Rep. 2022, 12, 11325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zeng, X.; Wu, Y.; Liu, Y.; Zhang, X.; Song, Z. Cuproptosis-Related Risk Score Predicts Prognosis and Characterizes the Tumor Microenvironment in Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 925618. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, X.; Zeng, X.; Liu, Y.; Zhang, C.; Zhang, Q.; Xu, J. Comprehensive Analysis of Cuproptosis-Related Genes in Immune Infiltration and Prognosis in Melanoma. Front. Pharmacol. 2022, 13, 930041. [Google Scholar] [CrossRef]
- Yun, Y.; Wang, Y.; Yang, E.; Jing, X. Cuproptosis-Related Gene—SLC31A1, FDX1 and ATP7B—Polymorphisms are Associated with Risk of Lung Cancer. Pharmgenomics Pers. Med. 2022, 15, 733–742. [Google Scholar] [CrossRef]
- Shang, C.; Huang, J.; Guo, H. Identification of an Metabolic Related Risk Signature Predicts Prognosis in Cervical Cancer and Correlates With Immune Infiltration. Front. Cell Dev. Biol. 2021, 9, 677831. [Google Scholar] [CrossRef]
- Li, D.; Wu, X.; Fan, X.; Cheng, C.; Li, D.; Zhang, W. Comprehensive analysis of cuproptosis-related lncRNAs in the prognosis and therapy response of patients with bladder cancer. Ann. Transl. Med. 2022, 10, 1232. [Google Scholar] [CrossRef]
- Fan, C.N.; Ma, L.; Liu, N. Systematic analysis of lncRNA-miRNA-mRNA competing endogenous RNA network identifies four-lncRNA signature as a prognostic biomarker for breast cancer. J. Transl. Med. 2018, 16, 264. [Google Scholar] [CrossRef]
- Xing, X.L.; Liu, Y.; Liu, J.; Zhou, H.; Zhang, H.; Zuo, Q.; Bu, P.; Duan, T.; Zhou, Y.; Xiao, Z. Comprehensive Analysis of Ferroptosis- and Immune-Related Signatures to Improve the Prognosis and Diagnosis of Kidney Renal Clear Cell Carcinoma. Front. Immunol. 2022, 13, 851312. [Google Scholar] [CrossRef] [PubMed]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhou, F. Cuproptosis: A new form of programmed cell death. Cell Mol. Immunol. 2022, 19, 867–868. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Luo, J.; Chen, X.; Ma, K.; He, H.; Li, W.; Cui, J. Serum Copper Level and the Copper-to-Zinc Ratio Could Be Useful in the Prediction of Lung Cancer and Its Prognosis: A Case-Control Study in Northeast China. Nutr. Cancer 2021, 73, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.A.K.; Adly, H.M.; Abdelkhaliq, A.A.; Nassir, A.M. Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Curr. Urol. 2020, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, V.; Sathisha, T.G.; Kasturi, K.; Mallika, D.S.; Amos, S.J.; Ragunatha, S. Serum levels of metal ions in female patients with breast cancer. J. Clin. Diagn. Res. 2015, 9, BC25–BC27. [Google Scholar] [CrossRef]
- Basu, S.; Singh, M.K.; Singh, T.B.; Bhartiya, S.K.; Singh, S.P.; Shukla, V.K. Heavy and trace metals in carcinoma of the gallbladder. World J. Surg. 2013, 37, 2641–2646. [Google Scholar] [CrossRef]
- Yaman, M.; Kaya, G.; Yekeler, H. Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J. Gastroenterol. 2007, 13, 612–618. [Google Scholar] [CrossRef]
- Kosova, F.; Cetin, B.; Akinci, M.; Aslan, S.; Seki, A.; Pirhan, Y.; Ari, Z. Serum copper levels in benign and malignant thyroid diseases. Bratisl. Lek. Listy 2012, 113, 718–720. [Google Scholar] [CrossRef]
