SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency
Abstract
:1. Introduction
2. Materials and Methods
3. Results
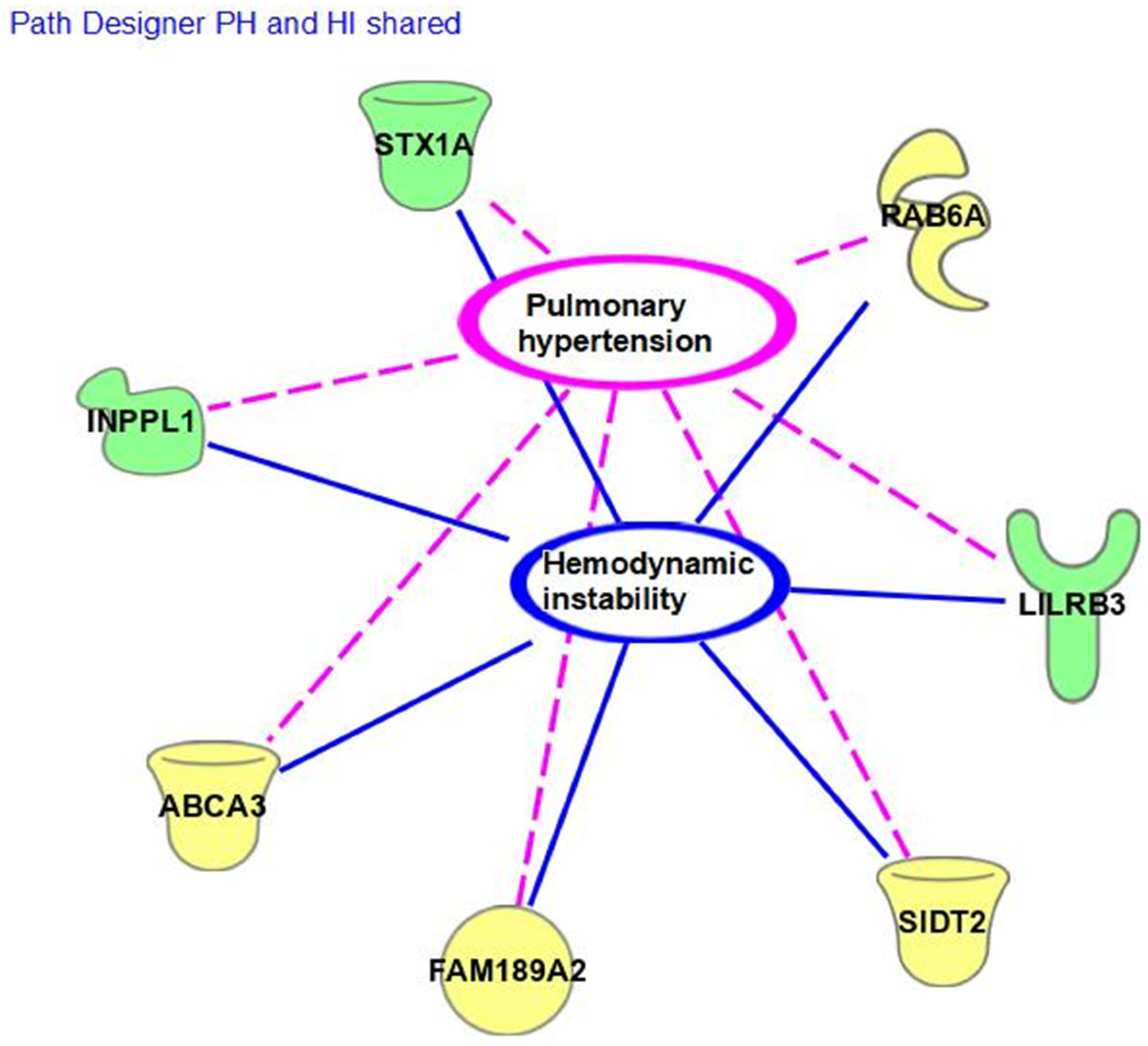
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, K.; McNally, J.D.; Choong, K.; Lawson, M.L.; Ramsay, T.; Wong, H.R. A cohort study of pediatric shock: Frequency of corticosteriod use and association with clinical outcomes. Shock 2015, 44, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gajarski, R.J.; Stefanelli, C.B.; Graziano, J.N.; Kaciroti, N.; Charpie, J.R.; Vazquex, D. Adrenocortical response in infants undergoing cardiac surgery with cardiopulmonary bypass and circulatory arrest. Pediatr. Crit. Care Med. 2010, 11, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rivera, C.L.; Schwartz, S.M.; Sawyer, J.E.; Macrae, D.J.; Agus, M.S. Endocrinologic diseases in pediatric cardiac intensive care. Pediatr. Crit. Care Med. 2016, 17 (Suppl. S1), S296–S301. [Google Scholar] [CrossRef] [PubMed]
- Schulman, D.; Palmert, M.; Kemp, S. Adrenal insufficiency: Still a cause of morbidity and death in childhood. Pediatrics 2007, 119, e484–e494. [Google Scholar] [CrossRef] [PubMed]
- Schiller, O.; Dagan, O.; Birk, E.; Bitan, S.; Amir, G.; Frenkel, G.; Nahum, E. Adrenal insufficiency in children undergoing heart surgery does not correlate with more complex postoperative course. Pediatr. Cardiol. 2013, 34, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Wald, E.L.; Backer, C.L.; Dearani, J.A.; Li, Z.; Oliver, W.C.; Crow, S.S. Total and free cortisol responses and their relation to outcomes after cardiopulmonary bypass in infants. J. Thorac. Cardiov. Surg. 2017, 153, 1155–1163. [Google Scholar] [CrossRef]
- Zanas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-stress-epigenetic regulation of FKBP5: Clinical and translational implications. Neuropsychopharmacology 2016, 41, 261–274. [Google Scholar] [CrossRef]
- Fudulu, D.P.; Gibbison, B.; Upton, T.; Stoica, S.C.; Caputo, M.; Lightman, S.; Angelini, G.D. Corticosteroids in Pediatric Heart Surgery: Myth or Reality. Front. Pediatr. 2018, 20, 112. [Google Scholar] [CrossRef]
