Variation of FMRP Expression in Peripheral Blood Mononuclear Cells from Individuals with Fragile X Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Samples
2.2. PBMC Collection and Storage
2.3. CGG Genotyping and Methylation Status
2.4. FMR1 mRNA Expression Levels
2.5. TR-FRET Assay
2.6. FMRP Quantification
2.6.1. Calculations
2.6.2. Significance of the Presence of FMRP
2.7. Statistical Analyses
3. Results
3.1. PBMCs Express Lower Levels of FMRP Relative to Fibroblasts
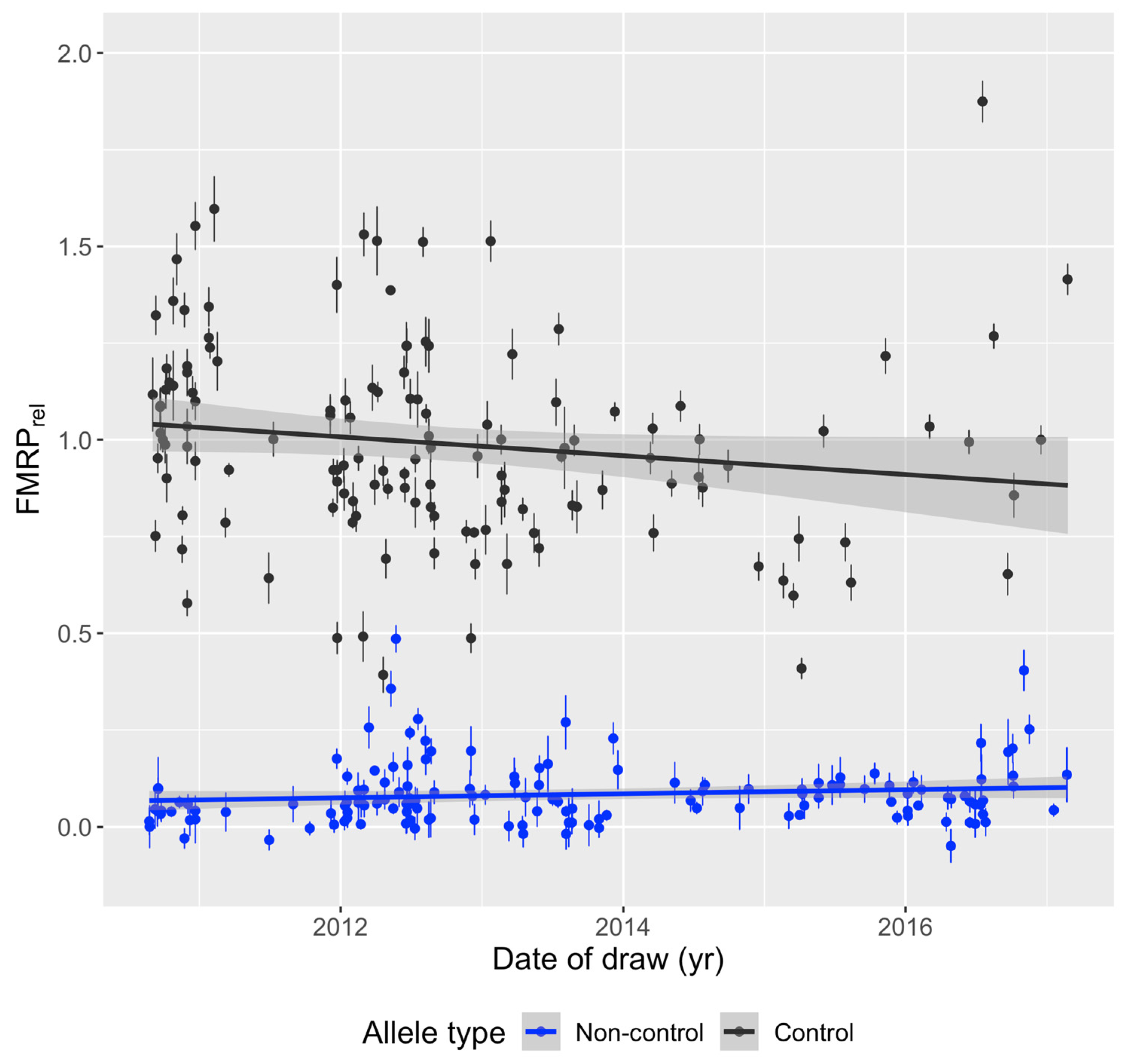
3.2. FMRP Levels Are Independent of Year of Blood Draw and Age
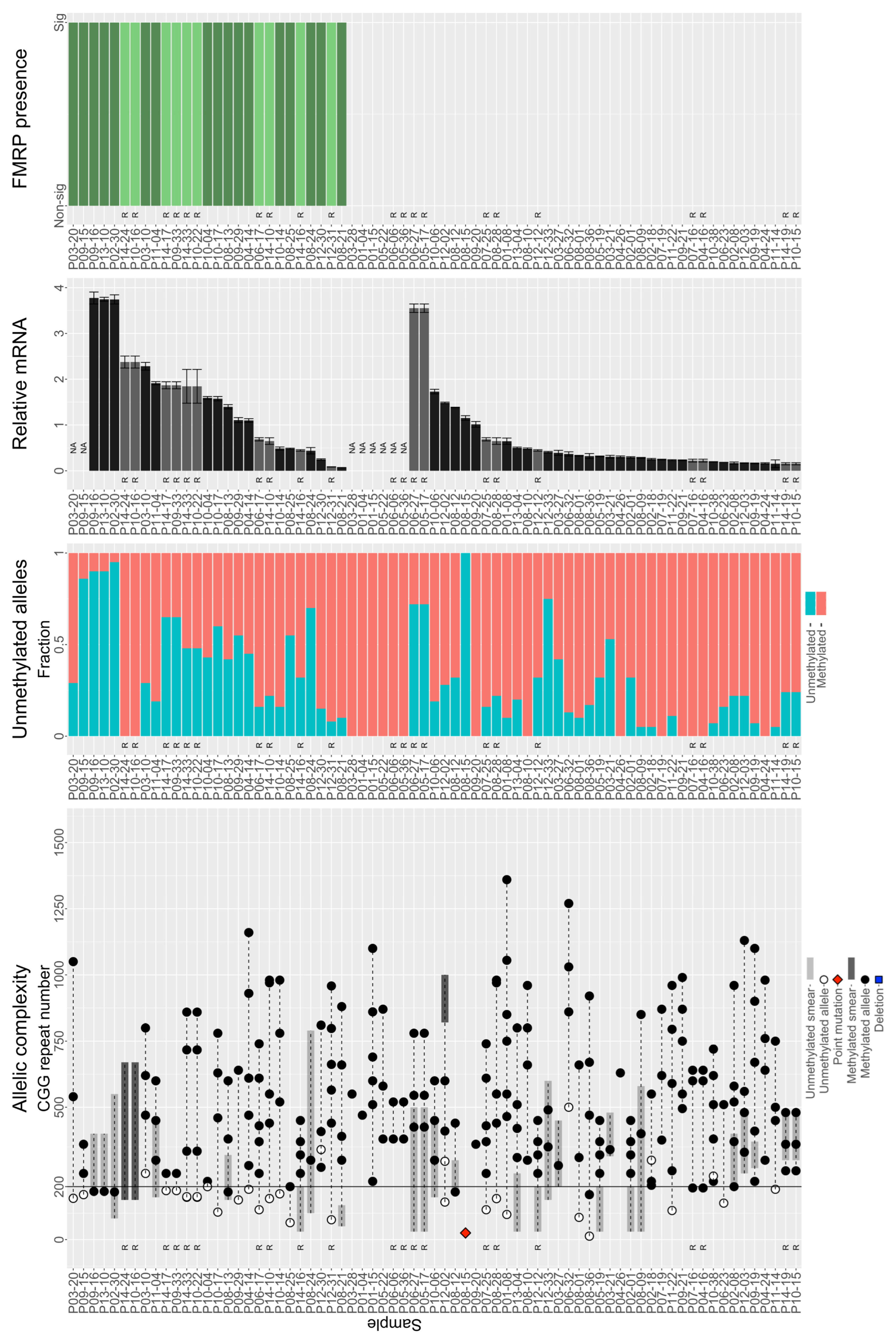
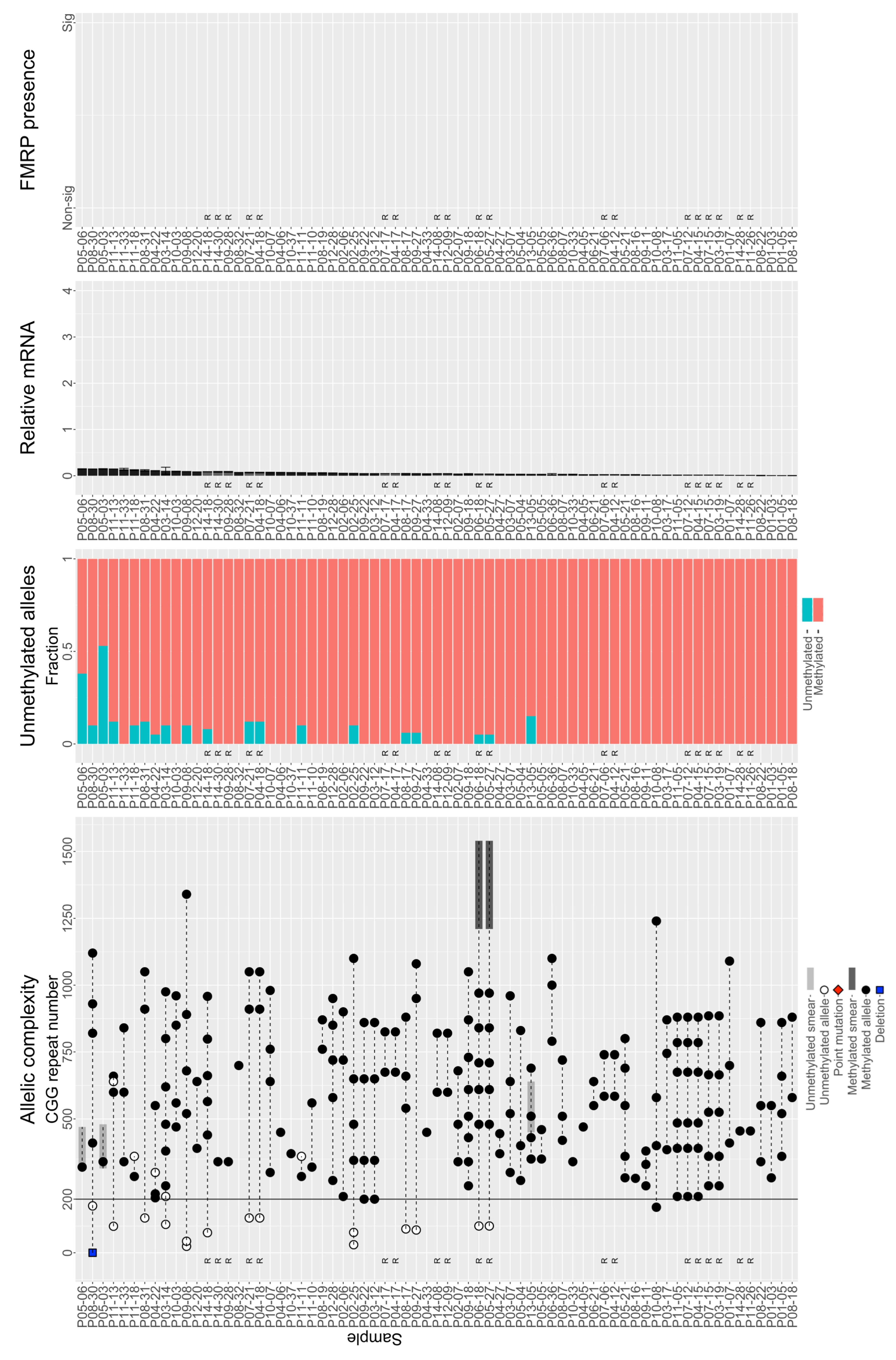
3.3. Individuals with FM Alleles Generally Have a Complex Genotype
3.4. Large Unmethylated and Methylated Alleles Produce mRNA
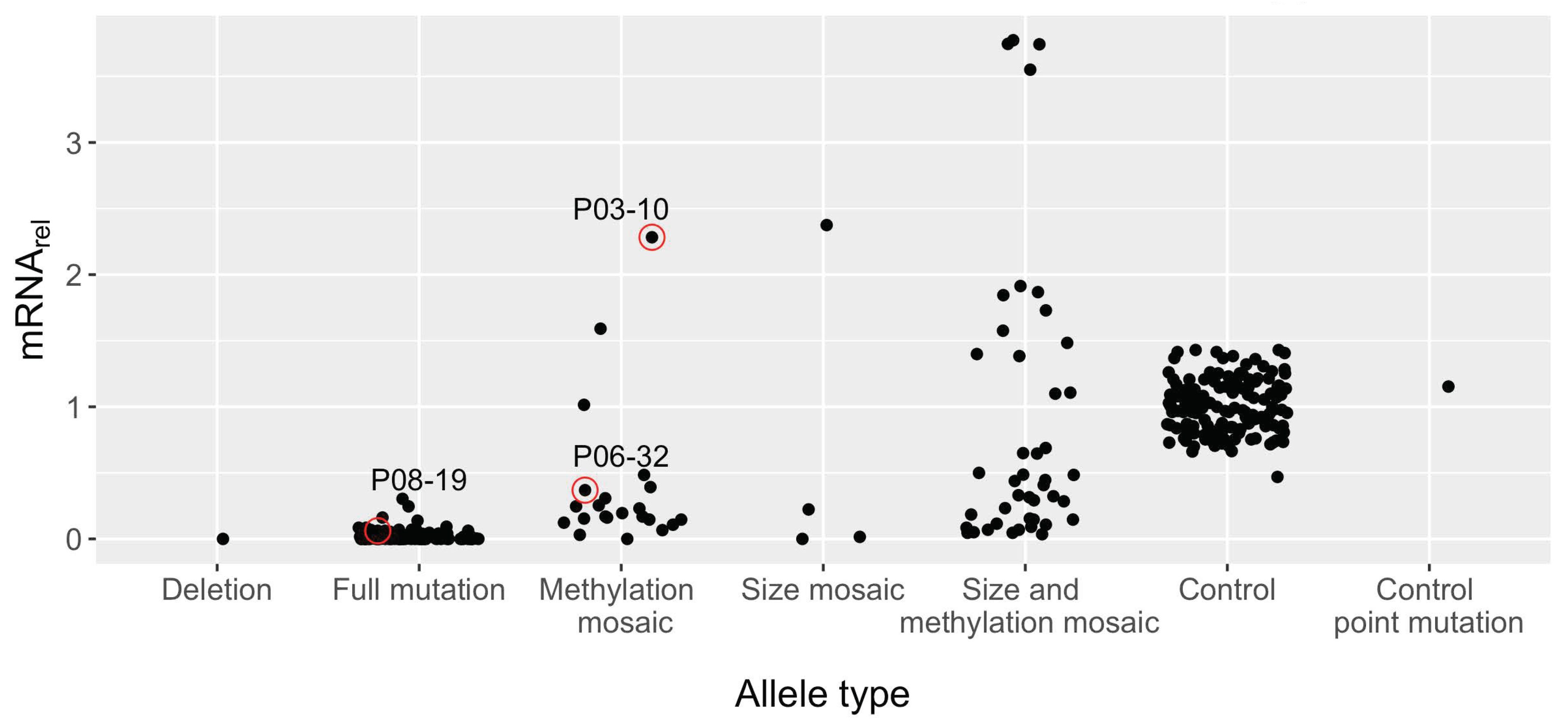
3.5. Large FM Alleles Produce Little to No FMRP, Rarely Approaching Control Levels despite Excess mRNA
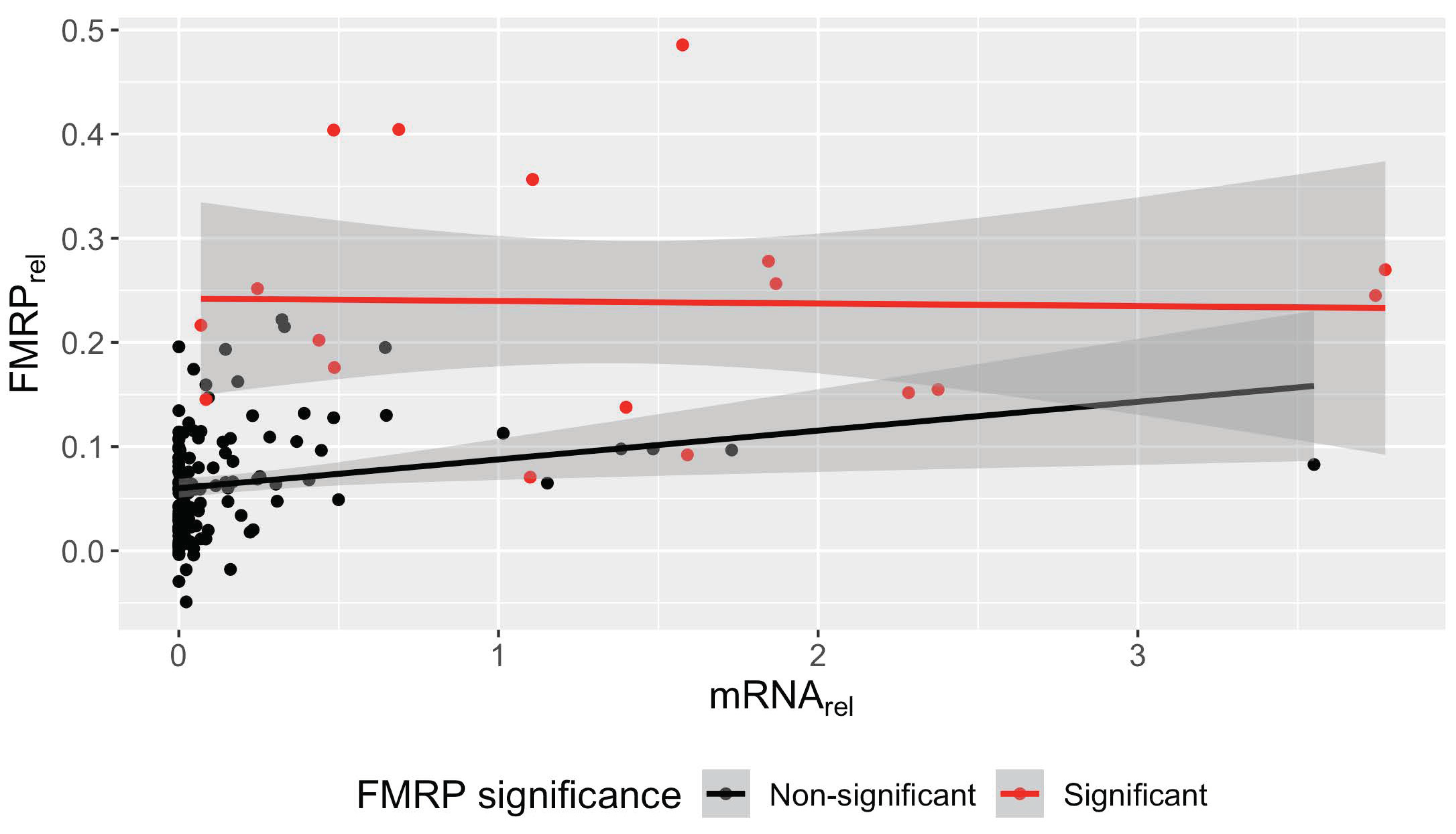
3.6. Significant Association between FMR1 mRNA and FMRP in Non-Control PBMCs
3.7. Translation Efficiency Is Negatively Associated with the Smallest CGG Repeat Size in Samples with FM Alleles
3.8. No Significant FMRP Production Was Detected for Alleles Greater Than ~270 CGG Repeats
3.9. FMR1 mRNA Significantly Affects FMRP and Unmethylated CGG Repeat Size Trends toward Significance in Nested Mixed-Effects Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef] [PubMed]
- De Rubeis, S.; Bagni, C. Regulation of molecular pathways in the Fragile X Syndrome: Insights into Autism Spectrum Disorders. J. Neurodev. Disord. 2011, 3, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.Y.; Klyachko, V.A. Channelopathies in fragile X syndrome. Nat. Rev. Neurosci. 2021, 22, 275–289. [Google Scholar] [CrossRef] [PubMed]
- McCary, L.M.; Roberts, J.E. Early identification of autism in fragile X syndrome: A review. J. Intellect. Disabil. Res. 2013, 57, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Kidd, S.A.; Andrews, H.F.; Budimirovic, D.B.; Esler, A.; Haas-Givler, B.; Stackhouse, T.; Riley, C.; Peacock, G.; Sherman, S.L.; et al. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 2017, 139, S194–S206. [Google Scholar] [CrossRef] [PubMed]