- de Groot, P.M.; Carter, B.W.; Betancourt Cuellar, S.L.; Erasmus, J.J. Staging of lung cancer. Clin. Chest Med. 2015, 36, 179–196, vii–viii. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Ostrakhovitch, E.A.; Lordnejad, M.R.; Schliess, F.; Sies, H.; Klotz, L.O. Copper ions strongly activate the phosphoinositide-3-kinase/Akt pathway independent of the generation of reactive oxygen species. Arch. Biochem. Biophys. 2002, 397, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.L.; Kampkotter, A.; Eckers, A.; Barthel, A.; Schmoll, D.; Sies, H.; Klotz, L.O. Modulation of FoxO signaling in human hepatoma cells by exposure to copper or zinc ions. Arch. Biochem. Biophys. 2006, 454, 107–113. [Google Scholar] [CrossRef]
- Turski, M.L.; Brady, D.C.; Kim, H.J.; Kim, B.E.; Nose, Y.; Counter, C.M.; Winge, D.R.; Thiele, D.J. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell Biol. 2012, 32, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Fan, R.; Xie, L. A Novel Cuproptosis-Related Prognostic Gene Signature and Validation of Differential Expression in Clear Cell Renal Cell Carcinoma. Genes 2022, 13, 851. [Google Scholar] [CrossRef]
- Li, J.; Wu, F.; Li, C.; Sun, S.; Feng, C.; Wu, H.; Chen, X.; Wang, W.; Zhang, Y.; Liu, M.; et al. The cuproptosis-related signature predicts prognosis and indicates immune microenvironment in breast cancer. Front. Genet. 2022, 13, 977322. [Google Scholar] [CrossRef]
- Chen, Y. Identification and Validation of Cuproptosis-Related Prognostic Signature and Associated Regulatory Axis in Uterine Corpus Endometrial Carcinoma. Front. Genet. 2022, 13, 912037. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, J.; Liu, X. Development of six immune-related lncRNA signature prognostic model for smoking-positive lung adenocarcinoma. J. Clin. Lab. Anal. 2022, 36, e24467. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, H.X.; Ma, B.; Meng, Q.K. Identification of a Genomic Instability-Related Long Noncoding RNA Prognostic Model in Colorectal Cancer Based on Bioinformatic Analysis. Dis. Markers 2022, 2022, 4556585. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Mu, J.; Xin, D.; Wang, L.; Fan, Y.; Zhang, S.; Xu, Y. Glycosyltransferase-related long non-coding RNA signature predicts the prognosis of colon adenocarcinoma. Front. Oncol. 2022, 12, 954226. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Zhang, J.; Li, L.; Wang, X.; Zhu, Y.; Guo, L.; Wang, J. Six mutator-derived lncRNA signature of genome instability for predicting the clinical outcome of colon cancer. J. Gastrointest. Oncol. 2021, 12, 2157–2171. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Jiang, S.; Wang, Y.; Sun, G.; Cao, J.; Hu, D.; Wang, G.; Liang, X.; Ding, J.; Du, J. Identification and validation of a cellular senescence-related lncRNA signature for prognostic prediction in patients with multiple myeloma. Cell Cycle 2023, 22, 1434–1449. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Wang, J.; Cui, N.; Liu, X.; Wang, H. Autophagy-related long non-coding RNA signature for potential prognostic biomarkers of patients with cervical cancer: A study based on public databases. Ann. Transl. Med. 2021, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Montano, M.A.; Cisneros-Villanueva, M.; Coales, I.; Hidalgo-Miranda, A. LINC00426 is a potential immune phenotype-related biomarker and an overall survival predictor in PAM50 luminal B breast cancer. Front. Genet. 2023, 14, 1034569. [Google Scholar] [CrossRef]