- Fudulu, D.P.; Angelini, G.D.; Papadopoulou, F.F.; Evans, J.; Walker-Smith, T.; Kema, I.; van Faassen, M.; Stoica, S.; Caputo, M.; Lightman, S.; et al. The Peacock study: Feasibility of the dynamic characterisation of the paediatric hypothalamic-pituitary-adrenal function during and after cardiac surgery. BMC Cardiovasc. Disord. 2020, 20, 245. [Google Scholar] [CrossRef]
- Scott, S.M.; Watterberg, K.L. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr. Res. 1995, 37, 112–116. [Google Scholar] [CrossRef]
- National Library of Medicine (NLM). Reference SNP (rs) Report rs1798718160. 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1798718160#frequency_tab (accessed on 1 December 2023).
- Virdi, M. Characterizing the Role of Syntaxin 1A in the Heart. Master’s Thesis, York University Graduate Program of Biology, Toronto, ON, Canada, 2019. [Google Scholar]
- Cao, F.; Hata, R.; Zhu, P.; Niinobe, M.; Sakanaka, M. Up-regulation of syntaxin1 in ischemic cortex after permanent focal ischemia in rats. Brain Res. 2009, 1272, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, H.; Zhang, Q.; Huang, C.; Shi, X. Syntaxin 1A mediates isoflurane but not hypoxia preconditioning-induced alleviation of hypoxia-reoxygenation injury in rat cardiomyocytes. Am. J. Transl. Res. 2015, 7, 1883–1895. [Google Scholar] [PubMed]
- Li, W.; Xia, Z.; Lei, S.; Zhan, L.; Zhao, B.; Liu, M. MiR-34a-5p mediates sevoflurane preconditioning-induced inhibition of hypoxia/reoxygenation injury through STX1A in cardiomyocytes. Biomed. Pharmacother. 2018, 102, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.H.; Xiao, Q.R.; Yang, Y.; Xu, J.L.; Zhang, F.; Liu, C.M.; Zhang, Z.M.; Lu, Y.Q.; Huang, N.P. MicroRNA-34a modulates the notch signaling pathway in mice with congenital heart disease and its role in heart development. J. Mol. Cell. Cardiol 2017, 114, 300–308. [Google Scholar] [CrossRef]
- Pierpont, M.E.; Brueckner, M.; Chung, W.K.; Garg, V.; Lacro, R.V.; McGuire, A.L.; Mital, S.; Priest, J.R.; Pu, W.T.; Roberts, A.; et al. Genetic basis for congenital heart disease. Circulation 2018, 138, e653–e711. [Google Scholar] [CrossRef]
- Pober, B.R. Williams-Beuren syndrome. NEJM 2010, 362, 239–252. [Google Scholar] [CrossRef]
- INPPL1. GeneCards the Human Gene Database. INPPL1 Gene-Inositol Polyphosphate Phosphatase Like 1. 2022. Available online: http://www.genecards.org/cgi-bin/carddisp.pl?gene=INPPL1&keywords=INPPL1 (accessed on 26 January 2023).