- Kosinovsky, B.; Hermon, S.; Yoran-Hegesh, R.; Golomb, A.; Senecky, Y.; Goez, H.; Kramer, U. The yield of laboratory investigations in children with infantile autism. J. Neural Transm. 2005, 112, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Iossifov, I.; Ronemus, M.; Levy, D.; Wang, Z.; Hakker, I.; Rosenbaum, J.; Yamrom, B.; Lee, Y.H.; Narzisi, G.; Leotta, A.; et al. De novo gene disruptions in children on the autistic spectrum. Neuron 2012, 74, 285–299. [Google Scholar] [CrossRef]
- Muhle, R.; Trentacoste, S.V.; Rapin, I. The genetics of autism. Pediatrics 2004, 113, e472–e486. [Google Scholar] [CrossRef]
- Schaefer, G.B.; Mendelsohn, N.J. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet. Med. 2013, 15, 399–407. [Google Scholar] [CrossRef]
- Richter, J.D.; Zhao, X. The molecular biology of FMRP: New insights into fragile X syndrome. Nat. Rev. Neurosci. 2021, 22, 209–222. [Google Scholar] [CrossRef]
- Kaufmann, W.E.; Raspa, M.; Bann, C.M.; Gable, J.M.; Harris, H.K.; Budimirovic, D.B.; Lozano, R. Latent Class Analysis Identifies Distinctive Behavioral Subtypes in Children with Fragile X Syndrome. J. Autism. Dev. Disord. 2024, 54, 725–737. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Raspa, M.; Bishop, E.; Mitra, D.; Martin, S.; Wheeler, A.; Sacco, P. Health and economic consequences of fragile X syndrome for caregivers. J. Dev. Behav. Pediatr. JDBP 2012, 33, 705–712. [Google Scholar] [CrossRef]
- Wheeler, A.C.; Gwaltney, A.; Raspa, M.; Okoniewski, K.C.; Berry-Kravis, E.; Botteron, K.N.; Budimirovic, D.; Hazlett, H.C.; Hessl, D.; Losh, M.; et al. Emergence of Developmental Delay in Infants and Toddlers with an FMR1 Mutation. Pediatrics 2021, 147, e2020011528. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Schlageter, A.; Filipovic-Sadic, S.; Protic, D.D.; Bram, E.; Mahone, E.M.; Nicholson, K.; Culp, K.; Javanmardi, K.; Kemppainen, J.; et al. A Genotype-Phenotype Study of High-Resolution FMR1 Nucleic Acid and Protein Analyses in Fragile X Patients with Neurobehavioral Assessments. Brain Sci. 2020, 10, 694. [Google Scholar] [CrossRef]