- Du, W.; Sun, J.; Gu, J.; Zhang, S.; Zhang, T. Bioinformatics analysis of LINC00426 expression in lung cancer and its correlation with patients’ prognosis. Thorac. Cancer 2020, 11, 150–155. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Y.; Liang, B. Comprehensive analysis of microenvironment-related genes in lung adenocarcinoma. Future Oncol. 2020, 16, 1825–1837. [Google Scholar] [PubMed]
- Xiang, Z.; Shen, E.; Li, M.; Hu, D.; Zhang, Z.; Yu, S. Potential prognostic biomarkers related to immunity in clear cell renal cell carcinoma using bioinformatic strategy. Bioengineered 2021, 12, 1773–1790. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, H.L.; Xing, T.J.; Wang, X.Q. A Novel Prognostic Risk Model for Cervical Cancer Based on Immune Checkpoint HLA-G-Driven Differentially Expressed Genes. Front. Immunol. 2022, 13, 851622. [Google Scholar] [CrossRef]
- Nie, C.; Qin, H.; Zhang, L. Identification and validation of a prognostic signature related to hypoxic tumor microenvironment in cervical cancer. PLoS ONE 2022, 17, e0269462. [Google Scholar] [CrossRef]
- Zhi, S.; Jiang, B.; Yu, W.; Li, Y.; Wang, H.; Zhou, T.; Li, N. Identification of candidate miRNA biomarkers correlated with the prognosis of cell carcinoma and endocervical adenocarcinoma via integrated bioinformatics analysis. Am. J. Transl. Res. 2022, 14, 8146–8155. [Google Scholar]
- Li, L.; Guo, Q.; Lan, G.; Liu, F.; Wang, W.; Lv, X. Construction of a four-mRNA prognostic signature with its ceRNA network in CESC. Sci. Rep. 2022, 12, 10691. [Google Scholar] [CrossRef] [PubMed]
- Bing, F.; Zhao, Y. Screening of biomarkers for prediction of response to and prognosis after chemotherapy for breast cancers. Onco Targets Ther. 2016, 9, 2593–2600. [Google Scholar]
- Weng, S.; Liu, Z.; Ren, X.; Xu, H.; Ge, X.; Ren, Y.; Zhang, Y.; Dang, Q.; Liu, L.; Guo, C.; et al. SCG2: A Prognostic Marker That Pinpoints Chemotherapy and Immunotherapy in Colorectal Cancer. Front. Immunol. 2022, 13, 873871. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Li, T.; Zhao, J.; Guo, M.; Zhao, W.; Gu, W.; Sun, C.; Yue, Y.; Zhong, Z.; Nan, K.; et al. mRNAsi-related metabolic risk score model identifies poor prognosis, immunoevasive contexture, and low chemotherapy response in colorectal cancer patients through machine learning. Front. Immunol. 2022, 13, 950782. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Mao, L.; Shi, R.; Chang, L.; Wang, G.; Cheng, J.; Chen, R. Prognostic Value and Immune Infiltration of HPV-Related Genes in the Immune Microenvironment of Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma. Cancers 2023, 15, 1419. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Y.; Deng, Y.; Zhou, L.; Yang, P.; Zheng, Y.; Zhang, D.; Zhai, Z.; Li, N.; Hao, Q.; et al. Identification of a prognostic immune signature for cervical cancer to predict survival and response to immune checkpoint inhibitors. Oncoimmunology 2019, 8, e1659094. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Qiu, J.; Qu, X.; Peng, J.; Lu, C.; Zhang, M.; Zhang, M.; Qi, X.; Li, G.; et al. Targeting CD96 overcomes PD-1 blockade resistance by enhancing CD8+ TIL function in cervical cancer. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Borcoman, E.; Le Tourneau, C. Pembrolizumab in cervical cancer: Latest evidence and clinical usefulness. Ther. Adv. Med. Oncol. 2017, 9, 431–439. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, T.; Cai, P. Sclareol inhibits cell proliferation and sensitizes cells to the antiproliferative effect of bortezomib via upregulating the tumor suppressor caveolin-1 in cervical cancer cells. Mol. Med. Rep. 2017, 15, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Peng, S.; Knoff, J.; Lee, S.Y.; Yang, B.; Wu, T.C.; Hung, C.F. Combination of proteasome and HDAC inhibitor enhances HPV16 E7-specific CD8+ T cell immune response and antitumor effects in a preclinical cervical cancer model. J. Biomed. Sci. 2015, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Brem, G.J.; Mylonas, I.; Bruning, A. Eeyarestatin causes cervical cancer cell sensitization to bortezomib treatment by augmenting ER stress and CHOP expression. Gynecol. Oncol. 2013, 128, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Elghouche, A.; Shokri, T.; Qin, Y.; Wargo, S.; Citrin, D.; Van Waes, C. Unilateral Cervical Polyneuropathies following Concurrent Bortezomib, Cetuximab, and Radiotherapy for Head and Neck Cancer. Case Rep. Otolaryngol. 2016, 2016, 2313714. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Xie, X.F.; Li, G.M.; Chen, J.R.; Li, M.T.; Xu, X.; Xiong, Q.Y.; Chen, G.R.; Yin, Y.P.; Peng, F.; et al. Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytother. Res. 2021, 35, 4511–4525. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qing, J.; Xiao, Y.; Huang, X.; Chi, Y.; Chen, Z. TIM-1 promotes proliferation and metastasis, and inhibits apoptosis, in cervical cancer through the PI3K/AKT/p53 pathway. BMC Cancer 2022, 22, 370. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Hu, M.; Deng, M. Effect of MiR-10b on Cervical Cancer Rats Through mTOR/P70S6K Signaling Pathway. Cell Mol. Biol. 2022, 68, 63–67. [Google Scholar] [CrossRef]
- Piotrowska, Z.; Costa, D.B.; Oxnard, G.R.; Huberman, M.; Gainor, J.F.; Lennes, I.T.; Muzikansky, A.; Shaw, A.T.; Azzoli, C.G.; Heist, R.S.; et al. Activity of the Hsp90 inhibitor luminespib among non-small-cell lung cancers harboring EGFR exon 20 insertions. Ann. Oncol. 2018, 29, 2092–2097. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Anghel, A.; Rogers, A.M.; Desai, A.J.; Kalous, O.; Conklin, D.; Ayala, R.; O’Brien, N.A.; Quadt, C.; Akimov, M.; et al. Inhibition of HSP90 with AUY922 induces synergy in HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol. Cancer Ther. 2013, 12, 509–519. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Xu, L.; Zhou, H.; Wang, J.; Xing, X. Prediction of Prognosis and Chemotherapeutic Sensitivity Based on Cuproptosis-Associated lncRNAs in Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma. Genes 2023, 14, 1381. https://doi.org/10.3390/genes14071381
Zhou J, Xu L, Zhou H, Wang J, Xing X. Prediction of Prognosis and Chemotherapeutic Sensitivity Based on Cuproptosis-Associated lncRNAs in Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma. Genes. 2023; 14(7):1381. https://doi.org/10.3390/genes14071381
Chicago/Turabian StyleZhou, Jianghong, Lili Xu, Hong Zhou, Jingjin Wang, and Xiaoliang Xing. 2023. "Prediction of Prognosis and Chemotherapeutic Sensitivity Based on Cuproptosis-Associated lncRNAs in Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma" Genes 14, no. 7: 1381. https://doi.org/10.3390/genes14071381
APA StyleZhou, J., Xu, L., Zhou, H., Wang, J., & Xing, X. (2023). Prediction of Prognosis and Chemotherapeutic Sensitivity Based on Cuproptosis-Associated lncRNAs in Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma. Genes, 14(7), 1381. https://doi.org/10.3390/genes14071381