- FAM189A/ENTREP1. GeneCards the Human Gene Database. ENTREP1 Gene-Endosomal Transmembrane Epsin Interactor 1. 2022. Available online: http://www.genecards.org/cgi-bin/carddisp.pl?gene=ENTREP1 (accessed on 10 January 2023).
- Charmet, R.; Duffy, S.; Keshavarzi, S.; Gyorgy, B.; Marre, M.; Rossing, P.; McKnight, A.J.; Maxwell, A.P.; Ahluwalia, T.V.S.; Paterson, A.D.; et al. Novel risk genes identified in a genome-wide association study for coronary artery disease in patients with type 1 diabetes. Cardiovasc. Diabetol. 2018, 17, 61. [Google Scholar] [CrossRef]
- National Library of Medicine (NLM). Reference SNP (rs) Report rs78542404. 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs78542404#frequency_tab (accessed on 1 December 2023).
- Yang, L.; Liu, G.; Li, X.; Xia, Z.; Wang, Y.; Lin, W.; Zhang, W.; Zhang, W.; Li, X. Small GTPase RAB6 deficiency promotes alveolar progenitor cell renewal and attenuates PM2.5-induced lung injury and fibrosis. Cell Death Dis. 2020, 11, 827. [Google Scholar] [CrossRef]
- León-Mimila, P.; Villamil-Ramírez, H.; Macías-Kauffer, L.R.; Jacobo-Albavera, L.; López-Contreras, B.E.; Posadas-Sánchez, R.; Posadas-Romero, C.; Romero-Hidalgo, S.; Morán-Ramos, S.; Domínguez-Pérez, M.; et al. Genome-wide association study identifies a functional SIDT2 variant associated with HDL-C (High-density lipoprotein cholesterol) levels and premature coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2494–2508. [Google Scholar] [CrossRef]
- Onnée, M.; Fanen, P.; Callebaut, I.; de Becdelièvre, A. Structure-based understanding of ABCA3 Variants. Int. J. Mol. Sci. 2021, 22, 10282. [Google Scholar] [CrossRef]
- Truong, A.D.; Hong, Y.; Lee, J.; Lee, K.; Tran, H.T.T.; Dang, H.V.; Nguyen, V.K.; Lillehoj, H.S.; Hong, Y.H. Chicken novel leukocyte immunoglobulin-like receptor subfamilies B1 and B3 are transcriptional regulators of major histocompatibility complex class I genes and signaling pathways. Asian-Aust. J. Anim. Sci. 2019, 32, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, S.; Kamoshita, N.; Nakayama, J.; Teramoto, R.; Pishesha, N.; Ohba, K.; Sato, N.; Kozawa, K.; Abe, H.; Semba, K.; et al. Epithelial cells remove precancerous cells by cell competition via MHC class I–LILRB3 interaction. Nat. Immunol. 2021, 22, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
| n (%) or Median (IQR) | Cohort (n = 16) | CIRCI (n = 8) | Non-CIRCI (n = 8) | p-Value |
|---|---|---|---|---|
| Male gender (%) | 13 (81.25) | 7 (87.5) | 6 (75) | 0.500 |
| Race | ||||
| White (%) Black (%) Multi-racial (%) | 14 (87.5) 1 (6.25) 1 (6.25) | 7 (87.5) 1 (12.5) 0 | 1 (12.5) 0 1 (12. 5) | 0.767 |
| Hispanic ethnicity (%) | 3 (18.75) | 2 (25) | 1 (12.5) | 0.500 |
| Gestational age (weeks) | 39 (1) | 39 (0.5) | 39 (1) | 0.505 |
| Single ventricle cardiac diagnosis (%) | 13 (81.25) | 7 (87.5) | 6 (75) | 0.500 |
| Cardiac surgery type | ||||
| Central/BTT Shunt (%) COA/Aortic Arch (%) Hybrid (%) Norwood (%) TAPVR/Shunt (%) | 8 (50) 2 (12.5) 2 (12.5) 3 (18.75) 1 (6.25) | 3 (37.5) 0 2 (25) 2 (25) 1 (12.5) | 5 (62.5) 2 (25) 0 1 (12.5) 0 | 0.825 |
| Age at surgery (days) | 10 (7.25) | 10 (6.5) | 9.5 (5) | 0.328 |
| Weight at surgery (kg) | 3 (0.66) | 3.25 (0.65) | 3.42 (0.75) | 0.798 |
| Intubated pre-operatively (%) | 4 (25) | 3 (37.5) | 1(4.25) | 0.285 |
| STAT category | ||||