- Kim, K.; Hessl, D.; Randol, J.L.; Espinal, G.M.; Schneider, A.; Protic, D.; Aydin, E.Y.; Hagerman, R.J.; Hagerman, P.J. Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat expansions. PLoS ONE 2019, 14, e0226811. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Folsom, T.D. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology 2011, 60, 1221–1226. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Kneeland, R.E.; Liesch, S.B.; Folsom, T.D. Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr. Res. 2010, 124, 246–247. [Google Scholar] [CrossRef]
- Kelemen, O.; Kovacs, T.; Keri, S. Contrast, motion, perceptual integration, and neurocognition in schizophrenia: The role of fragile-X related mechanisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Protic, D.; Allen, E.G.; Archibald, A.D.; Baud, A.; Brown, T.W.; Budimirovic, D.B.; Cohen, J.; Dufour, B.; Eiges, R.; et al. Insight and Recommendations for Fragile X-Premutation-Associated Conditions from the Fifth International Conference on FMR1 Premutation. Cells 2023, 12, 2330. [Google Scholar] [CrossRef] [PubMed]
- LaFauci, G.; Adayev, T.; Kascsak, R.; Brown, W.T. Detection and Quantification of the Fragile X Mental Retardation Protein 1 (FMRP). Genes 2016, 7, 121. [Google Scholar] [CrossRef]
- LaFauci, G.; Adayev, T.; Kascsak, R.; Kascsak, R.; Nolin, S.; Mehta, P.; Brown, W.T.; Dobkin, C. Fragile X screening by quantification of FMRP in dried blood spots by a Luminex immunoassay. J. Mol. Diagn. 2013, 15, 508–517. [Google Scholar] [CrossRef]
- Roth, M.; Ronco, L.; Cadavid, D.; Durbin-Johnson, B.; Hagerman, R.J.; Tassone, F. FMRP Levels in Human Peripheral Blood Leukocytes Correlates with Intellectual Disability. Diagnostics 2021, 11, 1780. [Google Scholar] [CrossRef]
- Schutzius, G.; Bleckmann, D.; Kapps-Fouthier, S.; di Giorgio, F.; Gerhartz, B.; Weiss, A. A quantitative homogeneous assay for fragile X mental retardation 1 protein. J. Neurodev. Disord. 2013, 5, 8. [Google Scholar] [CrossRef]
- Sitzmann, A.F.; Hagelstrom, R.T.; Tassone, F.; Hagerman, R.J.; Butler, M.G. Rare FMR1 gene mutations causing fragile X syndrome: A review. Am. J. Med. Genet. A 2018, 176, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Jiraanont, P.; Hagerman, R.J.; Neri, G.; Zollino, M.; Murdolo, M.; Tassone, F. Germinal mosaicism for a deletion of the FMR1 gene leading to fragile X syndrome. Eur. J. Med. Genet. 2016, 59, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Filipovic-Sadic, S.; Sah, S.; Chen, L.; Krosting, J.; Sekinger, E.; Zhang, W.; Hagerman, P.J.; Stenzel, T.T.; Hadd, A.G.; Latham, G.J.; et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin. Chem. 2010, 56, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Pan, R.; Amiri, K.; Taylor, A.K.; Hagerman, P.J. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J. Mol. Diagn. 2008, 10, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Longshore, J.; Zunich, J.; Steinbach, P.; Salat, U.; Taylor, A.K. Tissue-specific methylation differences in a fragile X premutation carrier. Clin. Genet. 1999, 55, 346–351. [Google Scholar] [CrossRef]
- Tassone, F.; Hagerman, R.J.; Taylor, A.K.; Gane, L.W.; Godfrey, T.E.; Hagerman, P.J. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 2000, 66, 6–15. [Google Scholar] [CrossRef]
- Tassone, F.; Hagerman, R.J.; Chamberlain, W.D.; Hagerman, P.J. Transcription of the FMR1 gene in individuals with fragile X syndrome. Am. J. Med. Genet. 2000, 97, 195–203. [Google Scholar] [CrossRef]
- Kenneson, A.; Zhang, F.; Hagedorn, C.H.; Warren, S.T. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet. 2001, 10, 1449–1454. [Google Scholar] [CrossRef]