| 1 (%) 2 (%) 3 (%) 4 (%) 5 (%) | 1 (6.25) - - 8 (50) 7 (43.75) | - - - 4 (50) 4 (50) | 1 (12.5) - - 4 (50) 3 (37.5) | 0.48 |
| Cortisol level | 5.45 (2.95) | 2.15 (3.3) | 5.95 (9.98) | <0.0001 |
| Time of day cortisol drawn (00:00) | 12:54 (8:33) | 15:32 (6:11) | 10:32 (10:11) | 0.234 |
| Lowest cortisol level drawn (days) | 2 (2.55) | 2.71 (2.44) | 1.11 (2.4) | 0.505 |
| Hydrocortisone started after level (%) | 15 (93.75) | 8 (100) | 7 (87.5) | 0.500 |
| Length of hydrocortisone (days) | 10 (11.25) | 10 (7.5) | 6 (11) | 0.491 |
| ECMO required post-operatively (%) | 4 (25) | 2 (25) | 2 (25) | 0.715 |
| Total mechanical ventilation (days) | 11 (12.25) | 13 (11.5) | 9.5 (7) | 0.574 |
| Reintubation after initial extubation (%) | 9 (56.25) | 6 (75) | 3 (37.5) | 0.442 |
| CBP time (minutes) | 123 (29.5) | 118.5 (23) | 127 (27) | 0.394 |
| Open-chest post-operatively | 9 (56.25) | 5 (62.5) | 4 (50) | 0.500 |
| Post-operative chest closure (days) | 2.5 (8.5) | 6 (10.25) | 1 (4.25) | 0.442 |
| Max VIS first post-operative day | 10.5 (12.75) | 13 (10.5) | 8 (14.75) | 0.721 |
| Max Sv02 first post-operative day | 48.15 (29.85) | 44.3 (18.35) | 60.75 (31.2) | 0.328 |
| Min right rS02 first post-operative day | 36.5 (16) | 36.5 (15.25) | 35 (24.5) | 0.382 |
| Min left rS02 first post-operative day | 51.5 (21.25) | 48 (16.25) | 53 (17) | 1.00 |
| Max lactate first post-operative day | 3.65 (3.1) | 2.8 (1.63) | 4.6 (1.63) | 0.328 |
| Highest BUN post-operatively | 9 (0.25) | 9 (0) | 9 (1) | 0.574 |
| Highest creatinine post-operatively | 0.40 (0.24) | 0.35 (0.13) | 0.43 (0.26) | 0.328 |
| Length of CICU stay (days) | 26.5 (16) | 33 (22.25) | 19.5 (12.75) | 0.083 |
| Length of neonatal stay (days) | 60 (97.25) | 59.5 (91.75) | 54.5 (90.25) | 0.645 |
| Transplant-free survival discharge (%) | 13 (81.25) | 6 (75) | 7 (87.5) | 0.500 |
| Status | Alteration | Impact/Function | ||
|---|---|---|---|---|
| CIRCI | Non-CIRCI | |||
| STX1A | `x x x x x x x x | - - - - - - - - | c.541-8A>C | Splicing/Loss |
| RAB6A | `- x X X X x X X | - - - - - - - - | c.496-9T>C | Unknown |
| ABCA3 | `x x x x - x x - | - - - - - - - - | c.2695A>C (p.T899P) | Missense/Normal |
| SIDT2 | `- x x x x x - x | - - - - - - - - | c. 1015 + 17T>G | Unknown |
| LILRB3 | `x - x x - x x x | - - - - - - - - | c.940G>Tp.D314Y | Missense/Normal |
| INPPL1 | `x x x x - -x x | - - - - - x - - | c.2329T>Ap.Y777N | Splicing/Loss |
| FAM189A2 | `- x x x x x x x | x - - - - - - - | c.742T>Ap.S248T | Missense/Normal |
| `x - x x x x x - | - - x - x - - - | c.704-3C>A | Splicing/Loss | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diehl, N.; Kibiryeva, N.; Marshall, J.; Tsai, S.L.; Farias, J.S.; Silva-Gburek, J.; Erickson, L.A. SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency. Genes 2024, 15, 128. https://doi.org/10.3390/genes15010128
Diehl N, Kibiryeva N, Marshall J, Tsai SL, Farias JS, Silva-Gburek J, Erickson LA. SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency. Genes. 2024; 15(1):128. https://doi.org/10.3390/genes15010128
Chicago/Turabian StyleDiehl, Nicholas, Natalia Kibiryeva, Jennifer Marshall, Sarah L. Tsai, Juan S. Farias, Jaime Silva-Gburek, and Lori A. Erickson. 2024. "SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency" Genes 15, no. 1: 128. https://doi.org/10.3390/genes15010128