- Boggs, A.E.; Schmitt, L.M.; McLane, R.D.; Adayev, T.; LaFauci, G.; Horn, P.S.; Dominick, K.C.; Gross, C.; Erickson, C.A. Optimization, validation and initial clinical implications of a Luminex-based immunoassay for the quantification of Fragile X Protein from dried blood spots. Sci. Rep. 2022, 12, 5617. [Google Scholar] [CrossRef]
- Genc, B.; Muller-Hartmann, H.; Zeschnigk, M.; Deissler, H.; Schmitz, B.; Majewski, F.; von Gontard, A.; Doerfler, W. Methylation mosaicism of 5′-(CGG)(n)-3′ repeats in fragile X, premutation and normal individuals. Nucleic Acids Res. 2000, 28, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- McConkie-Rosell, A.; Lachiewicz, A.M.; Spiridigliozzi, G.A.; Tarleton, J.; Schoenwald, S.; Phelan, M.C.; Goonewardena, P.; Ding, X.; Brown, W.T. Evidence that methylation of the FMR-I locus is responsible for variable phenotypic expression of the fragile X syndrome. Am. J. Hum. Genet. 1993, 53, 800–809. [Google Scholar]
- Nolin, S.L.; Glicksman, A.; Houck, G.E., Jr.; Brown, W.T.; Dobkin, C.S. Mosaicism in fragile X affected males. Am. J. Med. Genet. 1994, 51, 509–512. [Google Scholar] [CrossRef]
- Pretto, D.; Yrigollen, C.M.; Tang, H.T.; Williamson, J.; Espinal, G.; Iwahashi, C.K.; Durbin-Johnson, B.; Hagerman, R.J.; Hagerman, P.J.; Tassone, F. Clinical and molecular implications of mosaicism in FMR1 full mutations. Front. Genet. 2014, 5, 318. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Hagerman, R.J.; Loesch, D.Z.; Lachiewicz, A.; Taylor, A.K.; Hagerman, P.J. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. Am. J. Med. Genet. 2000, 94, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Kaufmann, W.E.; Frye, R.E.; Ong, K.; Kaminski, J.W.; Velinov, M.; Berry-Kravis, E. The association between mosaicism type and cognitive and behavioral functioning among males with fragile X syndrome. Am. J. Med. Genet. A 2022, 188, 858–866. [Google Scholar] [CrossRef]
- Usdin, K.; Hayward, B.E.; Kumari, D.; Lokanga, R.A.; Sciascia, N.; Zhao, X.N. Repeat-mediated genetic and epigenetic changes at the FMR1 locus in the Fragile X-related disorders. Front. Genet. 2014, 5, 226. [Google Scholar] [CrossRef]
- Zhao, X.N.; Usdin, K. Ups and Downs: Mechanisms of Repeat Instability in the Fragile X-Related Disorders. Genes 2016, 7, 70. [Google Scholar] [CrossRef]
- Tabolacci, E.; Nobile, V.; Pucci, C.; Chiurazzi, P. Mechanisms of the FMR1 Repeat Instability: How Does the CGG Sequence Expand? Int. J. Mol. Sci. 2022, 23, 5425. [Google Scholar] [CrossRef] [PubMed]
- Nolin, S.L.; Glicksman, A.; Tortora, N.; Allen, E.; Macpherson, J.; Mila, M.; Vianna-Morgante, A.M.; Sherman, S.L.; Dobkin, C.; Latham, G.J.; et al. Expansions and contractions of the FMR1 CGG repeat in 5,508 transmissions of normal, intermediate, and premutation alleles. Am. J. Med. Genet. A 2019, 179, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Hayward, B.E.; Zafarullah, M.; Kumar, J.; Durbin Johnson, B.; Holmans, P.; Usdin, K.; Tassone, F. Both cis and trans-acting genetic factors drive somatic instability in female carriers of the FMR1 premutation. Sci. Rep. 2022, 12, 10419. [Google Scholar] [CrossRef] [PubMed]
- Pretto, D.I.; Mendoza-Morales, G.; Lo, J.; Cao, R.; Hadd, A.; Latham, G.J.; Durbin-Johnson, B.; Hagerman, R.; Tassone, F. CGG allele size somatic mosaicism and methylation in FMR1 premutation alleles. J. Med. Genet. 2014, 51, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.Z.; Huggins, R.M.; Bui, Q.M.; Taylor, A.K.; Pratt, C.; Epstein, J.; Hagerman, R.J. Effect of fragile X status categories and FMRP deficits on cognitive profiles estimated by robust pedigree analysis. Am. J. Med. Genet. A 2003, 122A, 13–23. [Google Scholar] [CrossRef]
- Menon, V.; Leroux, J.; White, C.D.; Reiss, A.L. Frontostriatal deficits in fragile X syndrome: Relation to FMR1 gene expression. Proc. Natl. Acad. Sci. USA 2004, 101, 3615–3620. [Google Scholar] [CrossRef] [PubMed]
- de Vries, B.B.; Jansen, C.C.; Duits, A.A.; Verheij, C.; Willemsen, R.; van Hemel, J.O.; van den Ouweland, A.M.; Niermeijer, M.F.; Oostra, B.A.; Halley, D.J. Variable FMR1 gene methylation of large expansions leads to variable phenotype in three males from one fragile X family. J. Med. Genet. 1996, 33, 1007–1010. [Google Scholar] [CrossRef]
- Jacquemont, S.; Pacini, L.; Jonch, A.E.; Cencelli, G.; Rozenberg, I.; He, Y.; D’Andrea, L.; Pedini, G.; Eldeeb, M.; Willemsen, R.; et al. Protein synthesis levels are increased in a subset of individuals with fragile X syndrome. Hum. Mol. Genet. 2018, 27, 2039–2051. [Google Scholar] [CrossRef]
- Santa Maria, L.; Pugin, A.; Alliende, M.A.; Aliaga, S.; Curotto, B.; Aravena, T.; Tang, H.T.; Mendoza-Morales, G.; Hagerman, R.; Tassone, F. FXTAS in an unmethylated mosaic male with fragile X syndrome from Chile. Clin. Genet. 2014, 86, 378–382. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, F.; Lokey, L.K.; Chastain, J.L.; Lakkis, L.; Eberhart, D.; Warren, S.T. Translational suppression by trinucleotide repeat expansion at FMR1. Science 1995, 268, 731–734. [Google Scholar] [CrossRef]
- Weisman-Shomer, P.; Cohen, E.; Fry, M. Interruption of the fragile X syndrome expanded sequence d(CGG)(n) by interspersed d(AGG) trinucleotides diminishes the formation and stability of d(CGG)(n) tetrahelical structures. Nucleic Acids Res. 2000, 28, 1535–1541. [Google Scholar] [CrossRef]
- Peprah, E.; He, W.; Allen, E.; Oliver, T.; Boyne, A.; Sherman, S.L. Examination of FMR1 transcript and protein levels among 74 premutation carriers. J. Hum. Genet. 2010, 55, 66–68. [Google Scholar] [CrossRef]
- Kumari, D.; Gazy, I.; Usdin, K. Pharmacological Reactivation of the Silenced FMR1 Gene as a Targeted Therapeutic Approach for Fragile X Syndrome. Brain Sci. 2019, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, E.; Palumbo, F.; Nobile, V.; Neri, G. Transcriptional Reactivation of the FMR1 Gene. A Possible Approach to the Treatment of the Fragile X Syndrome. Genes 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Wu, H.; Krzisch, M.; Wu, X.; Graef, J.; Muffat, J.; Hnisz, D.; Li, C.H.; Yuan, B.; Xu, C.; et al. Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene. Cell 2018, 172, 979–992.e976. [Google Scholar] [CrossRef] [PubMed]






| Allele Class | n | Age (Year) | CGG Repeat Size | FMRPrel | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Med. | SD | Min | Max | Mean | Med. | SD | Min | Max | Mean | Med. | SD | |||
| Control | 152 | 1 | 80 | 33.9 | 30.5 | 21.4 | 19 | 46 | 29.7 | 30 | 4.8 | 0.39 | 1.87 | 0.99 | 0.98 | 0.26 | |
| Non-control | 170 | 0 | 57 | 11.1 | 8.5 | 10.2 | 13 | 1400 | 542.5 | 505 | 282.4 | −0.05 | 0.49 | 0.09 | 0.07 | 0.09 | |
| Full mutation | 97 | 1 | 44 | 10.7 | 9 | 9.2 | 200 | 1400 | 610 | 590 | 245.3 | −0.05 | 0.42 | 0.05 | 0.04 | 0.06 | |
| Methylation mosaicism | 22 | 1 | 32 | 10.6 | 5.5 | 10.2 | 200 | 1270 | 482.9 | 380 | 253.4 | −0.02 | 0.25 | 0.09 | 0.08 | 0.06 | |
| Size mosaicism | 4 | 9 | 53 | 29 | 27 | 18.4 | 170 | 1240 | 532.8 | 440 | 364.9 | 0.02 | 0.15 | 0.11 | 0.12 | 0.06 | |
| Size and methylation mosaicism | 45 | 0 | 57 | 10.5 | 8 | 10.6 | 13 | 1540 | 477 | 440 | 314.7 | 0.01 | 0.49 | 0.17 | 0.14 | 0.12 | |
| Point mutation * | 1 | - | - | 10 | - | - | - | - | 25 | - | - | - | - | 0.06 | - | - | |
| Deletion * | 1 | - | - | 6 | - | - | - | - | - | - | - | - | - | 0.08 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randol, J.L.; Kim, K.; Ponzini, M.D.; Tassone, F.; Falcon, A.K.; Hagerman, R.J.; Hagerman, P.J. Variation of FMRP Expression in Peripheral Blood Mononuclear Cells from Individuals with Fragile X Syndrome. Genes 2024, 15, 356. https://doi.org/10.3390/genes15030356
Randol JL, Kim K, Ponzini MD, Tassone F, Falcon AK, Hagerman RJ, Hagerman PJ. Variation of FMRP Expression in Peripheral Blood Mononuclear Cells from Individuals with Fragile X Syndrome. Genes. 2024; 15(3):356. https://doi.org/10.3390/genes15030356
Chicago/Turabian StyleRandol, Jamie L., Kyoungmi Kim, Matthew D. Ponzini, Flora Tassone, Alexandria K. Falcon, Randi J. Hagerman, and Paul J. Hagerman. 2024. "Variation of FMRP Expression in Peripheral Blood Mononuclear Cells from Individuals with Fragile X Syndrome" Genes 15, no. 3: 356. https://doi.org/10.3390/genes15030356






